Abstract
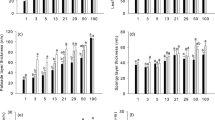
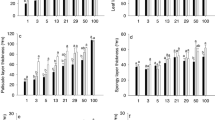
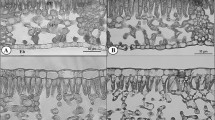
The photosynthetic light acclimation of fully expanded leaves of tree seedlings in response to gap formation was studied with respect to anatomical and photosynthetic characteristics in a natural cool-temperate deciduous forest. Eight woody species of different functional groups were used; two species each from mid-successional canopy species (Kalopanax pictus and Magnolia obovata), from late-successional canopy species (Quercus crispula and Acer mono), from sub-canopy species (Acer japonicum and Fraxinus lanuginosa) and from vine species (Schizophragma hydrangeoides and Hydrangea petiolaris). The light-saturated rate of photosynthesis (P max) increased significantly after gap formation in six species other than vine species. Shade leaves of K. pictus, M. obovata and Q. crispula had vacant spaces along cell walls in mesophyll cells, where chloroplasts were absent. The vacant space was filled after the gap formation by increased chloroplast volume, which in turn increased P max. In two Acer species, an increase in the area of mesophyll cells facing the intercellular space enabled the leaves to increase P max after maturation. The two vine species did not significantly change their anatomical traits. Although the response and the mechanism of acclimation to light improvement varied from species to species, the increase in the area of chloroplast surface facing the intercellular space per unit leaf area accounted for most of the increase in P max, demonstrating the importance of leaf anatomy in increasing P max.





Similar content being viewed by others
References
Bazzaz FA, Pickett STA (1980) Physiological ecology of tropical succession: a comparative review. Annu Rev Ecol Syst 11:287–310
Cassab GI, Varner JE (1988) Cell-wall proteins. Annu Rev Plant Physiol Plant Mol Biol 39:321–353
Clinton BD (2003) Light, temperature, and soil moisture responses to elevation, evergreen understory, and small, canopy gaps in the southern Appalachians. For Ecol Manage 186:243–255
Denslow JS (1987) Tropical rainforest gaps and tree species diversity. Annu Rev Ecol Syst 18:431–451
Denslow JS, Ellison AM, Sanford RE (1998) Treefall gap size effects on above- and below-ground processes in a tropical wet forest. J Ecol 86:597–609
Distefano JF, Gholz HL (1986) A proposed use of ion-exchange resins to measure nitrogen mineralization and nitrification in intact soil cores. Commun Soil Sci Plant Anal 17:989–998
Evans JR, Von Caemmerer S, Setchell BA, Hudson GS (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust J Plant Physiol 21:475–495
Farquhar GD, Von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Fujimoto S, Motai T (1981) An analysis of growth and form for tree seedlings in a natural forest. In: Igarashi T (ed) Some analyses of community structure and regeneration for natural forests in Hokkaido. Hokkaido Forestry Service, Sapporo, pp 123–141
Hanba YT, Miyazawa SI, Terashima I (1999) The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm-temperate forests. Funct Ecol 13:632–639
Hanba YT, Kogami H, Terashima I (2002) The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light demand. Plant Cell Environ 25:1021–1030
Hikosaka K, Hanba YT, Hirose T, Terashima I (1998) Photosynthetic nitrogen-use efficiency in leaves of woody herbaceous species. Funct Ecol 12:896–905
Hikosaka K, Kato MC, Hirose T (2004) Photosynthetic rates and partitioning of absorbed light energy in photoinhibited leaves. Physiol Plant 121:699–708
Hiura T (2001) Stochasticity of species assemblage of canopy trees and understorey plants in a temperate secondary forest created by major disturbances. Ecol Res 16:887–893
JSSSPN (Japanese Society of Soil Science and Plant Nutrition) (1990) Analysis methods of nitrogen compounds. In: Editors commission of experimental methods of plant nutrition (eds) Experimental methods of plant nutrition. Hakuyu-sya, Tokyo, pp 174–203
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420:829–832
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Chemical and microbiological properties, 2nd edn. American Society of Agronomy, Soil Science Society of America, Madison, Wis., pp 643–698
Keller B (1993) Structural cell-wall proteins. Plant Physiol 101:1127–1130
Kikuzawa K (1983) Leaf survival of woody-plants in deciduous broadleaved forests. 1. Tall trees. Can J Bot 61:2133–2139
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Krause GH, Grube E, Koroleva OY, Barth C, Winter K (2004) Do mature shade leaves of tropical tree seedlings acclimate to high sunlight and UV radiation? Funct Plant Biol 31:743–756
Kursar TA, Coley PD (1999) Contrasting modes of light acclimation in two species of the rainforest understory. Oecologia 121:489–498
Martindale W, Bowes G (1996) The effects of irradiance and CO2 on the activity and activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in the aquatic plant Spirodela polyrhiza. J Exp Bot 47:781–784
