Abstract
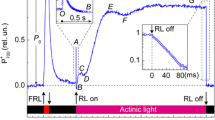
Tradescantia is a good model for assaying induction events in higher plant leaves. Chlorophyll (Chl) fluorescence serves as a sensitive reporter of the functional state of photosynthetic apparatus in chloroplasts. The fluorescence time-course depends on the leaf growth conditions and actinic light quality. In this work, we investigated slow induction of Chl a fluorescence (SIF) excited by blue light (BL, λmax = 455 nm) or red light (RL, λmax = 630 nm) in dark-adapted leaves of Tradescantia fluminensis acclimated to high light (~ 1000 µmol photons m−2 s−1; HL) or low light (~ 100 µmol photons m−2 s−1; LL). Our special interest was focused on the contribution of the avoidance response to SIF kinetics. Bearing in mind that BL and RL have different impacts on photoreceptors that initiate chloroplast movements within the cell (accumulation/avoidance responses), we have compared the SIF patterns during the action of BL and RL. The time-courses of SIF and kinetics of non-photochemical quenching (NPQ) of Chl a fluorescence revealed a certain difference when leaves were illuminated by BL or RL. In both cases, the yield of fluorescence rose to the maximal level P and then, after the lag-phase P–S–M1, the fluorescence level decreased toward the steady state T (via the intermediate phases M1–M2 and M2–T). In LL-acclimated leaves, the duration of the P–S–M1 phase was almost two times longer that in HL-grown plants. In the case of BL, the fluorescence decay included the transient phase M1–M2. This phase was obscure during the RL illumination. Non-photochemical quenching of Chl a fluorescence has been quantified as \( {\text{NPQ}} = F_{\text{m}}^{ 0} /F^{\prime}_{\text{m}} - 1 \), where \( F_{\text{m}}^{ 0} \) and \( F^{\prime}_{\text{m}} \) stand for the fluorescence response to saturating pulses of light applied to dark-adapted and illuminated samples, respectively. The time-courses of such a formally determined NPQ value were markedly different during the action of RL and BL. In LL-grown leaves, BL induced higher NPQ as compared to the action of RL. In HL-grown plants, the difference between the NPQ responses to BL and RL illumination was insignificant. Comparing the peculiarities of Chl a fluorescence induced by BL and RL, we conclude that the avoidance response can provide a marked contribution to SIF and NPQ generation. The dependence of NPQ on the quality of actinic light suggests that chloroplast movements within the cell have a noticeable impact on the formally determined NPQ value. Analyzing kinetics of post-illumination decay of NPQ in the context of solar stress resistance, we have found that LL-acclimated Tradescantia leaves are more vulnerable to strong light than the HL-grown leaves.













Similar content being viewed by others
Notes
The fluorescence parameters of Tradescantia leaves reveal seasonable variability. We have to note, however, that general peculiarities of the SIF kinetics upon the RL or BL illumination were similar in plants grown in different seasons.
