Abstract
Trees regenerating in the understory respond to increased availability of light caused by gap formation by undergoing a range of morphological and physiological adjustments. These adjustments include the production of thick, sun-type leaves containing thicker mesophyll and longer palisade cells than in shade-type leaves. We asked whether in the shade-regenerating tree Acer pseudoplatanus, the increase in leaf thickness and expansion of leaf tissues are possible also in leaves that had been fully formed prior to the increase in irradiance, a response reported so far only for a handful of species. We acclimated potted seedlings to eight levels (from 1 to 100%) of solar irradiance and, in late summer, transferred a subset of them to full sunlight. Within 30 days, the shaded leaves increased leaf mass per area and became thicker mostly due to elongation of palisade cells, except for the most shaded individuals which suffered irreversible photo-oxidative damage. This anatomical acclimation was accompanied by partial degradation of chlorophyll and a transient decline in photosynthetic efficiency of PSII (Fv/FM). These effects were related to the degree of pre-shading. The Fv/FM recovered substantially within the re-acclimation period. However, leaves of transferred plants were shed significantly earlier in the fall, indicating that the acclimation was not fully effective. These results show that A. pseudoplatanus is one of the few known species in which mature leaves may re-acclimate anatomically to increased irradiance. This may be a potentially important mechanism enhancing utilization of gaps created during the growing season.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Survival and growth of trees regenerating in the understory is made possible by adjustments of plant architecture and leaf anatomy, and physiological regulation, together increasing the efficiency of light harvesting and improving the whole plant’s energy budget (Givnish 1988; Valladares and Niinemets 2008). Especially important is the acclimatory modification of leaf structure and physiology, allowing optimization of photosynthetic capacity with respect to light harvesting (Oguchi et al. 2005). Anatomical acclimation to low irradiance manifests itself with the production of thin leaves containing single-layered palisade mesophyll, with short cells and a small number of chloroplasts. In contrast, leaves produced under high light often contain several layers of strongly elongated palisade cells, capable of accommodating large numbers of chloroplasts needed for absorption and utilization of the incoming energy (Terashima et al. 2005). While shade leaves tend to be richer in chlorophyll and contain a similar amount of nitrogen on a mass basis as sun leaves, the sun leaves, because of their greater thickness, contain more nitrogen on an area basis and have a reduced chlorophyll/nitrogen ratio, reflecting the shifting balance between factors limiting photosynthesis (Poorter et al. 2019). Other structural modifications in shade may include lower vein and stomatal density (Scoffoni et al. 2015; Poorter et al. 2019), reduced development of features associated with phloem loading (Amiard et al. 2005) and increased lamina size as well as reduced lobation or dentation (Nicotra et al. 2011), relative to sun leaves.
Leaves are determinate organs, with an early stage of area expansion and tissue formation lasting usually up to 6 weeks in temperate trees (Niinemets et al. 2004; Ding et al. 2014) followed by a developmental plateau (Pantin et al. 2012). According to this accepted view, formation of the final leaf shape, thickness and tissue structure takes place during its early stage of growth. Afterward no further anatomical change is possible, other than accumulation of storage and cell wall material (Amiard et al. 2005). Accordingly, structural acclimation to light must take place during that early time window, prior to reaching the stasis stage. Yet the light environment in the temperate forest canopy is dynamic, with, e.g., gradual canopy defoliation increasing the penetration of light late in the season. Occasional formation of canopy gaps, whether natural or man-made, causes wholesale increases of irradiance in the understory. In fact, gap formation is required by many shade-tolerant trees for growth into the canopy layer. Such sudden increases in irradiance may cause photo-oxidative stress in unacclimated leaves (Oguchi et al. 2006), setting off complex defensive and acclimative responses (Tognetti et al. 1998; Campa et al. 2017; Oguchi et al. 2017) or causing premature leaf death. Developmental plasticity allows new leaves of understory plants produced after an increase in irradiance to match the new conditions physiologically and structurally. On the other hand, in pre-existing leaves the extent of physiological, particularly photosynthetic, re-acclimation varies among species (Yamashita et al. 2000; Calzavara et al. 2017; Martinez and Friedley 2017; Oguchi et al. 2017) and anatomical re-acclimation is considered rare (Oguchi et al. 2018).
Only a handful of studies appear to have actually tested the ability of mature, fully expanded leaves to adjust anatomically to increased irradiance. Although such leaves may respond to increased irradiance by increasing leaf mass per area (LMA), this usually occurs in the absence of any significant increase in thickness (Naidu and DeLucia 1997; Yamashita et al. 2000; Yamashita et al. 2002; Niinemets et al. 2003; Oguchi et al. 2006 but see Frak et al. 2001). Such increases in LMA could be explained by accumulation of carbohydrates and various other protoplast components, as well as cell wall material, leading to increased leaf tissue density. In contrast, the majority of studies that examined the anatomical traits that typically contribute to sun vs. shade leaf anatomy (such as lamina thickness, mesophyll or epidermal thickness or palisade cell length), showed a lack of adjustment of these traits. Examples include seedlings of shade-tolerant trees: Acer saccharum and Quercus rubra (Naidu and DeLucia 1998), Fagus sylvatica (Tognettti et al. 1998), Abies grandis, A. lasiocarpa and Picea engelmannii (Youngblood and Fergusson 2003), Betula ermanii and F. crenata (Oguchi et al. 2005), Q. petraea and Q. cerris (Rodriguez Calcerrada et al. 2008), six other species of trees and two lianas (Oguchi et al. 2006) as well as herbs Alocasia macrorrhiza (Sims and Pearcy 1992) and Chenopodium album (Oguchi et al. 2003). Notable exceptions, however, have been reported. Thickness of fully expanded leaves increased in response to increased irradiance in Glycine max (Bunce et al. 1977), Bischofia javanica, Trema orientalis and Schima mertensiana (Yamashita et al. 2002), and Acer rufinerve (Oguchi et al. 2005). In G. max and A. rufinerve, the increase in lamina thickness was explainable by an increase in mesophyll thickness, which in A. rufinerve was accompanied by an increased area of cell walls contacting intercellular spaces. Another type of anatomical adjustment, found in B. ermanii and A. rufinerve, as well as in C. album involved expansion of surface area of chloroplasts leading to their increased contact with cell walls and resulting in enhanced mesophyll conductance to CO2 (Oguchi et al. 2003, 2005). Moreover, in a single case documented to-date, palisade cells in shade-acclimated leaves of Hedera helix exposed to an increased irradiance underwent divisions leading to formation of an additional palisade layer (Watson 1942; Bauer and Thöni 1988). Results of the above studies indicate that the capacity of fully developed leaves for anatomical acclimation to increased light is species-specific and probably rather rare. On the other hand, it appears that only a small number of species have been investigated using an experimental design that analyzed anatomical changes in mature leaves after a shift in irradiance. Moreover, it may be expected that the extent of re-acclimation should depend on the original growth irradiance, as a high magnitude increase in light may trigger photo-oxidative stress leading to cellular damage and lack of response. Applying multiple levels of growth irradiance may thus be useful in experimental tracking of anatomical responses of mature leaves.
