Abstract
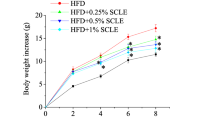
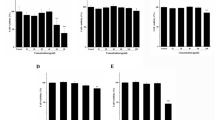
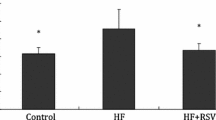
Smilax china L. is an important herb used in traditional Chinese medicine. In this study, the mechanism of Smilax china L. polyphenols (SCP) on insulin resistance and anti-obesity in mice induced by a high-fat diet (HFD) was investigated. Fifty female mice were randomly divided into five groups: control, HFD and low, medium, and high doses of SCP for 70 d. SCP significantly decreased intraperitoneal adipose tissue index, body weight gain, liver lipids, and serum inflammatory factor levels. Blood glucose and insulin concentrations, as well as insulin resistance index in SCP, were significantly lower than those in HFD. In addition, SCP markedly up-regulated the gene expression of glucose transporter 4 (GLUT4), insulin receptor substrate 1 (IRS1), insulin receptor substrate 2 (IRS2), serine-threonine kinase (AKT), Acyl-CoA oxidase (ACO), and protein kinase A (PKA), and down-regulated the expression of mammalian target of rapamycin complex 1 (mTORC1), sterol-responsive element-binding protein-1c (SREBP1c), fatty acid synthase (FAS), 3-hydroxy-3-methyl glutaryl coenzyme A reductase (HMGCR), and forkhead box protein O1 (FOXO1). SCP significantly increased the protein expression of AKT, GLUT4, AMP-activated protein kinase (AMPK), phosphorylated-AMPK (p-AMPK), phosphorylated-AKT (p-AKT), and uncoupling protein 1 (UCP-1), and decreased the expression of SREBP1c, FAS, HMGCR, phosphorylation of IKBα (p-IKBα), and nuclear factor kappa B subunit p65 (P65) in the liver. Overall, SCP effectively reduced HFD-induced insulin resistance and obesity in mice, partly through NF-κB and IRS/AKT-AMPK signaling pathways to regulate inflammatory factors. Therefore, SCP may improve lifestyle diseases.




Similar content being viewed by others
Data Availability
The authors are confident that all data and materials conform to domain standards.
References
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 348(17):1625–1638. https://doi.org/10.1056/NEJMoa021423
Ruan H, Miles PD, Ladd CM, Ross K, Golub TR, Olefsky JM, Lodish HF (2002) Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-α: implications for insulin resistance. Diabetes 51(11):3176–3188. https://doi.org/10.2337/diabetes.51.11.3176
Tanti J-F, Ceppo F, Jager J, Berthou F (2013) Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front Endocrinol 3:181–195. https://doi.org/10.3389/fendo.2012.00181
Kurutas EB, Cetinkaya A, Bulbuloglu E, Kantarceken B (2005) Effects of antioxidant therapy on leukocyte myeloperoxidase and Cu/Zn-superoxide dismutase and plasma malondialdehyde levels in experimental colitis. Mediators Inflamm 2005(6):390–394. https://doi.org/10.1155/MI.2005.390
Fleischman A, Shoelson SE, Bernier R, Goldfine AB (2008) Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care 31(2):289–294. https://doi.org/10.2337/dc07-1338
Guo S (2014) Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models to disease mechanisms. J Endocrinol 220(2):T1–T23. https://doi.org/10.1530/JOE-13-0327
Liu Z, Sun Y, Qiao Q, Zhao T, Zhang W, Ren B, Liu Q, Liu X (2017) Sesamol ameliorates high-fat and high-fructose induced cognitive defects via improving insulin signaling disruption in the central nervous system. Food Funct 8(2):710–719. https://doi.org/10.1039/c6fo01562j
Vazirian M, Nabavi SM, Jafari S, Manayi A (2018) Natural activators of adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK) and their pharmacological activities. Food Chem Toxicol 122:69–79. https://doi.org/10.1016/j.fct.2018.09.079
Sun X, Zhang H (2007) NFKB and NFKBI polymorphisms in relation to susceptibility of tumour and other diseases. Histol Histopathol 22:1387–1398. https://doi.org/10.14670/HH-22.1387
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N (2014) Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105(2):141–150. https://doi.org/10.1016/j.diabres.2014.04.006
Shu X-S, Gao Z-H, Yang X-L (2006) Anti-inflammatory and anti-nociceptive activities of Smilax china L. aqueous extract. J Ethnopharmacol 103(3):327–332. https://doi.org/10.1016/j.jep.2005.08.004
Yang L, Zhao Y, Pan Y, Li D, Zheng G (2019) Dietary supplement of Smilax china L. ethanol extract alleviates the lipid accumulation by activating AMPK pathways in high-fat diet fed mice. Nutr Metab 16(6):1–11. https://doi.org/10.1186/s12986-019-0333-z