Abbreviations
- b 6 f :
-
Cytochrome b6f complex
- BL:
-
Blue light
- CBC:
-
Calvin–Benson cycle
- Chl:
-
Chlorophyll
- EPR:
-
Electron paramagnetic resonance
- ETC:
-
Electron transport chain
- Fd:
-
Ferredoxin
- FNR:
-
Ferredoxin-NADP-oxidoreductase
- HL:
-
High light
- LL:
-
Low light
- NPQ:
-
Non-photochemical quenching
- PAM:
-
Pulse amplitude modulation
- PFD:
-
Photon flux density
- Pmf :
-
Proton motive force
- PSA:
-
Photosynthetic apparatus
- PSI:
-
Photosystem I
- PSII:
-
Photosystem II
- P700 :
-
Primary electron donor in PSI
- P680 :
-
Primary electron donor in PSII
- Pc:
-
Plastocyanin
- PQ:
-
Plastoquinone
- PQH2 :
-
Plastoquinol
- qE :
-
Energy-dependent component of NPQ
- qI :
-
Residual component of NPQ
- qZ :
-
Long-term component of NPQ
- RL:
-
Red light
- SIF:
-
Slow induction of fluorescence
- SP:
-
Short saturating pulse of light
- Vx:
-
Violaxanthin
- Zx:
-
Zeaxanthin
- ΔpH:
-
Trans-thylakoid pH difference
References
Adamson HY, Chow WS, Anderson JM, Vesk M, Sutherland MW (1991) Photosynthetic acclimation of Tradescantia albiflora to growth irradiance: morphological, ultrastructural and growth responses. Physiol Plant 82:353–359
Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1657:23–32
Allen JF (1992) Protein phosphorylation in regulation of photosynthesis. Biochim Biophys Acta 1098:275–335
Anderson JM (1986) Photoregulation of the composition, function, and structure of thylakoid membranes. Annu Rev Plant Physiol 37:93–136
Anderson JM, Chow WS, Park Y, Franklin LA, Robinson SP-A, van Hasselt PR (2001) Response of Tradescantia albiflora to growth irradiance: change versus changeability. Photosynth Res 67:103–112
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Balsera M, Schürmann P, Buchanan BB (2016) Redox regulation in chloroplasts. In: Kirchhoff H (ed) Chloroplasts: current research and future trends. Caister Academic Press, Norfolk, pp 187–207
Banaś AK, Aggarwal C, Łabuz J, Sztatelman O, Gabrys H (2012) Blue light signalling in chloroplast movements. J Exp Botany 63:1559–1574
Barber J (2009) Photosynthetic energy conversion: natural and artificial. Chem Soc Rev 38:185–196
Benkov MA, Yatsenko AM, Tikhonov AN (2019) Light acclimation of shade-tolerant and sun-resistant Tradescantia species: photochemical activity of PSII and its sensitivity to heat treatment. Photosynth Res 139:203–214
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in Hedera canariensis. Photosynth Res 25:173–185
Blankenship RE (2002) Molecular mechanisms of photosynthesis. Blackwell Science, Oxford
Buchanan BB (1980) Role of light in the regulation of chloroplast enzymes. Annu Rev Plant Physiol 31:341–374
Casal JJ (2013) Photoreceptor signaling networks in plant responses to shade. Annu Rev Plant Biol 64:403–427
Chow WS, Melis A, Anderson JM (1990) Adjustment of photosystems stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci USA 87:7502–7506
Chow WS, Adamson HY, Anderson JM (1991) Photosynthetic acclimation of Tradescantia albiflora to growth irradiance: lack of adaptation of light-harvesting components and its consequences. Physiol Plant 81:175–182
Christie JM (2007) Phototropin blue-light receptors. Annu Rev Plant Biol 58:21–45
Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM (2001) Contribution of electric field (Δψ) to steady-state transthylakoid proton motive force (pmf) in vivo and in vitro. Control of pmf parsing into Δψ and ΔpH by ionic strength. Biochemistry 40:1226–1237
Cui M, Vogelmann TC, Smith WK (1991) Chlorophyll and light gradients in sun and shade leaves Spinacia oleracea. Plant Cell Environ 14:493–500
Dall’Osto L, Cazzaniga S, Wada M, Bassi R (2014) On the origin of a slowly reversible fluorescence decay component in the Arabidopsis npq4 mutant. Phil Trans R Soc B 369:20130221
Davis PA, Hangarter RP (2012) Chloroplast movement provides photoprotection to plants by redistributing PSII damage within leaves. Photosynth Res 112:153–161