In this study, we examine the ability for anatomical re-acclimation of leaves of sycamore maple (Acer pseudoplatanus L.), a shade-tolerant winter deciduous tree with a broad distribution in Europe. Typical of shade-tolerant species, A. pseudoplatanus shows high survival and slow growth at low light intensities, a low photosynthetic rate at high irradiance and a low light compensation point (Hättenschwiler and Körner 2000; Kazda et al. 2004). Seeds germinate usually before forest canopy closure in the spring but establish and survive under deep canopy shade. Higher irradiance is, however, required if they are to advance to the canopy layer (Helliwell and Harrison 1979). Remarkably, on fertile forest soil, seedlings can survive more than 15 years under deep shade (Hein et al. 2009). Sycamore maple is relatively tolerant to late spring frosts which explains the success of its establishment after the formation of large canopy gaps (Piovesan et al. 2005). We grew potted seedlings of sycamore maple under eight irradiance levels, ranging from deep shade to full sunlight. We then exposed plants to full solar irradiance and examined their response after 30 days to (1) test the hypothesis that mature leaves formed under the various growth irradiances are capable of anatomical acclimation to increased irradiance. We additionally (2) analyzed other responses indicative of acclimation (pigment and nitrogen and concentrations and photochemical efficiency of photosystem II) and (3) monitored leaf persistence on the plants for the remainder of the growing season to evaluate the extent of permanent damage caused by photo-oxidative stress.
Materials and methods
Plant cultivation
In late April 2009, 80 two-year old cold-stored, dormant bare-rooted seedlings of Acer pseudoplatanus L. were obtained from Poznań University of Life Sciences experimental nursery in Zielonka. Plants were transported to an experimental garden at Polish Academy of Sciences Institute of Dendrology in Kórnik (Poland, 55° 15′ N 17° 6′ E). Plants were immediately potted into 5L pots filled with commercial peat-based compost with 10% (v/v) addition of topsoil from a mature A. pseudoplatanus stand and 3 kg m−3 of slow-release fertilizer (Osmocote 15-10-12) and temporarily placed in a shaded spot. Eight levels of growth irradiance were created by erecting outdoor shade house frames and covering them with polyethylene shading cloth of different densities according to manufacturer’s specifications or by combining cloth layers. Relative irradiance in each shade house was determined by taking simultaneous measurements of photosynthetic photon flux density (PPFD, covering wavelengths between 400 and 700 nm) inside the house and under open sky using two sensors (Apogee Instruments, Utah, USA) that gave nearly identical readings (N = 5 readings per house, Supplementary Information Table S1). Measurements were conducted on a sunny day with average (± SD) outside PPFD = 1570 ± 254 µmol m−2 s−1 (n = 35). Average relative irradiances for the different houses were 1.1%, 3.4%, 4.6%, 12.7%, 21.5%, 29.4% and 50.0% of full solar irradiance (hereafter rounded to full numbers). On May 4, when buds were beginning to break, plants were randomly distributed among the shade houses (N = 10 plants for each). An additional set of 10 plants were left unshaded (100% irradiance). Average plant height at that time was 37.6 ± 12.9 cm (range 12.5–74 cm; Supplementary Information Table S2) and there was no significant difference in mean height among irradiance levels (Anova F7;79 = 1.09, P = 0.380). During the experiment, plants were watered regularly to field capacity. They were rearranged haphazardly within each shade house at 3–4 week intervals and kept apart to minimize mutual shading. On August 6, when all terminal buds had formed, leaf samples were collected from all plants (hereafter the PRE treatment). Half of the plants from each shade level were randomly selected and transferred to full irradiance (hereafter, the transferred group, TRANS), with the rest remaining under original growth irradiance (the control group, CONT). Plants were maintained for the rest of the season. Daily minimal and maximal temperatures and direct sunshine hours for the location were obtained from a local weather station (Supplementary Information Fig. S1).
Chlorophyll fluorescence
Chlorophyll a fluorescence was measured in the morning on August 6, i.e., immediately prior to transfer of plants to full irradiance (day 0), then on the following day (day 1) and finally on September 4 (day 29 after transfer) using a Plant Efficiency Analyzer (PEA, Hansatech, Norfolk, UK). Measurements were conducted using the same permanently marked leaves (one per plant). In the evening before measurements, darkening clips were placed on the leaves. The following morning, the PEA fiberoptic was inserted into the clip and a 1 s pulse of saturating light (4000 µmol m−2 s−1) was delivered to the leaf surface. Maximal quantum yield (FV/FM) was calculated from the leaf fluorescence emission according to the formula FV/FM = (FM−F0)/FM where FM is maximal fluorescence under saturating pulse and F0 is fluorescence in dark adapted state (Maxwell and Johnson 2000).
Leaf sampling
On August 5, i.e., one day before transfer to full irradiance, a leaf from one of the lower nodes was collected from each plant. Care was taken to select a leaf that was not shaded by other leaves of the same plant. A ca. 6 cm2 segment not containing midrib was first cut for determination of leaf mass per area. A 5 × 2 mm fragment was excised from the middle part of the lamina, excluding major veins, for anatomical analysis. Remaining leaf material, after removing major veins, was dried and used for determination of nitrogen and carbohydrates. An adjacent, usually opposite, leaf was collected for chlorophyll determination. Sampling was repeated on September 5 (30th day after transfer), where possible using leaves from equivalent positions on the stem.
LMA and leaf anatomy
The exact area of the ca. 6 cm2 leaf segment was determined using a desk-top scanner and the sample was dried in a forced circulation drier at 65 °C for 72 h. Each dry sample was weighed. Leaf mass per area (LMA, g m−2) was determined as ratio of lamina mass to lamina area after subtracting the content of nonstructural carbohydrates from lamina mass. The 5 × 2 mm leaf fragments were fixed overnight at 4 °C in a solution consisting of 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.0). Samples were then dehydrated in a graded ethanol series, transferred to acetone and infiltrated with Spur’s resin in a stepwise manner using three overnight incubations in solutions of resin in acetone at graded concentrations followed by 48 h incubation in 100% resin. Infiltration steps were conducted at 4 °C followed by polymerization in molds at 45 °C, 60 °C and 75 °C for 48 h at each temperature. The embedded tissue was cut to 2 µm sections using an ultramicrotome (Leica, Austria). Sections were stained with 0.1% toluidine blue in 1% borate buffer and photographed through a light microscope (Axioskop, Zeiss, Germany) using Powershot G5 digital camera (Canon, Japan). Anatomical measurements were taken from digital images using LSM510 software (Zeiss, Germany). Two sections per leaf were analyzed.