Xu M, Xue H, Li X, Zhao Y, Lin L, Yang L, Zheng G (2019) Chemical composition, antibacterial properties, and mechanism of Smilax china L. polyphenols. Appl Microbiol Biotechnol 103:9013–9022. https://doi.org/10.1007/s00253-019-10100-0
Folch J, Lees M, Sloane G (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509. https://doi.org/10.1016/S0021-9258(18)64849-5
Zhao Y, Yang L, Huang Z, Lin L, Zheng G (2017) Synergistic effects of caffeine and catechins on lipid metabolism in chronically fed mice via the AMP-activated protein kinase signaling pathway. Eur J Nutr 56(7):2309–2318. https://doi.org/10.1007/s00394-016-1271-4
Chen L, Chen R, Wang H, Liang F (2015) Mechanisms linking inflammation to insulin resistance. Int J Endocrinol 2015:508409. https://doi.org/10.1155/2015/508409
Ben J, Jiang B, Wang D, Liu Q, Zhang Y, Qi Y, Tong X, Chen L, Liu X, Zhang Y (2019) Major vault protein suppresses obesity and atherosclerosis through inhibiting IKK–NF-κB signaling mediated inflammation. Nat Commun 10(1):1801–1815. https://doi.org/10.1038/s41467-019-09588-x
Catrysse L, van Loo G (2017) Inflammation and the metabolic syndrome: the tissue-specific functions of NF-κB. Trends Cell Biol 27(6):417–429. https://doi.org/10.1016/j.tcb.2017.01.006
Chen Y, Nie Y-c, Luo Y-l, Lin F, Zheng Y-f, Cheng G-h, Wu H, Zhang K-j, Su W-w, Shen J-g (2013) Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem Toxicol 58:133–140. https://doi.org/10.1016/j.fct.2013.04.024
Huang H, Cheng Z, Shi H, Xin W, Wang TT, Yu L (2011) Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties. J Agric Food Chem 59(9):4562–4569. https://doi.org/10.1021/jf2002969
Mohanraj L, Kim H-S, Li W, Cai Q, Kim KE, Shin H-J, Lee Y-J, Lee WJ, Kim JH, Oh Y (2013) IGFBP-3 inhibits cytokine-induced insulin resistance and early manifestations of atherosclerosis. PLoS ONE 8(1):e55084. https://doi.org/10.1371/journal.pone.0055084
Daniele G, Guardado Mendoza R, Winnier D, Fiorentino T, Pengou Z, Cornell J, Andreozzi F, Jenkinson C, Cersosimo E, Federici M (2014) The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol 51(1):123–131. https://doi.org/10.1007/s00592-013-0543-1
Osborn O, Olefsky JM (2012) The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 18(3):363–374. https://doi.org/10.1038/nm.2627
Boura-Halfon S, Zick Y (2009) Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol-Endoc M 296(4):E581–E591. https://doi.org/10.1152/ajpendo.90437.2008
Tanti J-F, Jager J (2009) Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol 9(6):753–762. https://doi.org/10.1016/j.coph.2009.07.004
Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB (2001) A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci 98(8):4640–4645. https://doi.org/10.1073/pnas.051042298
Samuel VT, Shulman GI (2016) The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest 126(1):12–22. https://doi.org/10.1172/JCI77812
Hotamisligil GS (2017) Inflammation, metaflammation and immunometabolic disorders. Nat 542(7640):177–185. https://doi.org/10.1038/nature21363
Mack R, Skurnick B, Sterling-Jean Y, Pedra-Nobre M, Bigg D (2003) Fasting insulin levels as a measure of insulin resistance in american blacks. J Med 34(1–6):31–38. https://doi.org/10.5694/j.1326-5377.2006.tb00506.x
Caron A, Richard D, Laplante M (2015) The roles of mTOR complexes in lipid metabolism. Annu Rev Nutr 35(1):321–348. https://doi.org/10.1146/annurev-nutr-071714-034355
Funding
This work was sponsored by the National Natural Science Foundation of China (No: 82060165 and 81760157).
Author information
Authors and Affiliations
Contributions
Meng Xu: Formal analysis, Writing-original draft, Investigation, Data curation. Hui Xue: Writing - review & editing, Data curation. Li Kong: Data curation, Formal analysis. Lezhen Lin: Writing - review, Formal analysis. Guodong Zheng: Funding acquisition, Conceptualization, Writing - review & editing, Project administration, Validation.
Corresponding author
Ethics declarations
Ethics Approval
The use of the animals and experimental methods were in compliant with the Guide for the Care and Use of Laboratory Animals of the Chinese Association for Laboratory Animal Science. The experimental protocols were approved by the Animal Care and Use Committee of the Jiangxi Agricultural University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, M., Xue, H., Kong, L. et al. Smilax china L. Polyphenols Improves Insulin Resistance and Obesity in High-fat Diet-induced Mice Through IRS/AKT-AMPK and NF-κB Signaling Pathways. Plant Foods Hum Nutr 78, 299–306 (2023). https://doi.org/10.1007/s11130-023-01052-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-023-01052-y