Mott KA, Woodrow IE (2000) Modeling the role of Rubisco activase in limiting non-steady-state photosynthesis. J Exp Bot 51:399–406
Murchie EH, Horton P (1997) Acclimation of photosynthesis to irradiance and spectral quality in British plant species: chlorophyll content, photosynthetic capacity and habitat preference. Plant Cell Environ 20:438–448
Murchie EH, Horton P (1998) Contrasting patterns of photosynthetic acclimation to the light environment are dependent on the differential expression of the responses to altered irradiance and spectral quality. Plant Cell Environ 21:139–148
Naidu SL, Delucia EH (1997a) Growth, allocation and water relations of shade-grown Quercus rubra L. saplings exposed to a late-season canopy gap. Ann Bot 80:335–344
Naidu SL, Delucia EH (1997b) Acclimation of shade-developed leaves on saplings exposed to late-season canopy gaps. Tree Physiol 17:367–376
Naidu SL, Delucia EH (1998) Physiological and morphological acclimation of shade-grown tree seedlings to late-season canopy gap formation. Plant Ecol 138:27–40
Nobel PS, Zaragoza LJ, Smith WK (1975) Relation between mesophyll surface area, photosynthetic rate, and illumination level during development for leaves of Plectranthus parviflorus Henckel. Plant Physiol 55:1067–1070
Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ 26:505–512
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927
Pearcy RW, Sims DA (1994) Photosynthetic acclimation to changing light environments: scaling from the leaf to the whole plant. In: Caldwell MM, Pearcy RW (eds) Exploitation of environmental heterogeneity by plants: ecophysiological processes above and below ground. Academic Press, San Diego, Calif., pp 145–174
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Prescott CE (2002) The influence of the forest canopy on nutrient cycling. Tree Physiol 22:1193–1200
Romme WH, Martin WH (1982) Natural disturbance by tree falls in old-growth mixed mesophytic forest: Lilley Cornett woods, Kentucky. In: Proceedings from the Fourth Central Hardwood Forest Conference. University of Kentucky, Lexington, Ky., pp 367–383
Ryel RJ, Beyschlag W (2000) Gap dynamics. In: Marshall B, Roberts JA (eds) Leaf development and canopy growth. Sheffield Academic Press, Sheffield, pp 250–279
Schmidt MG, Ogden AE, Lertzman KP (1998) Seasonal comparison of soil temperature and moisture in pits and mounds under vine maple gaps and conifer canopy in a coastal western hemlock forest. Can J Soil Sci 78:291–300
Seemann JR, Kirschbaum MUF, Sharkey TD, Pearcy RW (1988) Regulation of ribulose-1,5-bisphosphate carboxylase activity in Alocasia-macrorrhiza in response to step changes in irradiance. Plant Physiol 88:148–152
Shibata H, Kirikae M, Tanaka Y, Sakuma T, Hatano R (1998) Proton budgets of forest ecosystems on volcanogenous Regosols in Hokkaido, northern Japan. Water Air Soil Pollut 105:63–72
Sims DA, Pearcy RW (1992) Response of leaf anatomy and photosynthetic capacity in Alocasia-macrorrhiza Araceae to a transfer from low to high light. Am J Bot 79:449–455
Sommer-Knudsen J, Bacic A, Clarke AE (1998) Hydroxyproline-rich plant glycoproteins. Phytochemistry 47:483–497
Terashima I, Miyazawa S, Hanba Y (2001) Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. J Plant Res 114:93–105
Thain JF (1983) Curvature correction factors in the measurement of cell surface areas in plant tissues. J Exp Bot 34:87–94
Turnbull MH, Doley D, Yates DJ (1993) The dynamics of photosynthetic acclimation to changes in light quantity and quality in three Australian rainforest tree species. Oecologia 94:218–228
Whitmore TC (1996) A review of some aspects of tropical rain forest seedling ecology with suggestions for further enquiry. In: Swaine MD (ed) The ecology of tropical forest tree seedlings (Man and the biosphere series vol. 17). UNESCO, Paris, pp 3–39
Yamamoto S (1989) Gap dynamics in climax Fagus crenata forests. Bot Mag Tokyo 102:93–114
Yamashita N, Ishida A, Kushima H, Tanaka N (2000) Acclimation to sudden increase in light favoring an invasive over native trees in subtropical islands, Japan. Oecologia 125:412–419
Acknowledgements
We thank the staff at Tomakomai Experimental Forest for their technical support and the experimental set-up, T. Koike, T. Kohyama and S. Tsuyuzaki for the generous offer of instruments, and S. Kitaoka, T. Aikawa, S. Kosuge, S. Takahashi, O. Muller, E. Nabeshima and Y. Miyazaki for support for the experiments, advice and discussion. This work was financially supported in part by Grants-in-Aid of the Japan Ministry of Education, Culture, Sports, Science and Technology and by a JSPS Research Fellowship for Young Scientists.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marilyn Ball
Rights and permissions
About this article
Cite this article
Oguchi, R., Hikosaka, K., Hiura, T. et al. Leaf anatomy and light acclimation in woody seedlings after gap formation in a cool-temperate deciduous forest. Oecologia 149, 571–582 (2006). https://doi.org/10.1007/s00442-006-0485-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0485-1