Carbohydrates and N
Leaf material was dried as above and ground to fine powder using a Culatti mill (IKE Labortechnik Staufen, Germany). The concentrations of total nonstructural carbohydrates (TNC, the sum of reducing sugars and starch) were determined by a modification of the method described by Haissig and Dickson (1979) and Hansen and Møller (1975). Sugars were extracted in methanol-chloroform-water, and tissue residuals were used for determination of starch content. Soluble sugars were determined colorimetrically with anthrone reagent at 625 nm within 30 min. Starch in the tissue residue was gelatinized and converted to glucose with amyloglucosidase. Glucose concentrations were measured using the peroxidase-glucose oxidase-o-dianisidine dihydrochloride reagent. Absorbance was measured at 450 nm after a 30 min. incubation at 37 °C against glucose standards. For determination of nitrogen concentration, powdered tissue was subjected to analysis in an Elemental Combustion System CHNS-O 4010 (Costech Instruments, Italy/USA). Nitrogen concentration was expressed on a nonstructural-carbohydrate-free leaf mass basis (Nmass) and a leaf area basis (Narea).
Pigments
For analysis of photosynthetic pigments, tissue samples of 40–50 mg fresh weight per leaf were collected while excluding major nerves. The samples were cut into small pieces and incubated in 5 ml of 100% DMSO saturated with CaCO3 at 60 °C until the solution became clear (approximately 5 h). The absorbance of the extract was measured at 665, 648, and 470 nm. Calculations of chlorophyll a, b, and total carotenoid concentration followed Barnes et al. (1992). Concentrations were expressed on a leaf fresh mass basis (Chlmass, Carmass) and an area basis (Chlarea Cararea).
Leaf shedding
Prior to the beginning of autumnal leaf shedding, petioles of ten leaves per plant were tied to the stem using dental floss to facilitate keeping track of individual leaves. Abscised leaves were censused at 3–4 day intervals until no leaves remained attached to stems. The percentage of leaves retained on the plants in each treatment was calculated for a given day. Mean day-of-year of abscission was calculated for each growth irradiance in the TRANS and the CONT group.
Statistics
Individual plants were considered independent replicates, with typically ten samples representing the PRE treatment and five samples in each TRANS and CONT group. All plants survived the experiment; however, sporadic sample mishandling resulted in the final sample number being lower in some treatments and for some variables (Supplementary Information Table S3). Since plants from the lowest (1%) irradiance level lost all leaves upon transfer to full irradiance, and because the 100% irradiance plants formed one group, our experimental set-up was unbalanced. For analysis of variance, we therefore excluded the 1% irradiance plants (but still show the data). Additionally, we randomly divided the 100% irradiance plants into two subgroups, assigning one as TRANS and the other as CONT, to produce a complete factorial design. This was, however, only possible for LMA and chemical traits, for which all plants were sampled. For anatomical analysis, only 5 plants were sampled from the 100% irradiance plants, we therefore excluded this group from analysis of variance and for anatomical traits conducted Anova on growth irradiances from 3 to 50% (but still show the complete data). We thus applied two-way Anovas with growth irradiance at six or five levels and three types of transfer treatment (PRE, CONT and TRANS), followed by pre-planned contrasts between treatments within each growth irradiance. For growth irradiances not included in the Anova design (i.e., 1% irradiance for both anatomical and chemical traits, and 100% irradiance for anatomical traits), we calculated contrasts between PRE vs. CONT plants. Significant contrasts between PRE and CONT plants indicated change in time, whereas contrasts between CONT and TRANS plants indicated the effect of an increased irradiance. Morpho-anatomical and chemical trait data were log transformed prior to analysis of variance, except for the chlorophyll a/b ratio. Linear regression relationships between pigment and N concentrations and LMA were obtained using individual leaves as replicates, and the effects of treatments on slopes and intercepts were evaluated using analysis of covariance. Where non-significant Ancova interaction indicated that slopes were not different, the interaction term was dropped from the model and intercepts were compared.
For analysis of the leaf shedding data, half of the 100% irradiance plants were assigned to the TRANS group to obtain a complete design, as described above. Analysis of covariance was performed, with mean day-of-year of leaf fall as the dependent variable, transfer treatment as main effect and growth irradiance as continuous covariate. Because the interaction was not significant, it was excluded from the model and the analysis repeated.
Results
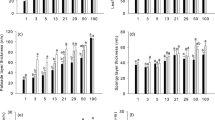
Analysis of variance showed that LMA and all leaf tissues adjusted to growth irradiance (Table 1). LMA increased from lowest to highest PPFD without showing saturation (Fig. 1a). When plants maintained under the same shading intensities were resampled after 30 days (black vs. gray bars), the LMA value was significantly higher while retaining the positive relationship with irradiance. In plants that were transferred to full sun, LMA increased further, except for those originating from the 29% and 50% treatment. Adjustment of LMA to growth irradiance and to change in irradiance was to a large extent caused by adjustment in lamina thickness (Figs. 1b, 2, 3a) and was strongly related to thickness of the palisade layer (Figs. 1c, 2, 3b). Specifically, palisade cells became slightly longer in mature leaves between the two sampling dates at most irradiances and responded to transfer to full light with further elongation. This additional elongation response was especially strong in leaves formed at the lowest irradiance levels. Adjustment to growth irradiance was also found with respect to the thickness of spongy mesophyll, and upper and lower epidermis (Fig. 1d–f). However, there was no clear increment between sampling dates, except for spongy layer and upper epidermis in the highest irradiances. There was also no clear effect of transfer to high light on the thickness of these three tissue layers.