Davis PA, Caylor S, Whippo CW, Hangarter RP (2011) Changes in leaf optical properties associated with light-dependent chloroplasts movements. Plant Cell Environ 34:2047–2059
Demmig-Adams B (1990) Carotenoids and photoprotection in plants: a role for the xanthophylls zeaxanthin. Biochim Biophys Acta 1020:1–24
Demmig-Adams B (1998) Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol 39:474–482
Demmig-Adams B, Cohu CM, Muller O, Adams WW (2012) Modulation of photosynthetic energy conversion efficiency in nature: from seconds to seasons. Photosynth Res 113:75–88
Dietzel L, Brautigam K, Pfannschmidt T (2008) Photosynthetic acclimation: state transitions and adjustment of photosystem stoichiometry-functional relationships between short-term and long-term light quality acclimation in plants. FEBS J 275:1080–1088
Edwards G, Walker D (1983) C3, C4: mechanisms, and cellular and environmental regulation, of photosynthesis. Univ of California Press, Berkeley
Fan M, Li M, Liu Z, Cao P, Pan X, Zhang H, Zhao X, Zhang J, Chang W (2015) Crystal structures of the PsbS protein essential for photoprotection in plants. Nat Struct Mol Biol 22:729–735
Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63:1637–1661
Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Gilmore AM, Hazlett TL, Debrunner PG, Govindjee (1996) Comparative time-resolved photosystem II chlorophyll a fluorescence analyses reveal distinctive differences between photoinhibitory reaction center damage and xanthophyll cycle-dependent energy dissipation. Photochem Photobiol 64:557–563
Goltsev VN, Kalaji HM, Paunov M, Bąba W, Horaczek T, Mojski J, Kociel H, Allakhverdiev SI (2016) Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ J Plant Physiol 63:869–893
Goral TK, Johnson MP, Duffy CDP, Brain APR, Ruban AV, Mullineaux CW (2012) Light-harvesting antenna composition controls the macrostructure and dynamics of thylakoid membranes in Arabidopsis. Plant J 69:289–301
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Haehnel W (1984) Photosynthetic electron transport in higher plants. Annu Rev Plant Physiol 35:659–693
Herbstová M, Tietz S, Kinzel C, Turkina MV, Kirchhoff H (2012) Architectural switch in plant photosynthetic membranes induced by light stress. Proc Natl Acad Sci USA 109:20130–20135
Horton P (2012) Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences. Philos Trans R Soc Lond B Biol Sci 367:3455–3465
Ikeuchi M, Uebayashi N, Sato F, Endo T (2014) Physiological functions of PsbS-dependent and PsbS-independent NPQ under naturally fluctuating light conditions. Plant Cell Physiol 55:1286–1295
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Johnson MP, Ruban AV (2014) Rethinking the existence of a steady-state Δψ component of the proton motive force across plant thylakoid membranes. Photosynth Res 119:233–242
Johnson MP, Davison PA, Ruban AV, Horton P (2008) The xanthophyll cycle pool size controls the kinetics of nonphotochemical quenching in Arabidopsis thaliana. FEBS Lett 582:262–266
Kadota A, Yamada N, Suetsugu N, Hirose M, Saito C, Shoda K, Ichikawa S, Kagawa T, Nakano A, Wada M (2009) Short actin-based mechanism for light-directed chloroplast movement in Arabidopsis. Proc Natl Acad Sci USA 106:13106–13111
Kagawa T, Wada M (1996) Phytochrome- and blue-light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted prothallial cells of fern Adiantum as analyzed by microbeam irradiation. Planta 198:488–493
Kagawa T, Wada M (1999) Chloroplast-avoidance response induced by high-fluence blue light in prothallial cells of the fern Adiantum capillus-veneris as analyzed by microbeam irradiation. Plant Physiol 119:917–923
Kagawa T, Wada M (2000) Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol 41:84–93
Kagawa T, Wada M (2002) Blue light-induced chloroplast relocation. Plant Cell Physiol 43:367–371
