Abstract
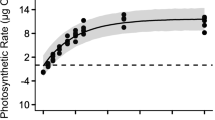
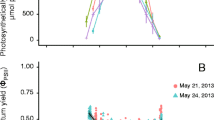
The effects of temperature, irradiance, and desiccation on the photosynthesis of a cultivated Japanese green alga Caulerpa lentillifera (Caulerpaceae) were determined by a pulse amplitude modulation (PAM)-chlorophyll fluorometer and dissolved oxygen sensors. The photochemical efficiency in the photosystem II (Fv/Fm and ΔF/Fm') during the 72-h temperature exposures (8, 12, 16, 20, 24, 28, 32, 36, and 40°C) was generally stable at 16–32°C but quickly dropped at lower and higher temperatures. The photosynthesis–temperature curve at 200 μmol photons m−2 s−1 also revealed that the maximum gross photosynthesis (GPmax) occurred at 30.7°C (30.5–30.9, 95% highest density credible intervals). Photosynthesis–irradiance curves at 16, 24, and 32°C quickly saturated, then expressed photoinhibition, and revealed that the maximum net photosynthetic rates (NPmax) and saturation irradiance (Ek) were highest at 32°C and lowest at 16°C. Continuous 6-h exposure to irradiances of 200 (low) and 400 (high) μmol photons m−2 s−1 at 16, 24, and 32°C expressed greater declines in their ΔF/Fm' at 16°C, revealing chronic chilling-light stress. The response to continuous desiccation (~480 min) under 50% humidity at 24°C showed that ΔF/Fm' dropped to zero at 480-min aerial exposure, and the treatments of more than 60-min desiccation did not return to the initial level even after 24-h subsequent rehydration in seawater. Likewise, ΔF/Fm' fell when the absolute water content (AWC) of the frond dropped below AWC of 90% and mostly did not return to the initial level even after 24-h subsequent rehydration in seawater, signifying a low tolerance to desiccation.






Similar content being viewed by others
References
Alexandrov GA, Yamagata Y (2007) A peaked function for modeling temperature dependence of plant productivity. Ecol Model 200:189–192
Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1657:23–32
Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550
Bellasio C, Burgess SJ, Griffiths H, Hibberd JM (2014) A high throughput gas exchange screen for determining rates of photorespiration or regulation of C4 activity. J Exp Bot 65:3769–3779
Borlongan IA, Nishihara GN, Shimada S, Terada R (2017a) Effects of temperature and PAR on the photosynthesis of Kappaphycus sp. (Solieriaceae, Rhodophyta) from Okinawa, Japan, as the northern limit of native Kappaphycus distribution in the western Pacific. Phycologia 56:444–453
Borlongan IA, Nishihara GN, Shimada S, Terada R (2017b) Photosynthetic performance of the red alga Solieria pacifica (Solieriaceae) from two different depths in the sublittoral waters of Kagoshima, Japan. J Appl Phycol 29:3077–3088
Borlongan IA, Maeno Y, Kozono J, Endo H, Shimada S, Nishihara GN, Terada R (2019a) Photosynthetic performance of Saccharina angustata (Laminariales, Phaeophyceae) at the southern boundary of distribution in Japan. Phycologia 58:300–309
Borlongan IA, Nishihara GN, Shimada S, Terada R (2019b) Assessment of photosynthetic performance in the two life history stages of Alaria crassifolia (Laminariales, Phaeophyceae). Phycol Res 67:28–38
Borlongan IA, Arita R, Nishihara GN, Terada R (2020a) The effects of temperature and irradiance on the photosynthesis of two heteromorphic life history stages of Saccharina japonica (Laminariales) from Japan. J Appl Phycol 32:4175–4187
Borlongan IA, Suzuki S, Nishihara GN, Kozono J, Terada R (2020b) Effects of light quality and temperature on the photosynthesis and pigment content of a subtidal edible red alga Meristotheca papulosa (Solieriaceae, Gigartinales) from Japan. J Appl Phycol 32:1329–1340
Bürkner PC (2018) Advanced Bayesian multilevel modeling with the R package brms. R J 10:395–411
Collado-Vides L, Robledo D (1999) Morphology and photosynthesis of Caulerpa (Chlorophyta) in relation to growth form. J Phycol 35:325–330
Collén J, Davison IR (1999) Stress tolerance and reactive oxygen metabolism in the intertidal red seaweeds Mastocarpus stellatus and Chondrus crispus. Plant Cell Environ 22:1143–1151
