Abstract
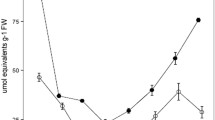
The seasonal temperature acclimation in crassulacean acid metabolism (CAM) and photosynthetic performance were investigated in the aquatic isoetid, Littorella uniflora. Plants were collected monthly from January to September, and CAM capacity and photosynthesis rates were measured at 5, 10, 15, and 20 °C. Seasonal acclimation was observed for CAM (Q 10 range: 0.6–1.8), and CAM was optimised close to ambient temperature throughout the season. Thus, in winter acclimated L. uniflora, the short-term response to raised temperature resulted in a decline in CAM capacity. Even though the ambient CAM increased from winter to spring/summer, CAM was present in cold acclimated plants, thus indicating an ecophysiological role for CAM even in winter. Similar to CAM, seasonal acclimation was observed in the light and carbon-saturated photosynthesis (Q 10 values ranged from 1.4 to 2.3), and the photosynthetic capacity was generally higher during the winter at all temperatures, indicating compensatory investments in the photosynthetic apparatus. Thus, L. uniflora displayed seasonal temperature acclimation with respect to both CAM and photosynthesis. The estimated in situ contribution of CAM to the carbon budget in L. uniflora was independent of season and varied from 23 to 46 %. A positive correlation between photosynthetic capacity and CAM capacity (both measured in the lab at temperature close to ambient temperature) was found, and the ratio of CAM activity to photosynthetic capacity was higher in summer compared with winter plants. Overall, the results from the present study support the suggested role of CAM as a carbon conserving mechanism of importance for survival in a carbon-limited habitat.




Similar content being viewed by others
References
Adams WW, Demming-Adams B, Rosenstiel TN, Brightwell AK, Ebbert V (2002) Photosynthesis and photoprotection in overwintering plants. Plant Biol 4:545–557
Aulio K (1985) Biomass and chlorophyll contents of Isoetes lacustris as related to water depth in the lake Pyhäjärvi, SW Finland. Aqua Fenn 15:127–131
Baatrup-Pedersen A, Madsen TV (1999) Interdependence of CO2 and inorganic nitrogen on crassulacean acid metabolism and efficiency of nitrogen use by Littorella uniflora (L.) Aschers. Plant Cell Environ 22:535–542
Bagger J, Madsen TV (2004) Morphological acclimation of aquatic Littorella uniflora to sediment CO2 concentration and wave exposure. Funct Ecol 18:946–951
Barko JW, Smart MS (1981) Comparative influences of light and temperature on the growth and metabolism of selected submerged freshwater macrophytes. Ecol Monogr 51:219–235
Barrat-Segretain MH (1996) Strategies of reproduction, dispersion, and competition in river plants: a review. Vegetatio 123:13–37
Behzadipour M, Ratajczak R, Faist K, Pawlitschek P, Trémoliéres A, Kluge M (1998) Phenotypic adaptation of tonoplast fluidity to growth temperature in the CAM plant Kalanchoë daigremontiana Ham. et Per. is accompanied by changes in the membrane phospholipids and protein composition. J Membr Biol 166:61–70
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Phys 31:491–543
Boston HL, Adams MS (1985) Seasonal diurnal acid rhythms in two aquatic crassulacean acid metabolism plants. Oecologia 65:573–579
Boston HL, Adams MS (1986) The contribution of crassulacean acid metabolism to the annual productivity of two vascular plants. Oecologia 68:615–622
Boston HL, Adams MS, Pienkowski TP (1987) Utilization of sediment CO2 by selected North American isoetids. Ann Bot 60:485–494
Bowes G (1991) Growth at elevated CO2: photosynthetic responses mediated through Rubisco. Plant Cell Environ 14:795–806
Bowes G, Salvucci ES (1989) Plasticity in the photosynthetic carbon metabolism of submerged aquatic macrophytes. Aquat Bot 34:233–266
Cushman B (2001) Crassulacean acid metabolism. A plastic photosynthetic adaptation to arid environments. Plant Phys 127:1439–1448
Davidson IR (1991) Environmental effects on algal photosynthesis: temperature. J Phycol 27:2–8
Farmer AM, Spence DHN (1987) Flowering, germination and zonation of the submerged aquatic plant Lobelia dortmanna L. J Ecol 75:1065–1076