Kalaji HM, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev SI et al (2014) Frequently asked questions about in vivo chlorophyll fluorescence: practical issues. Photosynth Res 122:121–158
Karavaev VA, Kukushkin AK (1975) Adaptation of higher plants leaves to dark and far-red light under conditions of oxygen deficiency. Biophysics 20:88–92
Karavaev VA, Kukushkin AK (1976) Application of fast fluorescence induction to study of electron-transport chain states in leaves of higher plants. Biophysics 21:862–866
Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Miyao M, Wada M (2002) Chloroplast avoidance movement reduces photodamage in plants. Nature 420:829–832
Kong S-G, Wada M (2014) Recent advances in understanding the molecular mechanism of chloroplast photorelocation movement. Biochim Biophys Acta 1837:522–530
Königer M, Bollinger N (2012) Chloroplast movement behavior varies widely among species and does not correlate with high light stress tolerance. Planta 236:411–426
Kono M, Terashima I (2014) Long-term and short-term responses of the photosynthetic electron transport to fluctuating light. J Photochem Photobiol B 137:89–99
Kraml M, Büttner G, Haupt W, Herrmann H (1988) Chloroplast orientation in Mesotaenium: the phytochrome effect is strongly potentiated by interaction with blue light. Protoplasma S1:172–179
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis. The basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kyle DJ, Staehelin LA, Arntzen CJ (1983) Lateral mobility of the light-harvesting complex in chloroplast membranes controls excitation energy distribution in higher plants. Arch Biochim Biophys 222:527–541
Lazár D (1999) Chlorophyll a fluorescence induction. Biochim Biophys Acta 1412:1–28
Lemeille S, Rochaix J-D (2010) State transitions at the crossroad of thylakoid signal pathways. Photosynth Res 106:33–46
Li X-P, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866-22874
Li Z, Wakao S, Fischer BB, Niyogi KK (2009) Sensing and responding to excess light. Annu Rev Plant Biol 60:239–260
Lichtenthaler HK, Babani F (2004) Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In: Chlorophyll a Fluorescence: Advances in Photosynthesis and Respiration (Papageorgiou GC and Govindjee, eds) vol. 19, pp 713–736. Springer, Dordrecht, Netherlands
Luesse DR, DeBlasio SL, Hangarter RP (2010) Integration of phot1, phot2, and PhyB signalling in light-induced chloroplast movements. J Exp Bot 61:4387–4397
Malkin S (1966) Fluorescence induction studies in isolated chloroplasts. II. Kinetic analysis of the fluorescence intensity dependence on time. Biochim Biophys Acta 126:433–442
Malkin S, Telfer A, Barber J (1986) Quantitative analysis of State 1-State 2 transitions in intact leaves using modulated fluorimetry—evidence for changes in the absorption cross-section of the two photosystems during state transitions. Biochim Biophys Acta 848:48–57
Mamedov M, Govindjee Nadtochenko V, Semenov A (2015) Primary electron transfer processes in photosynthetic reaction centers from oxygenic organisms. Photosynth Res 125:51–63
Mathur S, Jain L, Jajoo A (2018) Photosynthetic efficiency in sun and shade plants. Photosynthetica 56:354–365
Matsubara S, Förster B, Waterman M, Robinson SA, Pogson BJ, Gunning B, Osmond B (2012) From ecophysiology to phenomics: some implications of photoprotection and shade–sun acclimation in situ for dynamics of thylakoids in vitro. Phil Trans R Soc B 367:3503–3514
Minagawa J (2011) State transitions—the molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim Biophys Acta 1807:897–905
Mishanin VI, Trubitsin BV, Benkov MA, Minin AA, Tikhonov AN (2016) Light acclimation of shade-tolerant and light-resistant Tradescantia species: induction of chlorophyll a fluorescence and P700 photooxidation, expression of PsbS and Lhcb1 proteins. Photosynth Res 130:275–291