Responses of morpho-anatomical traits of mature leaves of A. pseudoplatanus seedlings to a sudden increase in irradiance. Means and standard errors for carbohydrate-free leaf mass per area (LMA; a), leaf lamina thickness (b), palisade mesophyll thickness (c), spongy mesophyll thickness (d), thickness of upper (e) and lower (f) epidermis are shown. Leaves were sampled on August 5, i.e., prior to transfer to full irradiance (PRE) and 30 days later (TRANS transferred plants, CONT plants left under the original irradiance). Significant contrasts between treatment groups within the same initial growth irradiance are indicated by different letters above bars. For analysis of variance see Table 1. Data for TRANS plants originally grown under lowest irradiance are missing because of the photo-oxidative damage to leaves
Anatomical adjustment of fully formed leaves of A. pseudoplatanus in response to transfer from low to high irradiance. Light microscope images represent leaf cross-sections from plants maintained for the whole season at 100% irradiance (a), at 3% irradiance (b) and first grown at 3% and then transferred for 30 days to 100% irradiance (c). All samples were collected 30 days after transfer
Linear relationships of leaf mass per area (LMA) vs. lamina thickness (a) and lamina thickness vs. palisade cell thickness (b) in A. pseudoplatanus leaves acclimated to eight levels of irradiance (PRE) and subsequently transferred to full solar irradiance (TRANS) or left under the original irradiance (CONT). In (a) points are means and bars indicate standard errors. In (b) values for individual leaves are plotted and lines are fitted to each treatment
Growth irradiance and transfer treatments significantly affected pigment and nitrogen contents, both when expressed on a leaf mass and on an area basis (Table 2). Total Chlmass was inversely related to original growth irradiance and declined between the mid- and late-season sampling dates, whereas Chlarea was positively related to growth irradiance and did not change between sampling dates (Fig. 4a, b). Transfer to full light caused a drastic decline in Chlmass, especially pronounced in plants from the deepest shade, with smaller declines in Chlarea (Fig. 4a,b). For plants transferred from 1% irradiance to full light, we observed rapid photobleaching (Fig. 5) and subsequent leaf drop. The chlorophyll a/b ratio was highest at low irradiance, increased between sampling dates across irradiance levels and increased drastically after transfer to full light, especially in the most strongly shaded plants (Fig. 4c). Concentration of carotenoids was inversely (Carmass) or positively (Cararea) related to growth irradiance, showed little change between sampling dates but, in case of Carmass, declined in leaves exposed to full irradiance (Fig. 4d, e). Nmass was also inversely related to growth irradiance and declined across shading levels between sampling dates (Fig. 4f). In contrast, Narea was positively related to growth irradiance and showed no significant change between sampling dates (Fig. 4g). Transfer to full light did not significantly affect the Nmass but slightly and erratically increased Narea (Fig. 4f, g).
Effect of growth irradiance and sudden increase in irradiance on leaf pigment and N content in mature A. pseudplatanus leaves. Means and standard errors for Chlmass (a), Chlarea (b), chlorophyll a/b ratio (c), Carmass (d), Cararea (e) as well as Nmass (f) and Narea (g) are shown. For analysis of variance see Table 2; other details as in Fig. 1
The diversification of LMA, pigment and N contents by differences in growth irradiance in the first part of the growing season resulted in significant relationships between individual leaf LMA chlorophyll, carotene and N concentrations. These relationships were negative for mass-based, and positive for area-based concentrations (Fig. 6a–f). The slopes of Chlmass and Carmass vs. LMA were reduced slightly between sampling dates. The exposure to higher light resulted in a further reduction in slope for Chlmass but not for Carmass (Fig. 6a, c). For area-based pigment concentrations, there were no differences in slopes. Intercepts of the Chlarea vs. LMA relationships decreased relative to the PRE leaves both because of time lapse and due to increased irradiance. There was no such change in the case of Cararea (Fig. 6b, d). Reductions in the intercepts of Nmass vs. LMA and slopes of Narea vs. LMA relationships were due only to time (Fig. 6e–f).
Relationships between LMA and mass-based (left-hand panels) and area-based (right-hand panels) concentrations of photosynthetic pigments (a, b chlorophyll, c, d carotenoids) and N (e, f) in leaves of A. pseudplatanus acclimated to eight levels of irradiance (PRE, P) and subsequently transferred to full solar irradiance (TRANS, T) or left under the original irradiance (CONT, C). Point are values for individual leaves. Results of Ancovas for pairwise comparisons between treatment groups are shown
Immediately prior to transfer, the chlorophyll fluorescence parameter Fv/FM was above 0.80 in shaded plants up to 25% of full irradiance, with values in more strongly illuminated plants being somewhat lower (Fig. 7a). Transfer to full light resulted in overall reduction of Fv/FM (to as low as 0.35 in plants from 1% irradiance) and the magnitude of this decline was in reverse proportion to growth irradiance (Fig. 7b). However, by day 29 after transfer, the Fv/FM recovered to 0.70 or more, except for the 1% irradiance plants that had lost all leaves due to photobleaching (Fig. 7c).
Efficiency of PSII in A. pseudplatanus leaves acclimated to eight levels of irradiance immediately before transfer to full sun (a), one day after transfer (b) and 29 days after transfer (c). Means ± standard errors for plants transferred to full solar irradiance (TRANS) or remaining under the original irradiance (CONT) are shown. Asterisks indicate significant contrasts between treatments within each irradiance level (*P < 0.05, ***P < 0.001)
Autumnal leaf shedding began in early September, and was complete in early November (Fig. 8a, b). The duration of leaf retention on the plants in the autumn (measured as mean day-of year of leaf fall) was scattered among irradiances (Fig. 8a, b); however, it showed no detectable relationship with irradiance intensity in TRANS (R2 = 0.33 P = 0.102) or CONT plants (R2 = 0.06 P = 0.820), or in both treatments analyzed jointly (Ancova F1,13 = 1.94 P = 0.181; Fig. 8c). The transfer to full light reduced leaf retention time across irradiances (Ancova F1,13 = 12.57 P = 0.004; Fig. 8c).
Effect of increased irradiance on autumnal shedding of leaves of A. pseudoplatanus plants acclimated to eight levels of irradiance. Proportion (%) of leaves remaining on plants in each treatment are plotted against day-of-year for plants remaining under the original growth irradiance (CONT; a) and transferred to full solar irradiance (TRANS; b). The mean day-of-year of leaf fall is shown for each treatment in relations to initial growth irradiance (c). Lines in (a) and (b) show exact determinations for c. 50 leaves in each treatment. Means per plant ± standard errors (N = 5) are shown in (c). Slopes of the regression lines in (c) are not significantly different (Ancova interaction P = 0.259) and the common slope Ancova indicated lack of irradiance effect (P = 0.181) but there was a significant transfer effect (P = 0.004). Note that a log scale for x-axis was used in (c)
Discussion
In this study, we acclimated A. pseudoplatanus plants to eight levels of solar irradiance, from extreme shade to full sun. We then used these plants to test the ability of fully mature leaves developed under the different irradiance levels to re-adjust anatomically toward the high-light phenotype. The results were consistent with our leading hypothesis. Although leaves on plants grown in the deepest shade (1% irradiance) experienced severe photo-oxidative stress as indicated by bleaching, and died within a few days, anatomical re-acclimation was evident in plants from higher irradiances. It involved near-doubling of the palisade cell length in plants from the 3% irradiance and relatively smaller increases of palisade tissues in leaves from higher light levels. In contrast, neither spongy tissue nor any of the epidermal tissues increased in thickness due to increased irradiance, making palisade tissue the sole structure capable of adjustment, and responsible for the increase in lamina thickness. It was likely also a major contributor to the increased LMA. This main result extends the list of known species capable of expanding palisade mesophyll in mature leaves (Bunce et al. 1977; Bauer and Thöni 1988; Yamashita et al. 2002; Oguchi et al. 2005) and suggests that this mechanism may be more widespread than is accepted (Oguchi et al. 2018). Although our study species belongs to genus Acer in which a similar mechanism was found previously (Oguchi et al. 2005), it may be representative of an entire guild of late successional, shade-tolerant broadleaf trees, more of which should be investigated for similar capabilities.