Garcia E, Ballesteros E (1994) Production of Isoetes lacustris in a Pyrenean lake: seasonality and ecological factors involved in the growing period. Aquat Bot 48:77–89
Hostrup O, Wiegleb G (1991a) Anatomy of leaves of submerged and emergent forms of Littorella uniflora (L.) Ascherson. Aquat Bot 39:195–209
Hostrup O, Wiegleb G (1991b) The influence of different CO2 concentrations in the light and the dark on diurnal malate rhythm and phosphoenolpyruvat carboxylase activities in leaves of Littorella uniflora (L.) Aschers. Aquat Bot 40:91–100
Keeley JE (1983) Crassulacean acid metabolism in the seasonally submerged aquatic Isoetes howelli. Oecologia 58:57–62
Keeley JE (1998) CAM photosynthesis in submerged aquatic plants. Bot Rev 64:121–175
Keeley JE, Busch G (1984) Carbon assimilation characteristics of the aquatic CAM plant, Isoetes howellii. Plant Phys 76:525–530
Keeley JE, Rundel PW (2003) Evolution of CAM and C4 carbon-concentrating mechanisms. Int J Plant Sci 164:55–77
Keeley JE, Walker CM, Mathewes RP (1983) Crassulacean acid metabolism in Isoetes bolanderi in high elevation oligotrophic lakes. Oecologia 58:63–69
Klavsen SK, Maberly SC (2009) Crassulacean acid metabolism contributes significantly to the in situ carbon budget in a population of the invasive aquatic macrophyte Crassula helmsii. Fresh Biol 54:105–188
Klavsen SK, Maberly SC (2010) Effect of light and CO2 on inorganic carbon uptake in the invasive aquatic CAM-plant Crassula helmsii. Funct Plant Biol 37:737–747
Klavsen SK, Madsen TV (2008) Effect of leaf age on CAM activity in Littorella uniflora. Aquat Bot 89:50–56
Klavsen SK, Madsen TV, Maberly SC (2011) Crassulacean acid metabolism (CAM) in the context of other carbon concentrating mechanisms in freshwater plants: a review. Photosynth Res 109:269–279
Kliemchen A, Schomburg M, Galla H-J, Lüttge U, Kluge M (1993) Phenotypic changes in the fluidity of the tonoplast membrane of crassulacean acid metabolism plants in response to temperature and salinity stress. Planta 189:403–409
Kluge M, Schomburg M (1996) The tonoplast as a target of temperature effects in crassulacean acid metabolism. In: Winter K, Smith JAC (eds) Crassulacean acid metabolism—biochemistry, ecophysiology and evolution. Springer, Berlin, pp 72–77
Kluge M, Ting IP (1978) Crassulacean acid metabolism—analysis of an ecological adaptation. Springer, Berlin
Koch KE, Kennedy RA (1980) Effects of seasonal changes in the Midwest on crassulacean acid metabolism (CAM) in Opuntia humifusa Raf. Oecologia 45:390–395
Lambers H, Chapin FS, Pons TL (1998) Photosynthesis, respiration, and long-distance transport. In: Lambers H, Chapin FS, Pons TL (eds) Plant physiological ecology. Springer, New York, pp 10–93
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lüttge U (2004) Ecophysiology of crassulacean acid metabolism (CAM). Ann Bot 93:629–652
Maberly SC (1985) Photosynthesis by Fontinalis antipyretica. I. Interactions between photon irradiance, concentration of carbon dioxide and temperature. New Phytol 100:127–140
Maberly SC (1996) Diel, episodic and seasonal changes in pH and concentration of inorganic carbon in a productive lake. Fresh Biol 35:579–598
Madsen TV (1987a) The effect of different growth conditions on dark and light carbon assimilation in Littorella uniflora. Physiol Plant 70:183–188
Madsen TV (1987b) Interactions between internal and external CO2 pools in the aquatic CAM plants Littorella uniflora (L.) Aschers and Isoetes lacustris L. New Phytol 106:35–50
Madsen TV, Brix H (1997) Growth, photosynthesis and acclimation by two submerged macrophytes in relation to temperature. Oecologia 110:320–327
Madsen TV, Olesen B, Bagger J (2002) Carbon acquisition and carbon dynamics by aquatic isoetids. Aquat Bot 73:351–371
Maxwell K, von Caemmerer S, Evans JR (1997) Is a low internal conductance to CO2 diffusion a consequence of succulence in plants with crassulacean acid metabolism? Aust J Plant Physiol 24:777–786
Murchie EH, Hubbart S, Peng S, Horton P (2005) Acclimation of photosynthesis to high irradiance in rice: gene expression and interactions with leaf development. J Exp Bot 56:449–460