Mishanin VI, Trubitsin BV, Patsaeva SV, Ptushenko VV, Solovchenko AE, Tikhonov AN (2017) Acclimation of shade-tolerant and light-resistant Tradescantia species to growth light: chlorophyll a fluorescence, electron transport, and xanthophyll content. Photosynth Res 133:87–102
Mitchell P (1966) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev 41:445–502
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Murata N, Nishimura M, Takamiya A (1966) Fluorescence of chlorophyll in photosynthetic systems. II. Induction of fluorescence in isolated spinach chloroplasts. Biochim Biophys Acta 120:23–33
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421
Nelson N, Junge W (2015) Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu Rev Biochem 84:659–683
Nelson N, Yocum CF (2006) Structure and function of photosystems I and II. Annu Rev Plant Biol 57:521–565
Noctor G, Rees D, Young A, Horton P (1991) The relationship between zeaxanthin, energy-dependent quenching of chlorophyll fluorescence and the trans-thylakoid pH-gradient in isolated chloroplasts. Biochim Biophys Acta 1057:320–330
Oguchi R, Terashima I, Kou J, Chow WS (2011a) Operation of dual mechanisms that both lead to photoinactivation of Photosystem II in leaves by visible light. Physiol Plant 142:47–55
Oguchi R, Douwstra P, Fujita T, Chow WS, Terashima I (2011b) Intra-leaf gradients of photoinhibition induced by different color lights: implications for the dual light mechanisms of photoinhibition and for the application of conventional chlorophyll fluorometers. New Phytol 191:146–159
Papageorgiou GC, Govindjee (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht, p 818
Papageorgiou GC, Govindjee (2011) Photosystem II fluorescence: slow changes—scaling from the past. J Photochem Photobiol B 104:258–270
Park Y, Chow WS, Anderson JM (1996) Chloroplast movement in the shade plant Tradescantia albiflora helps protect photosystem II against light stress. Plant Physiol 111:867–875
Ptushenko OS, Ptushenko VV (2019) Tradescantia-based models: a powerful looking glass for investigation of photoacclimation and photoadaptation in plants. Physiol Plant 166:120–133
Ptushenko VV, Ptushenko EA, Samoilova OP, Tikhonov AN (2013) Chlorophyll fluorescence in the leaves of Tradescantia species of different ecological groups: induction events at different intensities of actinic light. BioSystems 114:85–97
Ptushenko VV, Ptushenko OS, Samoilova OP, Solovchenko AE (2016) An exceptional irradiance-induced decrease of light trapping in two Tradescantia species: an unexpected relationship with the leaf architecture and zeaxanthin-mediated photoprotection. Biologia Plantarum 60:385–393
Ptushenko VV, Ptushenko OS, Samoilova OP, Solovchenko AE (2017) Analysis of photoprotection and apparent non-photochemical quenching of chlorophyll fluorescence in Tradescantia leaves based on the rate of irradiance-induced changes in optical transparence. Biochemistry (Moscow) 82:67–74
Ptushenko VV, Zhigalova TV, Avercheva OV, Tikhonov A.N. (2019) Three phases of energy-dependent induction of \( {\text{P}}_{700}^{ + } \) and Chl a fluorescence in Tradescantia fluminensis leaves. Photosynth Res 139:509–522
Quick WP, Stitt M (1989) An examination of factors contributing to nonphotochemical quenching of chlorophyll fluorescence in barley leaves. Biochim Biophys Acta 977:287–296
Rees D, Young A, Noctor G, Britton G, Horton P (1989) Enhancement of the ΔpH-dependent dissipation of excitation energy in spinach chloroplasts by light-activation: correlation with the synthesis of zeaxanthin. FEBS Lett 256:85–90
Rees D, Noctor G, Ruban AV, Crofts J, Young A, Horton P (1992) pH dependent chlorophyll fluorescence quenching in spinach thylakoids from light-treated or dark-adapted leaves. Photosynth Res 31:11–19
Ruban AV, Johnson MP, Duffy CDP (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817:167–181