The important question is whether and how this palisade cell growth contributes to dissipation of photo-oxidative stress and allows leaves to maintain or improve carbon uptake following a rapid increase in irradiance? The long palisade cells may facilitate acclimation of individual chloroplasts to local light intensity and provide additional volume for chloroplast placement and surface area for chloroplast contact with air spaces (Terashima et al. 2011). The high photosynthetic rates typical of sun leaves, however, are supported also by other anatomical features, such as densely spaced stomata and small veins, and a greater development of cell wall labyrinth in phloem parenchyma (Amiard et al. 2005; Poorter et al. 2019). Although we did not investigate most of these additional traits in our study, it is unlikely that new stomata or veins would have been formed upon exposure of mature leaves to stronger light. Thus, the additional thickness of palisade mesophyll would not have been matched by a larger number of stomata or by denser veins. It is interesting whether such structurally imbalanced leaves with sun-type palisade but retaining shade-type stomatal and vascular arrangement will nevertheless be able to perform faster photosynthesis. We posit that the answer to this question is yes, because increased photosynthetic capacity without accompanying anatomical changes has been documented in similarly designed studies (Mohammed and Parker 1999, Oguchi et al. 2006). However, it is also likely that such leaves will experience greater stomatal limitation of photosynthesis, especially under conditions of strong sunlight coupled with water stress. Leaf cooling ability may also be compromised. Additionally, the survival of sun leaves in microsites with high light, wind and water deficit exposure is improved by the various mechanical structures, thicker cuticle and more strongly developed epicuticular wax (Scoffoni et al. 2015; Coble and Cavaleri 2017). The acclimative potential of these traits in mature leaves needs yet to be examined.
Even though the increase in dimensions of palisade cells could provide space for the expansion of chloroplasts, we actually observed an accompanying decline in Chlmass, and to a lesser extent in Chlarea, in TRANS vs. CONT plants. Such decrease of chlorophyll concentration could indicate stress, although it might also partly result from dilution of chlorophyll by the accumulating biomass, as suggested by the smaller area-based reduction. This effect might be photoprotective because it reduced light absorption by the suddenly exposed leaves; however, Chlarea in transferred plants remained lower than in plants from full irradiance, indicating an over-response. It was also lower at comparable LMA, showing that exposure to high light disturbed the coordination between structure and function, at least with reference to undisturbed leaf phenotypes. Also, the strongly preferential degradation of chlorophyll b, a molecule associated mainly with peripheral antennas, was indicative of acclimative antenna size reduction, and consistent with the development of high-light leaf phenotype (Lichtenthaler and Babani 2004). However, the chlorophyll a/b ratios in light exposed leaves from the lowest irradiances exceed those typically reported in high-light leaves (Naidu and DeLucia 1998; Yamashita et al. 2000; Lichtenthaler and Babani 2004), suggesting that the experimental treatment induced a stoichiometric imbalance in the photosynthetic apparatus.
Although direct measurements of photosynthetic rate were not performed, the recovery of variable fluorescence to a level only slightly lower than control following a transient but drastic decline, indicated that partial photosynthetic recovery took place concomitantly with palisade development. This recovery might have occurred through structural mechanisms, with, e.g., the expansion of palisade cells providing an increased contact area between chloroplasts and intercellular spaces to facilitate CO2 access (Oguchi et al. 2005, 2006; Terashima et al. 2006) but also through activation of photoprotection mechanisms, changes in pigment composition (higher chlorophyll a/b ratio) and adjustments of other components of light harvesting and the carbon reduction cycle. The decline and recovery of Fv/FM upon rapid increase in irradiance has been reported in juveniles of other tree species (Yamashita et al. 2000; Oguchi et al. 2006), but they were not necessarily accompanied by recovery of CO2 uptake (Yamashita et al. 2000), implying activation of other photoprotective mechanisms.
The role of palisade expansion vs. biochemical adjustments in photosynthetic acclimation might be evaluated by studying the temporal dynamics of physiological vs. anatomical acclimation; however, currently few datasets with sufficient temporal resolution exist. Increases in photosynthetic capacity in tree seedlings may be detected as soon as 5 days after increased exposure (Oguchi et al., 2006), whereas in an experiment with walnut an accumulation of N and enhancement of carboxylation capacity began within 4 weeks and was coordinated with a gradual increase in LMA (Frak et al. 2001). Ultimately, the problem may be solved if tools permitting experimental manipulation of mesophyll development independently of external light level become available (Munekage et al. 2015).
Plasticity of traits controlling light harvesting, such as crown structure, leaf arrangement, leaf size and shape, as well as the anatomical structure of leaves, is a salient element of the ecological strategy of late successional trees that typically regenerate in shady forest understories and reach canopy or subcanopy layers by utilizing variously sized canopy gaps (Ashton and Berlyn 1992; Oguchi et al.2006). A mid- or late-season gap opening creates a problem of excessive irradiance as well as a chance to utilize the additional light in photosynthesis. The ability to expand the palisade cells in already-formed shade leaves may constitute a mechanism to cope with both. However, the time available for re-acclimation of leaf structure and function following canopy-gap formation during the growing season is limited, therefore, given the requirement for additional biomass allocation to the leaf structure, the benefit of such change is conditional. The fact that A. pseudoplatanus leaves from the transferred plants were shed earlier than control leaves suggests that the degree and rate of re-acclimation were not fully effective for protection against damage from photo-oxidative stress. Indeed, the re-acclimation at 30 days after transfer was still incomplete (e.g., palisade cells from transferred plants were still shorter and chlorophyll contents was lower than in leaves experiencing full irradiance during the whole season). Since our objective was to test leaves that were fully developed, i.e., not only expanded but also having fully formed cell walls, our experiment was conducted in the second half of the growing season. In experiments with walnuts, the timing of increased exposure was critical, with exposure at 58 d after bud burst resulting in significant structural and photosynthetic acclimation of existing leaves and exposure at 91 days resulting in no acclimation (Frak et al. 2001). It is thus likely that anatomical responses would have been stronger if the transfer to high light occurred significantly earlier, when cell walls were more elastic and there was more time remaining until autumn (Frak et al. 2001).
Although A. pseudoplatanus forms terminal buds relatively early in the growing season, it does have the ability for sylleptic (Lammas) growth, similarly to many other woody species. An alternative option to leaf re-acclimation is therefore production of new sun-type leaves (however often with a carry-over effect; Yamashita et al. 2000; Martinez & Friedley 2018). New leaves are certainly more costly in terms of biomass expense than the adjustment of pre-existing leaves. The balance between costs and benefits should depend on the extent of photo-oxidative damage to pre-existing leaves (itself dependent on initial growth irradiance) and on the timing of gap formation with respect to the anticipated length of the growing season, resulting in varied expected photosynthetic income. A trade-off may be expected, and needs to be tested, between the ability to modify pre-existing leaves and the efficiency of production of new, additional leaves in response to increased irradiance.