Contreras-Porcia L, Thomas D, Flores V, Correa JA (2011) Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J Exp Bot 62:1815–1829
Coppejans E, Prathep A, Lewmanomont K, Hayashizaki K, Clerck D, Leliaert F, Terada R (2017) Seaweeds and seagrasses of the Southern Andaman Sea coast of Thailand. Kagoshima University Museum, Kagoshima
Dring MJ, Brown FA (1982) Photosynthesis of intertidal brown algae during and after periods of emersion: a renewed search for physiological causes of zonation. Mar Ecol Prog Ser 8:301–308
Eggert A (2012) Seaweed responses to temperature. In: Wiencke C, Bischof K (eds) Seaweed Biology. Springer, Berlin, pp 47–66
Eggert A, Wiencke C (2000) Adaptation and acclimation of growth and photosynthesis of five Antarctic red algae to low temperatures. Polar Biol 23:609–618
Flores-Molina MR, Thomas D, Lovazzano C, Núñez A, Zapata J, Kumar M, Correa JA, Contreras-Porcia L (2014) Desiccation stress in intertidal seaweeds: effects on morphology, antioxidant responses and photosynthetic performance. Aquat Bot 113:90–99
Fukumoto R, Borlongan IA, Nishihara GN, Endo H, Terada R (2018) The photosynthetic responses to PAR and temperature including chilling-light stress on the heteromorphic life history stages of a brown alga, Cladosiphon okamuranus (Chordariaceae) from Ryukyu Islands, Japan. Phycol Res 66:209–217
Fukumoto R, Borlongan IA, Nishihara GN, Endo H, Terada R (2019) Effect of photosynthetically active radiation and temperature on the photosynthesis of two heteromorphic life history stages of a temperate edible brown alga, Cladosiphon umezakii (Chordariaceae, Ectocarpales), from Japan. J Appl Phycol 31:1259–1270
Gacia E, Littler MM, Littler DS (1996a) The relationships between morphology and photosynthetic parameters within the polymorphic genus Caulerpa. J Exp Mar Biol Ecol 204:209–224
Gacia E, Rodriguez-Prieto C, Delgado O, Ballesteros E (1996b) Seasonal light and temperature responses of Caulerpa taxifolia from the northwestern Mediterranean. Aquat Bot 53:215–225
Gao S, Wang G (2012) The enhancement of cyclic electron flow around photosystem I improves the recovery of severely desiccated Porphyra yezoensis (Bangiales, Rhodophyta). J Exp Bot 63:4349–4358
Gao S, Niu J, Chen W, Wang G, Xie X, Pan G, Gu W, Zhu D (2013) The physiological links of the increased photosystem II activity in moderately desiccated Porphyra haitanensis (Bangiales, Rhodophyta) to the cyclic electron flow during desiccation and rehydration. Photosynth Res 116:45–54
Gao D, Sun Z, Huang C, Yao J, Wang Y, Tan W, Chen F (2020) First record of Caulerpa lentillifera J. Agardh (Bryopsidales, Chlorophyta) from China. Mar Biol Res 16:44–49
Gayol P, Falconetti C, Chisholm JRM, Jaubert JM (1995) Metabolic responses of low-temperature-acclimated Caulerpa taxifolia (Chlorophyta) to rapidly elevated temperature. Bot Mar 38:61–67
Gelman A (2004) Parameterization and Bayesian Modeling. J Amer Stat Ass 99:537–545
Gelman A (2006) Prior distributions for variance parameters in hierarchical models. Bayesian Anal 1:515–533
Guo H, Yao J, Sun Z, Duan D (2015a) Effects of salinity and nutrients on the growth and chlorophyll fluorescence of Caulerpa lentillifera. Chinese J Oceanol Limnol 33:410–418
Guo H, Yao J, Sun Z, Duan D (2015b) Effect of temperature, irradiance on the growth of the green alga Caulerpa lentillifera (Bryopsidophyceae, Chlorophyta). J Appl Phycol 27:879–885
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photo inhibition and diel changes. J Phycol 29:729–739
Holzinger A, Karsten U (2013) Desiccation stress and tolerance in green algae: consequences for ultrastructure, physiological, and molecular mechanisms. Front Plant Sci 4:article 327
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Ji Y, Tanaka J (2002) Effect of desiccation on the photosynthesis of seaweeds from the intertidal zone in Honshu, Japan. Phycol Res 50:145–153
Kageyama A, Yokohama Y (1978) The function of siphonein in a siphonous green alga Dichotomosiphon tuberosus. Jap J Phycol 26:151–155