Ryzhikov SB, Tikhonov AN (1988) Regulation of electron transfer in photosynthetic membranes of higher plants. Biophysics 33:642–646
Schansker G, Tóth SZ, Kovács L, Holzwarth AR, Garab G (2011) Evidence for a fluorescence yield change driven by a light-induced conformational change within photosystem II during the fast chlorophyll a fluorescence rise. Biochim Biophys Acta 1807:1032–1043
Schansker G, Tóth SZ, Holzwarth AR, Garab G (2014) Chlorophyll a fluorescence: beyond the limits of the QA model. Photosynth Res 120:43–58
Schmitt F-J, Renger G, Friedrich T, Kreslavski VD, Zharmukhamedov SK, Los DA, Kuznetsov VV, Allakhverdiev SI (2014) Reactive oxygen species: re-evaluation of generation, monitoring and role in stress-signal in phototrophic organisms. Biochim Biophys Acta 1837:835–848
Solovchenko A (2010) Photoprotection in Plants. Springer Series in Biophysics 14. Springer-Verlag Berlin Heidelberg
Stirbet A, Govindjee (2011) On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: basics and applications of the OJIP fluorescence transient. J Photochem Photobiol, B 104:236–257
Stirbet A, Govindjee (2012) Chlorophyll a fluorescence induction: a personal perspective perspective of the thermal phase, the J-I-P rise. Photosynth Res 113:15–61
Stirbet A, Govindjee (2016) The slow phase of chlorophyll a fluorescence induction in silico: origin of the S-M fluorescence rise. Photosynth Res 130:193–213
Stirbet A, Riznichenko GY, Rubin AB, Govindjee (2014) Modeling chlorophyll a fluorescence transient: relation to photosynthesis. Biochemistry (Mosc.) 79:291–323
Suetsugu N, Wada M (2012) Chloroplast photorelocation movement: a sophisticated strategy for chloroplasts to perform efficient photosynthesis. In: Najafpour M (ed) Advances in Photosynthesis—Fundamental Aspects. InTech, Croatia, pp 215–234. ISBN 978-953-307-928-8
Suslichenko IS, Tikhonov AN (2019) Photo-reducible plastoquinone pools in chloroplasts of Tradescentia plants acclimated to high and low light. FEBS Lett 593:788–798
Tikhonov AN (2012) Energetic and regulatory role of proton potential in chloroplasts. Biochemistry (Moscow) 77:956–974
Tikhonov AN (2013) pH-Dependent regulation of electron transport and ATP synthesis in chloroplasts. Photosynth Res 116:511–534
Tikhonov AN (2015) Induction events and short-term regulation of electron transport in chloroplasts: an overview. Photosynth Res 125:65–94
Tikkanen M, Aro E-M (2012) Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim Biophys Acta 1817:232–238
Tóth SZ, Schansker G, Strasser RJ (2007) A non-invasive assay of the plastoquinone pool redox state based on OJIP-transient. Photosynth Res 93:193–203
Vener AV, van Kan PJM, Rich PR, Ohad I, Andersson B (1997) Plastoquinol at the quinol oxidation site of reduced cytochrome bf mediates signal transduction between light and protein phosphorylation: thylakoid protein kinase deactivation by a single turnover flash. Proc Natl Acad Sci USA 94:1585–1590
Vener AV, Ohad I, Andersson B (1998) Protein phosphorylation and redox sensing in chloroplast thylakoids. Curr Opin Plant Biol 1:217–223
Vogelmann TC, Evans JR (2002) Profiles of light absorption and chlorophyll within spinach leaves from chlorophyll fluorescence. Plant Cell Environ 25:1313–1323
Walters RG, Horton P (1991) Resolution of components of non-photochemical chlorophyll fluorescence quenching in barley leaves. Photosynth Res 27:121–133
Zurzycki J (1955) Chloroplasts arrangement as a factor in photosynthesis. Acta Soc Bot Pol 24:27–63
Acknowledgements
We thank Dr. A. N. Baranov for his help in measuring the real intensities of blue and red light produced by a PAM-2500 fluorometer. We also thank Dr. V. V. Ptushenko for valuable discussion of the questions concerning anatomical aspects of Tradescantia leaves. We would like to express our gratitude to Reviewers for thorough reading of the manuscript and constructive comments.