Niinemets Ü (2007) Photosynthesis and resource distribution through plant canopies. Plant Cell Environ 30:1052–1071
Nimmo HG (2000) The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci 5:75–80
Nobel PS, Hartsok TL (1983) Relationships between photosynthetically active radiation, nocturnal acid accumulation and CO2 uptake for a crassulacean acid metabolism plant, Opuntia ficus-indica. Plant Physiol 71:71–75
Olesen B, Madsen TV (2000) Growth and physiological acclimation to temperature and inorganic carbon availability by two submerged aquatic macrophyte species, Callitriche cophocarpa and Elodea canadensis. Funct Ecol 14:252–260
Öquist G, Huner NPA (2003) Photosynthesis of overwintering evergreen plants. Annu Rev Plant Biol 54:329–355
Pedersen O, Sand-Jensen K, Revsbech NP (1995) Dial pulses of O2 and CO2 in sandy lake sediments inhabited by Lobelia dortmanna. Ecol 76:1536–1545
Rattray MR, Webb DR, Brown JMA (1992) Light effects on crassulacean acid metabolism in the submerged plant Isoetes kirkii A Braun. N Z J Mar Freshw Res 26:465–470
Raven JA, Handley LL, MacFarlane JJ, McInroy S, McKenzie L, Richards JH, Samuelsson G (1988) The role of CO2 uptake by roots and CAM in acquisition of inorganic C by plants of the isoetid life-form: a review, with new data on Eriocaulon decangulare L. New Phytol 108:125–148
Robe WE, Griffith H (1990) Photosynthesis of Littorella uniflora grown under two PAR regimes: C3 and CAM gas exchange and the regulation of internal CO2 and O2 concentrations. Oecologia 85:128–136
Robe WE, Griffiths H (1992) Seasonal variation in the ecophysiology of Littorella uniflora (L.) Ascherson in acidic and eutrophic habitats. New Phytol 120:289–304
Robe WE, Grifftih H (1988) C-3 and CAM photosynthetic characteristics of the submerged aquatic macrophyte Littorella uniflora: regulation of leaf internal CO2 supply in response to variation in rooting substrate inorganic carbon concentration. J Exp Bot 39:1397–1410
Sand-Jensen K (1978) Metabolic adaptation and vertical zonation of Littorella uniflora (L.) Aschers and Isoetes lacustris L. Aquat Bot 4:1–10
Sand-Jensen K, Søndergaard M (1978) Growth and production of isoetids in oligotrophic Lake Kalgaard Denmark. Verh Int Verein Limnologie 20:659–666
Sand-Jensen K, Søndergaard M (1997) Plants and environmental conditions in Danish Lobelia-lakes. In: Sand-Jensen K, Pedersen O (eds) Freshwater biology. Priorities and Development in Danish Research, Gad, København, pp 54–73
Sculpthorpe CD (1967) The biology of aquatic vascular plants. Edwards Arnold, London
Skillman JB, Winter K (1997) High photosynthetic capacity in a shade-tolerant crassulacean acid metabolism plant. Plant phys 113:441–450
Smart RM, Barko JW (1985) Laboratory culture of submersed freshwater macrophytes on natural sediments. Aquat Bot 21:251–263
Søndergaard M, Bonde G (1988) Photosynthetic characteristics and pigment content and composition in Littorella uniflora (L.) Aschers in a depth gradient. Aquat Bot 307–319
Søndergaard M, Sand-Jensen K (1979) Carbon uptake by leaves and roots of Littorella uniflora (L.) Aschers. Aquat Bot 6:1–12
Staehr PA, Wernberg T (2009) Physiological responses of Ecklonia radiata (Laminariales) to a latitudinal gradient in ocean temperature. J Phycol 45:91–99
Zotz G, Winter K (1996) Seasonal changes in daytime versus night time CO2 fixation of Clusia uvitana in situ. In: Winter K, Smith JAC (eds) Crassulacean acid metabolism—biochemistry, ecophysiology and evolution. Springer, Berling, pp 312–323
Acknowledgments
The authors thank Stephen C. Maberly for helpful comments on the manuscript and the faculty of Natural Science, Aarhus University and SOAS (School of Aquatic Sciences), which funded the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klavsen, S.K., Madsen, T.V. Seasonal variation in crassulacean acid metabolism by the aquatic isoetid Littorella uniflora . Photosynth Res 112, 163–173 (2012). https://doi.org/10.1007/s11120-012-9759-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-012-9759-0