Kageyama A, Yokohama Y, Shimura S, Ikawa T (1977) An efficient excitation energy transfer from a carotenoid, siphonaxanthin to chlorophyll a observed in a deep-water species of chlorophycean seaweed. Plant Cell Physiol 18:477–480
Karsten U (2012) Seaweed acclimation to salinity and desiccation stress. In: Wiencke C, Bischof K (eds) Seaweed Biology. Springer, Berlin, pp 87–107
Kokubu S, Nishihara GN, Watanabe Y, Tsuchiya Y, Amano Y, Terada R (2015) The effect of irradiance and temperature on the photosynthesis of a native brown alga, Sargassum fusiforme (Fucales) from Kagoshima, Japan. Phycologia 54:235–247
Kurashima A, Serisawa Y, Kanbayashi T, Toma T, Yokohama Y (2003) Characteristics in photosynthesis of Caulerpa lentillifera J. Agardh and C. racemosa (Forsskål) J. Agardh var. laete-virens (Montagne) Weber-van Bosse with reference to temperature and light intensity. Jpn J Phycol 51:167–172 (in Japanese with English abstract)
Li H, Liu J, Zhang L, Pang T (2016) Effects of low temperature stress on the antioxidant system and photosynthetic apparatus of Kappaphycus alvarezii (Rhodophyta, Solieriaceae). Mar Biol Res 12:1064–1077
Liu H, Wang F, Wang Q, Dong S, Tian X (2016) A comparative study of the nutrient uptake and growth capacities of seaweeds Caulerpa lentillifera and Gracilaria lichenoides. J Appl Phycol 28:3083–3089
Matanjun P, Mohamed S, Mustapha NM, Muhammad K (2009) Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol 21:75–80
Minh NP, Nhi TTY, Tuyen LK, Phi TH, Khoa DT, Thuan TQ (2019) Technical factors affecting seagrape (Caulerpa lentillifera) production by cultivation and its stability by post-harvest treatment. J Pharm Sci Res 11:783–786
Ohno M, Largo DB (1998) The seaweed resources of Japan. In: Critchley AT, Ohno M (eds) Seaweed resources of the world. Japan International Cooperation Agency, Yokosuka, pp 1–14
Paul NA, Neveux N, Magnusson M, de Nys R (2014) Comparative production and nutritional value of “sea grapes” – the tropical green seaweeds Caulerpa lentillifera and C. racemosa. J Appl Phycol 26:1833–1844
Pereira L (2016) Edible seaweeds of the world. CRC Press, Boca Raton
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
R Development Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 10 May 2020
Raniello R, Lorenti M, Brunet C, Buia MC (2004) Photosynthetic plasticity of an invasive variety of Caulerpa racemosa in a coastal Mediterranean area: light harvesting capacity and seasonal acclimation. Mar Ecol Prog Ser 271:113–120
Ricketts TR (1971) The structures of siphonein and siphonaxanthin from Codium fragile. Phytochemistry 10:155–160
Robledo D, Freile-Pelegrín Y (2005) Seasonal variation in photosynthesis and biochemical composition of Caulerpa spp. (Bryopsidales, Chlorophyta) from the Gulf of Mexico. Phycologia 44:312–319
Roleda MY (2009) Photosynthetic response of Arctic kelp zoospores exposed to radiation and thermal stress. Photobiol Sci 8:1302–1312
Shiroma H (2012) Caulerpa lentillifera. In: Watanabe MM et al (eds) Handbook of algae. Their diversity and utilization. NTS, Tokyo, pp 568–571 (in Japanese)
Silva PC, Basson PW, Moe RL (1996) Catalogue of the benthic marine algae of the Indian ocean. University of California Publications in Botany 79. University of California Press, Ewing
Stan Development Team (2020) Stan: A C++ library for probability and sampling, Version 2.14.2. http://mc-stan.org. Accessed 10 May 2020
Sudrajat I, Hamsah, Hasbullah D, Lideman (2018) Peningkatan produksi lawi-lawi (Caulerpa sp.). Jurnal Pereka Yasaan Budidaya Air Pa Yau 4:91–101 (in Indonesian)
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13:178–182
Tcherkez G, Bligny R, Gout E, Mahé A, Hodges M, Cornic G (2008) Respiratory metabolism of illuminated leaves depends on CO2 and O2 conditions. Proc Natl Acad Sci USA 105:797–802
Terada R, Uchimura M, Tanaka T (2012) Morphology and distribution of Caulerpa lentillifera J. Agardh (Chlorophyceae) in Japanese waters, including the first record from southern Kyushu and northern Ryukyu Islands. J Jap Bot 87:260–267 (in Japanese with English abstract)