Funding
This work has been partly supported by the Russian Foundation for Basic Research (Grant 18-04-00214).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Chlorophyll fluorescence and anatomy peculiarities of Tradescantia leaves
Analysis of induction events in high plant leaves may be complicated by anatomical peculiarities of leaves. Cui et al. (1991), who measured chlorophyll and light gradients in sun and shade leaves of Spinacia oleracea, found that propagation of BL and RL within leaves appeared to be determined largely by light absorption. Light at 450, 550, and 680 nm (blue, green and red light) was greatly attenuated within the palisade layer by initial 150 μm of palisade tissue. In the context of BL and RL propagation into the depth of the Tradescantia leaf, we consider below how the initial rise of fluorescence to maximal value (parameter FP) depends on the photon flux density and light quality. Here, we have to mention one interesting and unusual anatomical property of Tradescantia leaves (T. fluminensis and T. sillamontana) described by Ptushenko and collaborators (Ptushenko et al. 2016; Ptushenko and Ptushenko 2019). It is well-established fact that in traditional model plants, Arabidopsis and Spinacia oleracia, chloroplast-containing cells are spread over the palisade and spongy mesophyll. The light intensity appears to be rapidly reduced and spectrally dispersed with the leaf depth in the direction perpendicular to the leaf surface (Vogelmann and Evans 2002; Oguchi et al. 2011a, b). In contrast to Arabidopsis and Spinacia oleracia, the mesophyll layer in T. fluminensis leaves is relatively thin (~ 40 μm and ~ 70 μm in LL and HL plants, respectively). In T. fluminensis, chloroplast-containing cells are concentrated in mesophyll, which comprises only ~ 10–13% (HL leaves) or ~ 24% (LL leaves) of the whole leaf thickness (Ptushenko et al. 2016). The mesophyll layer consists on average of 2–3 layers of mesophyll cells. The tissues adjacent to mesophyll (thick layers of adaxial and abaxial epidermis; see cartoon in Fig. 14) are almost free of chloroplasts. Thus, in T. fluminensis the vast majority of chloroplast-containing cells are clustered in the relatively thin layer inside the leaf. This circumstance (relatively thin mesophyll) might be important from the optical point of view: since mesophyll cells are located within a relatively thin layer, dispersion of photosynthetically active light absorbed by mesophyll should be reduced.
The top panel in Fig. 14 depicts the histogram, in which we compare the \( F_{\text{P}} /F_{\text{m}}^{ 0} \) ratios (for definition, see Fig. 1) determined in LL- and HL-acclimated leaves upon the action of weak (40 μmol photons m−2 s−1) or strong (500 μmol photons m−2 s−1) actinic light. Here, we refer to maximal intensity of fluorescence \( F_{\text{P}} \) measured at the very beginning of actinic light irradiation, when the regulatory effects associated with the NPQ and avoidance responses to be negligible. In LL-grown leaves illuminated by weak actinic light, the \( F_{\text{P}} /F_{\text{m}}^{ 0} \) ratio determined for BL was lower than that in the case of RL illumination. We can explain this result by more significant attenuation of the BL flux compared to RL. In HL-grown plants, however, both BL and RL induced an increase in fluorescence to the same level FP. The latter result could be explained by morphological peculiarities of Tradescantia leaves. Acclimation of Tradescantia to HL conditions causes the thickening of leaves and the formation of elongated column-type cells in adaxial epidermis (Ptushenko et al. 2016). Cui et al. (1991), who investigated light propagation within Spinacia oleracia leaves, put forward the curious hypothesis that thicker palisade tissue of sun leaves could facilitate the light propagation to spongy mesophyll, providing light penetration further into the leaf. They speculated that elongated cells might act as the lenses that promoted the transmission of light further into the mesophyll cells. In that event one could expect that in HL leaves both BL and RL would propagate more efficiently toward the mesophyll cells. Actually, for HL-grown T. fluminensis leaves, the \( F_{\text{P}} /F_{\text{m}}^{ 0} \) ratio was the same both for weak BL and weak RL (Fig. 14). In the case of strong actinic light, the \( F_{\text{P}} /F_{\text{m}}^{ 0} \) ratio was practically the same (\( F_{\text{P}} /F_{\text{m}}^{ 0} \) = 0.98–0.99), regardless of the growth light intensity and the quality of actinic light. This suggests that the fluxes of strong BL and strong RL in the vicinity of mesophyll were enough to excite fluorescence of maximal intensity FP.