Although the scenario we tested was equivalent to a sudden gap formation, light availability may also change slightly and gradually throughout the growing season due to upper canopy defoliation (Rozendal and Kobe 2016) and to the changing leaf or solar angle. Such changes may not induce sudden stress and allow a more gradual anatomical and physiological acclimation. Notably, our data indeed showed that some palisade cell expansion occurred also between August 6 and September 4 even in CONT plants. This effect was unexpected but was consistent with the higher number of daily sunshine hours during that period (see Supplementary Information Fig. S1). Adjustments of palisade cell length in developed leaves after increases in irradiance of various rate and magnitude may thus supplement the numerous mechanisms allowing acclimation to increased light in late successional species (Frak et al. 2001; Youngblood and Fergusson 2003; Oguchi et al. 2006; Wen et al. 2008; Cano et al. 2011).
By employing multiple levels of irradiance in our experimental design our study provides additional insights into the relationships between light and variation in leaf anatomy. Such variation has been traditionally described in terms of sun/shade dichotomy (Givnish 1988). Only recently, information on dose–response relationships between irradiance and leaf traits has been summarized through a multi-species and multi-experiment meta-analysis (Poorter et al. 2019). These authors demonstrated that the relative increase of leaf mass per area, leaf thickness, leaf density and stomatal density is strongest in low irradiance with trends approaching saturation at higher irradiance levels. Their dataset, however, combined plastic responses of individual species with the adaptive interspecific differences. Only a limited number of studies have attempted to determine the shape of analogous dose–response relationship for leaf anatomy within a single species. Most of such information has been provided by reports of leaf variability within the crowns of individual trees or along natural understory light gradients. Leaf mass per area as well as lamina thickness or, rarely, palisade thickness, have been shown to follow a saturating mode of response to irradiance (Bond et al. 1999; Poorter 1999; Posada et al. 2009; Coble et al. 2014; Legner et al. 2014; Coble and Cavaleri 2017). Only rarely have linear (Niinemets 1997) or power (Sprugel et al. 1996) relationships been reported. Variation of leaf phenotype within the canopy may, however, be controlled by other environmental parameters covarying with irradiance, such as temperature or level of water stress, particularly important in the exposed canopy positions (Zwieniecki et al. 2004). Furthermore, the determination of irradiance at the leaf position in the canopy or under the canopy is frequently only approximate. The second type of studies uses controlled exposure of whole individuals, usually seedlings, to light, generally applying two or three irradiance levels (e.g., Strauss-Debenedetti and Berlyn 1994). Seldom have larger number of levels been used (five in each Sims et al. 1998 and Pons and Poorter 2014, six in Poorter 1999, eight in Babaei Soustani et al. 2014). Moreover, studies using multiple levels of irradiance tend to focus on integrated traits, such as leaf mass per area (or its reciprocal, specific leaf area) and lamina density and thickness, rather than detailed tissue structure. With our eight levels of irradiance, we show that the thickness responses of all tissues contributing to lamina thickness are not saturated within the biologically relevant range of irradiance, similarly to the LMA. This finding is in contrast with the majority of the previously cited reports, mostly, however, based on within-canopy variation. Certainly, irradiance might not have been the only player in our experiment, as it probably co-varied with frequency of occurrence of high temperature, vapor pressure deficit and higher wind speed. All these factors alone, or by aggravating leaf-level water stress, are known to affect the leaf structure by increasing the leaf thickness (Wu et al. 2016; Oguchi et al. 2018) and the various components of density, especially the sizes of structural and vascular tissues (Poorter et al. 2009; Coble and Cavaleri 2015). Such multiple factors differ also among actual understory gaps, likely reinforcing the effect of increased irradiance. In our experiment, however, variability in leaf-level water stress was certainly reduced in comparison to canopies of mature trees or natural gaps due to regular watering and small plant size.
In summary, our study has demonstrated the ability of mature, shade-acclimated leaves of Acer pseudoplatanus to respond to increased irradiance by enlarging palisade cells resulting in increased leaf thickness. As part of the species’ ecological strategy, this mechanism has the potential to contribute to dissipation of photo-oxidative stress and to enable increased photosynthetic gain under greater availability of light. Both aspects need further study. The ability to re-acclimate mature leaves may be a significant component of the species’ response to gap formation during the growing season, with a potential bearing on forest dynamics and successional processes. In addition, we have documented a non-saturated light response relationship between growth irradiance and thickness of particular leaf tissues, contrasting with findings of the few analogous reports available.
Data availability
Data are available at https://figshare.com/s/d563b8019b0e9795206f
Change history
06 July 2022
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11120-022-00940-9
References
Amiard V, Mueah KE, Demming-Adams B, Ebbert V, Turgeon R, Adams WW III (2005) Anatomical and photosynthetic acclimation to the light environment in species with differing mechanisms of phloem loading. Proc Nat Acad Sci 102:12968–12973. https://doi.org/10.1073/pnas.0503784102
Ashton PMS, Berlyn GP (1992) Leaf adaptations of some Shorea species to sun and shade. New Phytol 121:587–596. https://doi.org/10.1111/j.1469-8137.1992.tb01130.x
Babaei Soustani F, Jalali SG, Sohrabi H, Shirvany A (2014) Growth responses to irradiance regime along an ecological gradient of Quercus castaneifolia seedlings of different provenance. Ecol Res 29:245–255. https://doi.org/10.1007/s11284-013-1119-9
Barnes JD, Balaguer L, Manrique E, Elvira S, Davison AW (1992) A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ Exp Bot 32:85–100. https://doi.org/10.1016/0098-8472(92)90034-Y
Bauer H, Thöni W (1988) Photosynthetic light acclimation in fully developed leaves of the juvenile and adult life phases of Hedera helix. Physiol Plantarum 73:31–37. https://doi.org/10.1111/j.1399-3054.1988.tb09189.x