Terada R, Matsumoto K, Borlongan IA, Watanabe Y, Nishihara GN, Endo H, Shimada S (2018a) The combined effects of PAR and temperature including the chilling-light stress on the photosynthesis of a temperate brown alga, Sargassum patens (Fucales), based on field and laboratory measurements. J Appl Phycol 30:1893–1904
Terada R, Nakazaki Y, Borlongan IA, Endo H, Nishihara GN (2018b) Desiccation effect on the PSII photochemical efficiency of cultivated Japanese Caulerpa lentillifera under the shipping package environment. J Appl Phycol 30:2533–2588
Terada R, Nakahara K, Borlongan IA, Watanabe Y, Mine T, Morikawa T, Igari T, Nishi H, Endo H, Nishihara GN (2019) Combined effects of irradiance and temperature on the PSII photochemical efficiency in the heteromorphic life history stages of cultivated Pyropia (Bangiales): P. yezoensis f. narawaensis and P. tenera from Japan. J Appl Phycol 31:1251–1257
Terada R, Nakashima Y, Borlongan IA, Shimabukuro H, Kozono J, Endo H, Shimada S, Nishihara GN (2020a) Photosynthetic activity including the thermal- and chilling-light sensitivities of a temperate Japanese brown alga Sargassum macrocarpum. Phycol Res 68:70–79
Terada R, Yuge T, Watanabe Y, Mine T, Morikawa T, Nishihara GN (2020b) Chronic effects of three different stressors, irradiance, temperature, and desiccation on the PSII photochemical efficiency in the heteromorphic life-history stages of cultivated Pyropia yezoensis f. narawaensis (Bangiales) from Japan. J Appl Phycol 32:3273–3284
Thornley JHM, Johnson IR (2000) Plant and crop modelling: a mathematical approach to plant and crop physiology. Blackburn Press, Caldwell
Titlyanov EA, Titlyanova TV (2012) Marine plants of the Asian Pacific region countries, their use and cultivation. A.V. Zhirmunsky Institute of Marine Biology, Far East Branch of the Russian Academy of Sciences, Dalnauka, Vladivostok
Toma T (1991) Caulerpa lentillifera. In: Miura A (ed) Cultivation of edible algae. Koseisha-Koseikaku, Tokyo, pp 69–80 (in Japanese)
Toma T (2012) Seaweed and seagrass in Okinawa. Mugen, Naha, Okinawa (in Japanese)
Trono GC (1998) The seaweed resources of the Philippines. In: Critchley AT, Ohno M (eds) Seaweed resources of the world. Japan International Cooperation Agency, Yokosuka, pp 47–61
Trono GC (1999) Diversity of the seaweed flora of the Philippines and its utilization. Hydrobiologia 398:1–6
Tsutsui I, Huynh QN, Nguyen HD, Arai S, Yoshida T (2005) The common marine plants of southern Vietnam. Japan Seaweed Association, Tosa, Kochi
Wang WJ, Wang FJ, Zhu JY, Sun XT, Yao CY, Xu P (2011) Freezing tolerance of Porphyra yezoensis (Bangiales, Rhodophyta) gametophyte assessed by chlorophyll fluorescence. J Appl Phycol 23:1017–1022
Watanabe Y, Morikawa T, Mine T, Kawamura Y, Nishihara GN, Terada R (2017) Chronological change and the potential of recovery on the photosynthetic efficiency of Pyropia yezoensis f. narawaensis (Bangiales) during the sporelings frozen storage treatment in the Japanese Nori cultivation. Phycol Res 65:265–271
Webb WL, Newton M, Starr D (1974) Carbon dioxide exchange of Alnus rubra: a mathematical model. Oecologia 17:281–291
Wichachucherd B, Pannak S, Saengthong C, Rodcharoen E, Koodkaew I (2019) Correlation between growth, phenolic content and antioxidant activity in the edible seaweed, Caulerpa lentillifera in open pond culture system. J Fish Environ Kasetsart Univ 43:66–75
Yoshida T (1998) Marine algae of Japan. Uchida Rokakuho Publishing Co., Ltd., Tokyo (in Japanese)
Acknowledgements
We thank Yoshiki Nakazaki and Tomohiro Ito of the Laboratory of Marine Botany, Kagoshima University, for their kind support in the preliminary experiment of the present study. All authors have provided consent.
Funding
This study was supported in part by the Grant-in-Aid for Scientific Research (category B, #16H02939, #20H03076) from Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Terada, R., Takaesu, M., Borlongan, I.A. et al. The photosynthetic performance of a cultivated Japanese green alga Caulerpa lentillifera in response to three different stressors, temperature, irradiance, and desiccation. J Appl Phycol 33, 2547–2559 (2021). https://doi.org/10.1007/s10811-021-02439-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-021-02439-7