a Effects of intensity of blue (BL) and red (RL) actinic light on the fluorescence parameter \( F_{\text{P}} /F_{\text{m}}^{ 0} \)(for definition, see Fig. 1) in LL- and HL-acclimated T. fluminensis leaves, as indicated. Mean values (n = 4–6) ± SE. b Cartoon illustrating the mesophyll layer position and light propagation across the leaf
The comparison of photo-reducible PQ pool capacities in LL and HL plants
One can evaluate the relative sizes of the photo-reducible PQ pool (parameter Q0) in LL- and HL-acclimated Tradescantia from the kinetics of fast induction of Chl a fluorescence (the OJIP curve) as described in (Suslichenko and Tikhonov 2019). Parameter Q0 was calculated as the integral \( Q_{0} = I_{2} \int_{{t_{1} }}^{{t_{2} }} {\alpha (t)dt = I_{2} \times W} \), where in I2 the PFD for PSII and W is the so-called work integral. The variable α(t) = 1 − F(t)/Fm contains F(t) and Fm, the values of which denote the current and maximal intensities of Chl a fluorescence, respectively. The twofold difference between the Q0 values for LL- and HL-acclimated Tradescantia leaves, Q0(LL)/Q0(HL) ≈ 2, suggests markedly increased capacity of the PQ pool in LL leaves compared to HL samples (for more details, see Suslichenko and Tikhonov 2019). A modified approach to the estimation of Q0 proposes the integration of the variable β(t) = 1 − [F(t) − F0]/[Fm − F0], instead of α(t) (Tóth et al. 2007). It is not difficult to show that both approaches lead to close values of parameter Q0. Elementary algebraic manipulations shows that α(t) = β(t)*[(Fm − F0)/Fm]. The correction factor, α(t)/β(t) = (Fm − F0)/Fm, was of the same value in LL and HL leaves, (Fm − F0)/Fm = 0.8 (see Fig. 1 in Suslichenko and Tikhonov 2019). This shows that both approaches would lead to the same proportionality between the PQ pool capacities in LL and HL samples, Q0(LL)/Q0(HL) ≈ 2.
Chlorophyll fluorescence and the avoidance response in Tradescantia leaves
In the context of the chloroplasts avoidance response, which can influence the time-course of SIF, we address to experimental data reported by Ptushenko et al. (2017), who investigated optical properties of T. fluminensis leaves in plants cultivated at LL and HL under experimental conditions close to those used in our work. Figure 15a demonstrates the spectrum of optical transmittance of dark-adapted T. fluminensis leaves grown under the HL conditions (800 μmol photons m−2 s−1). This spectrum was extracted and re-plotted from Fig. 1 presented in (Ptushenko et al. 2017). Figure 15b demonstrates how the leaf transmittance, determined at 455, 550, 630, and 680 nm, increased upon illumination of T. fluminensis leaves by continuous blue light (475 nm, 150 μmol photons m−2 s−1). Undoubtedly, the light-induced blooming of the leaf was caused by a decrease in the light absorptance caused by the chloroplast avoidance response. Actually, one can see that the most significant increase in the optical transmittance was observed at 455 and 680 nm (maximally absorbed by Chl molecules), rather than that in the green area of spectrum (~ 550 nm). Interestingly that the half-time of the light-induced increase in optical transparence in HL-grown leaves (t1/2 ~ 5–7 min) was similar to the appearance of the intermediate state M2 of SIF observed upon illumination by BL of LL- and HL-acclimated T. fluminensis leaves (Fig. 3).
Optical transmittance spectrum for dark-adapted T. fluminensis leaves grown under the HL conditions (a) and kinetics of the light-induced changes in optical transmittance at four wavelength (455, 550, 630 and 680 nm) induced by continuous blue light (b). All the data shown here were extracted from Fig. 1 in (Ptushenko et al. 2017) and then transformed into the kinetic curves shown in b
Rights and permissions
About this article
Cite this article
Kalmatskaya, O.A., Karavaev, V.A. & Tikhonov, A.N. Slow induction of chlorophyll a fluorescence excited by blue and red light in Tradescantia leaves acclimated to high and low light. Photosynth Res 142, 265–282 (2019). https://doi.org/10.1007/s11120-019-00663-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-019-00663-4