Bond BJ, Farnsworth BT, Robert A, Coulombe RA, Winner WE (1999) Foliage physiology and biochemistry in response to light gradients in conifers with varying shade tolerance. Oecologia 120:183–192. https://doi.org/10.1007/s004420050847
Bunce JA, Patterson DT, Peet MM, Alberte RS (1977) Light acclimation during and after leaf expansion in soybean. Plant Physiol 60:255–258. https://doi.org/10.1104/pp.60.2.255
Calzavara AK, Rocha JS, Lourenço G, Sanada K, Medri C, Bianchini E, Pimenta JA, Stolf-Moreira R, Oliveira HC (2017) Acclimation responses to high light by Guazuma ulmifolia Lam. (Malvaceae) leaves at different stages of development. Plant Biol 19:720–727. https://doi.org/10.1111/plb.12592
Campa C, Urban L, Mondolot L, Fabre D, Roques S, Lizzi Y, Aarrouf J, Doulbeau S, Breitler J-C, Letrez C, Toniutti L, Bertrand B, La Fisca P, Bidel LPR, Etienne H (2017) Juvenile coffee leaves acclimated to low light are unable to cope with a moderate light increase. Front Plant Sci 8:1126. https://doi.org/10.3389/fpls.2017.01126
Cano FJ, Sánchez-Gómez D, Gascó A, Rodríguez-Calcerrada J, Gil L, Warren CR, Aranda I (2011) Light acclimation at the end of the growing season in two broadleaved oak species. Photosynthetica 49 581–592. https://doi.org/10.1007/s11099-011-0066-3
Coble AP, Cavaleri MA (2015) Light acclimation optimizes leaf functional traits despite height-related constraints in a canopy shading experiment. Oecologia 177:1131–1143. https://doi.org/10.1007/s00442-015-3219-4
Coble AP, Cavaleri MA (2017) Vertical leaf mass per area gradient of mature sugar maple reflects both height-driven increases in vascular tissue and light-driven increases in palisade layer thickness. Tree Physiol 37:1337–1351. https://doi.org/10.1093/treephys/tpx016
Coble AP, Autio A, Cavaleri MA, Binkley D, Ryan MG (2014) Converging patterns of vertical variability in leaf morphology and nitrogen across seven Eucalyptus plantations in Brazil and Hawaii, USA. Trees 28:1–15. https://doi.org/10.1007/s00468-013-0925-6
Ding Y, Zheng Q-S, Zhang Y, He C, Xie B (2014) Observation of apparently unchanging mesophyll cell diameters throughout leaf ontogeny in woody species. J Plant Growth Regul 33:150–159. https://doi.org/10.1007/s00344-013-9357-1
Frak E, Le Roux X, Millard P, Dreyer E, Jaouen G, Saint-Joanis B, Wendler R (2001) Changes in total leaf nitrogen and partitioning of leaf nitrogen drive photosynthetic acclimation to light in fully developed walnut leaves. Plant Cell Environ 24:1279–1288. https://doi.org/10.1046/j.0016-8025.2001.00784.x
Givnish TJ (1988) Adaptation to sun and shade: a whole-plant perspective. Funct Plant Biol 15:63–92. https://doi.org/10.1071/PP9880063
Haissig BE, Dickson RE (1979) Starch measurement in plant tissue using enzymatic hydrolysis. Physiol Plant 47:151–157. https://doi.org/10.1111/j.1399-3054.1979.tb03207.x
Hansen J, Møller I (1975) Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal Biochem 68:87–94. https://doi.org/10.1016/0003-2697(75)90682-X
Hättenschwiler S, Körner C (2000) Tree seedling responses to in situ CO2 -enrichment differ among species and depend on understorey light availability. Glob Chang Biol 6:213–226. https://doi.org/10.1046/j.1365-2486.2000.00301.x
Hein S, Collet C, Ammer Ch, Le Goff N, Skovsgaard J, Savil P (2009) A review of growth and stand dynamics of Acer pseudoplatanus L. in Europe: implications for silviculture. Forestry 82:361–385. https://doi.org/10.1093/forestry/cpn043
Helliwell DR, Harrison AF (1979) Effects of light and weed competition on the growth of seedlings of four tree species on a range of soils. Quart J For 73:160–171
Kazda M, Salzer J, Schmid I, Von Wrangell P (2004) Importance of mineral nutrition for photosynthesis and growth of sessile oak, Fagus sylvatica and Acer pseudoplatanus planted under Norway spruce canopy. Plant Soil 264:25–34. https://doi.org/10.1023/B:PLSO.0000047715.95176.63
Legner N, Fleck S, Leuschner C (2014) Within-canopy variation in photosynthetic capacity, SLA and foliar N in temperate broad-leaved trees with contrasting shade tolerance. Trees 28:263–280. https://doi.org/10.1007/s00468-013-0947-0
Lichtenthaler HK, Babani F (2004) Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In: GC Papageorgiou & Govindjee (eds.) Chlorophyll fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 713–736
Martinez KA, Friedley JD (2018) Acclimation of leaf traits in seasonal light environments: are non-native species more plastic? J Ecol 106:2019–2030. https://doi.org/10.1111/1365-2745.12952
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence – a practical guide. J Exp Bot 345:659–668. https://doi.org/10.1093/jexbot/51.345.659
Mohammed GH, Parker WC (1999) Photosynthetic acclimation in eastern hemlock [Tsuga canadensis (L.) Carr.] seedlings following transfer of shade-grown seedlings to high light. Trees 13:117–124
Munekage YN, Inoue S, Yoneda Y, Yokota A (2015) Distinct palisade tissue development processes promoted by leaf autonomous signalling and long-distance signalling in Arabidopsis thaliana. Plant Cell Environ 38:116–1126. https://doi.org/10.1111/pce.12466
Naidu SL, DeLucia EH (1997) Acclimation of shade-developed leaves on saplings exposed to late-season canopy gaps. Tree Physiol 17:367–376. https://doi.org/10.1093/treephys/17.6.367
Naidu SL, DeLucia EH (1998) Physiological and morphological acclimation of shade-grown tree seedlings to late season canopy gap formation. Plant Ecol 138:27–40. https://doi.org/10.1023/A:1009780114992
Nicotra AB, Leigh A, Boyce CK, Jones CS, Niklas KJ, Royer DL, Tsykaya H (2011) The evolution and functional significance of leaf shape in the angiosperms. Funct Plant Biol 38:535–552. https://doi.org/10.1071/FP11057
Niinemets Ü (1997) Role of foliar nitrogen in light harvesting and shade tolerance of four temperate deciduous woody species. Funct Ecol 11:518–531. https://doi.org/10.1046/j.1365-2435.1997.00109.x
Niinemets Ü, Kollist H, García-Plazaola JI, Hernández A, Becerril JM (2003) Do the capacity and kinetics for modification of xanthophyll cycle pool size depend on growth irradiance in temperate trees? Plant Cell Environ 26:1787–1801. https://doi.org/10.1046/j.1365-3040.2003.01096.x
Niinemets Ü, Kull O, Tenhunen JD (2004) Within-canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell Environ 27:293–313. https://doi.org/10.1111/j.1365-3040.2003.01143.x
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ 26:505–512. https://doi.org/10.1046/j.1365-3040.2003.00981.x
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927. https://doi.org/10.1111/j.1365-3040.2005.01344.x
Oguchi R, Hikosaka K, Hiura T, Hirose T (2006) Leaf anatomy and light acclimation in woody seedlings after gap formation in a cool-temperate deciduous forest. Oecologia 149:571–582. https://doi.org/10.1007/s00442-006-0485-1
Oguchi R, Hiura T, Hikosaka K (2017) The effect of interspecific variation in photosynthetic plasticity on 4-year growth rate and 8-year survival of understorey tree seedlings in response to gap formations in a cool-temperate deciduous forest. Tree Physiol 37:1113–1127. https://doi.org/10.1093/treephys/tpx042
Oguchi R, Onoda Y, Terashima I, Tholen D (2018) Leaf anatomy and function. In: Adams III WW, Terashima I (eds.) The leaf: A platform for performing photosynthesis. Advances in Photosynthesis and Respiration 44. Springer International Publishing AG, pp. 97–139. https://doi.org/10.1007/978-3-319-93594-2_5
Pantin F, Simonneau T, Muller B (2012) Coming of leaf age: control of growth by hydraulics and metabolics during leaf ontogeny. New Phytol 196:349–366. https://doi.org/10.1111/j.1469-8137.2012.04273.x
Piovesan G, Di Filippo A, Alessandrini A, Biondi F, Schirone B (2005) Structure, dynamics and dendroecology of an old-growth Fagus forest in the Apennines. J Veg Sci 16:13–28. https://doi.org/10.1111/j.1654-1103.2005.tb02334.x
Pons TL, Poorter H (2014) The effect of irradiance on the carbon balance and tissue characteristics of five herbaceous species differing in shade tolerance. Front Plant Sci 5:12. https://doi.org/10.3389/fpls.2014.00012
Poorter L (1999) Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct Ecol 13:396–410
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588. https://doi.org/10.1111/j.1469-8137.2009.02830.x
Poorter H, Niinemets Ü, Ntagkas N, Siebenkäs A, Mäenpää M, Matsubara S, Pons T (2019) A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol 223:1073–1105. https://doi.org/10.1111/nph.15754
Posada JM, Lechowicz MJ, Kitajima K (2009) Optimal photosynthetic use of light by tropical tree crowns achieved by adjustment of individual leaf angles and nitrogen content. Ann Bot 103:795–805. https://doi.org/10.1093/aob/mcn265
Rodríguez-Calcerrada J, Reich PB, Rosenqvist E, Pardos JA, Cano FJ, Aranda I (2008) Leaf physiological versus morphological acclimation to high-light exposure at different stages of foliar development in oak. Tree Physiol 28:761–771. https://doi.org/10.1093/treephys/28.5.761
Rozendal DMA, Kobe RK (2016) A forest tent caterpillar outbreak increased resource levels and seedling growth in a northern hardwood forest. PLoS ONE 11:e0167139. https://doi.org/10.1371/journal.pone.0167139
Scoffoni C, Kunkle J, Pasquet-Kok J, Vuong C, Patel AJ, Montgomery RA, Givnish TJ, Sack L (2015) Light-induced plasticity in leaf hydraulics, venation, anatomy, and gas exchange in ecologically diverse Hawaiian lobeliads. New Phytol 207:43–58. https://doi.org/10.1111/nph.13346
Sims DA, Pearcy RW (1992) Response of leaf anatomy and photosynthetic capacity in Alocasia macrorhiza (Araceae) to a transfer from low to high light. Am J Bot 79:449–455. https://doi.org/10.1002/j.1537-2197.1992.tb14573.x
Sims DA, Seemann JR, Luo Y (1998) The significance of differences in the mechanisms of photosynthetic acclimation to light, nitrogen and CO2 for return on investment in leaves. Funct Ecol 12:185–194. https://doi.org/10.1046/j.1365-2435.1998.00194.x
Sprugel DG, Brooks JR, Hinckley TM (1996) Effects of light on shoot geometry and needle morphology in Abies amabilis. Tree Physiol 16:91–98. https://doi.org/10.1093/treephys/16.1-2.91
Strauss-Debenedetti S, Berlyn GP (1994) Leaf anatomical responses to light in five tropical Moraceae of different successional status. Am J Bot 81:1582–1591. https://doi.org/10.1002/j.1537-2197.1994.tb11470.x
Terashima I, Araya T, Miyazawa S-I, Sone K, Yano S (2005) Construction and maintenance of the optimal photosynthetic systems of the leaf, herbaceous plant and tree: an eco-developmental treatise. Ann Bot 95:507–519. https://doi.org/10.1093/aob/mci049
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57:343–354. https://doi.org/10.1093/jxb/erj014
Terashima I, Hanba YT, Tholen D, Niinemets Ü (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155:108–116. https://doi.org/10.1104/pp.110.165472
Tognetti R, Minotta G, Pinzauti S, Michelozzi M, Borgetti M (1998) Acclimation to changing light conditions of long-term shade-grown beech (Fagus sylvatica L.) seedlings of different geographic origins. Trees 12:326–333. https://doi.org/10.1007/PL00009719
Valladares F, Niinemets Ü (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol S 39:237–257. https://doi.org/10.1146/annurev.ecolsys.39.110707.173506
Watson RW (1942) The mechanism of elongation of palisade cells. New Phytol 41:206–221. https://doi.org/10.1111/j.1469-8137.1942.tb07074.x
Wen S, Fetcher N, Zimmerman JK (2008) Acclimation of tropical tree species to hurricane disturbance: ontogenetic differences. Tree Physiol 28:935–946. https://doi.org/10.1093/treephys/28.6.935
Wu T, Zhang P, Zhang L, Wang GG, Yu M (2016) Morphological response of eight Quercus species to simulated wind load. PLoS ONE 11:e0163613. https://doi.org/10.1371/journal.pone.0163613
Yamashita N, Ishida A, Kushima H, Tanaka N (2000) Acclimation to sudden increase in light favoring an invasive over native trees in subtropical islands, Japan. Oecologia 125:412–419. https://doi.org/10.1007/s004420000475
Yamashita N, Koike N, Ishida A (2002) Leaf ontogenetic dependence of light acclimation in invasive and native subtropical trees of different successional status. Plant Cell Environ 25:1341–1356. https://doi.org/10.1046/j.1365-3040.2002.00907.x
Youngblood A, Ferguson DE (2003) Changes in needle morphology of shade-tolerant seedlings after partial overstory canopy removal. Can J For Res 33:1315–1322. https://doi.org/10.1139/x03-060
Zwieniecki MA, Boyce CK, Holbrook NM (2004) Hydraulic limitations imposed by crown placement determine final size and shape of Quercus rubra L. leaves. Plant Cell Environ 37:357–365. https://doi.org/10.1111/j.1365-3040.2003.01153.x
Acknowledgements
We thank Zielonka nursery manager Mr. Marian Grodzki for donation of tree seedlings.
Funding
This research was supported by Faculty of Biology, Adam Mickiewicz University in Poznań, Poland and Polish Academy of Sciences, Institute of Dendrology in Kórnik, Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11120-022-00940-9
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wyka, T.P., Robakowski, P., Żytkowiak, R. et al. RETRACTED ARTICLE: Anatomical adjustment of mature leaves of sycamore maple (Acer pseudoplatanus L.) to increased irradiance. Photosynth Res 152, 55–71 (2022). https://doi.org/10.1007/s11120-022-00898-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-022-00898-8