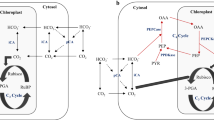
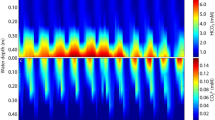
Abstract
Inorganic carbon can be in short supply in freshwater relative to that needed by freshwater plants for photosynthesis because of a large external transport limitation coupled with frequent depleted concentrations of CO2 and elevated concentrations of O2. Freshwater plants have evolved a host of avoidance, exploitation and amelioration strategies to cope with the low and variable supply of inorganic carbon in water. Avoidance strategies rely on the spatial variation in CO2 concentrations within and among lakes. Exploitation strategies involve anatomical and morphological features that take advantage of sources of CO2 outside of the water column such as the atmosphere or sediment. Amelioration strategies involve carbon-concentrating mechanisms based on uptake of bicarbonate, which is widespread, C4-fixation, which is infrequent, and crassulacean acid metabolism (CAM), which is of intermediate frequency. CAM enables aquatic plants to take up inorganic carbon in the night. Furthermore, daytime inorganic carbon uptake is generally not inhibited and therefore CAM is considered to be a carbon-conserving mechanism. CAM in aquatic plants is a plastic mechanism regulated by environmental variables and is generally downregulated when inorganic carbon does not limit photosynthesis. CAM is regulated in the long term (acclimation during growth), but is also affected by environmental conditions in the short term (response on a daily basis). In aquatic plants, CAM appears to be an ecologically important mechanism for increasing inorganic carbon uptake, because the in situ contribution from CAM to the C-budget generally is high (18–55%).



Similar content being viewed by others
References
Aulio K (1985) Differential expression of diel acid metabolism in two life forms of Littorella uniflora (L.) Aschers. New Phytol 100:533–536
Aulio K (1986) CAM-like photosynthesis in Littorella uniflora (L.) Aschers: the role of humidity. Ann Bot 58:273–275
Baatrup-Pedersen A (1996) Growth and photosynthesis of submerged plants—relations to nitrogen. Ph.D. Thesis, Aarhus University
Baatrup-Pedersen A, Madsen TV (1999) Interdependence of CO2 and inorganic nitrogen on crassulacean acid metabolism and efficiency of nitogen use by Littorella uniflora (L.) Aschers. Plant Cell Environ 22:535–542
Black MA, Maberly SC, Spence DHN (1981) Resistance to carbon dioxide fixation in four submerged freshwater macrophytes. New Phytol 89:557–568
Boston HL, Adams MS (1985) Seasonal diurnal acid rhythms in two aquatic Crassulacean acid metabolism plants. Oecologia 65:573–579
Boston HL, Adams MS (1986) The contribution of crassulacean acid metabolism to the annual productivity of two vascular plants. Oecologia 68:615–622
Boston HL, Adams MS, Pienkowski TP (1987) Utilization of sediment CO2 by selected north American isoetids. Ann Bot 60:485–494
Bowes G (1987) Aquatic plant photosynthesis: strategies that enhance carbon gain. In: Crawford RMM (ed) Plant life in aquatic and amphibious habitats. Blackwell Scientific Publications, Oxford, pp 79–98
Bowes G (1991) Growth at elevated CO2: photosynthetic responses mediated through Rubisco. Plant Cell Environ 14:795–806
Bowes G, Salvucci ME (1989) Plasticity in the photosynthetic carbon metabolism of submerged aquatic macrophytes. Aquat Bot 34:233–266
Casati P, Lara MV, Andreo CS (2000) Induction of a C4-like mechanisms of CO2 fixation in Egeria densa, a submersed aquatic species. Plant Physiol 123:1611–1621
Cushman JC (2001) Crassulacean Acid Metabolism. A plastic photosynthetic adaptation to arid environments. Plant Physiol 127:1439–1448
Dacey JWH (1980) Internal winds in water lilies-an adaptation for life in anaerobic sediments. Science 210:1017–1019
Dawson FH, Warman EA (1987) Crassula helmsii (T. Kirk) Cockayne: is it an aggressive alien aquatic plant in Britain? Biol Conserv 42:247–272
Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K (2002) Crassulacean acid metabolism: plastic, fantastic. J Exp Bot 53:569–580
Edwards GE, Franceschi VR, Vosnesenskaya EV (2004) Single-cell C-4 photosynthesis versus dual-cell (Kranz) anatomy. Ann Rev Plant Biol 55:173–196
Ehleringer JR, Monson RK (1993) Evolution and ecological aspects of photosynthetic pathway variation. Ann Rev 24:411–439
Farmer AM, Spence DHN (1985) Studies of diurnal acid fluctuations in British isoetid-type submerged aquatic macrophytes. Ann Bot 56:347–350
Groenhof AC, Smirnoff N, Bryant JA (1988) Enzymatic activities associated with the ability of aerial and submerged forms of Littorella uniflora(L.) Aschers to perform CAM. J Exp Bot 39:353–361
Herrera A (2009) Crassulacean acid metabolism and fitness under water deficit stress: if not for carbon gain, what is facultative CAM good for? Ann Bot London 103:645–653
Hostrup O, Wiegleb G (1991) The influence of different CO2 concentrations in the light and the dark on diurnal malate rhythm and phosphoenolpyruvat carboxylase activities in leaves of Littorella uniflora (L.) Aschers. Aquat Bot 40:91–100
Keeley JE (1996) Aquatic CAM photosynthesis. In: Winter K, Smith JAC (eds) Crassulacean Acid Metabolism–biochemistry, ecophysiology and evolution. Springer-Verlag, Berling, pp 281–295
Keeley JE (1998a) C4 photosynthetic modifications in the evolutionary transition from land to water in aquatic grasses. Oecologia 116:85–97
Keeley JE (1998b) CAM photosynthesis in submerged aquatic plants. Bot Rev 64:121–175
Keeley JE (1999) Photosynthetic pathway diversity in a seasonal pool community. Fun Ecol 13:106–118
Keeley JE, Bowes G (1982) Gas exchange characteristics of the submerged aquatic Crassulacean acid metabolism plant, Isoetes howellii. Plant Physiol 70:1455–1458
Keeley JE, Busch G (1984) Carbon assimilation characteristics of the aquatic CAM plant, Isoetes howellii. Plant Phys 76:525–530
Keeley JE, Rundel PW (2003) Evolution of CAM and C4 carbon-concentrating mechanisms. Int J Plant Sci 164:55–77
Keeley JE, Sandquist DR (1992) Carbon: freshwater plants. Plant Cell Environ 15:1021–1035
Keeley JE, Walker CM, Mathews RP (1983) Crassulacean acid metabolism in Isoetes bolanderi in high elevation oligotrophic lakes. Oecologia 58:63–69
Klavsen SK, Maberly SC (2009) Crassulacean acid metabolism contributes significantly to the in situ carbon budget in a population of the invasive aquatic macrophyte Crassula helmsii. Fresh Biol 54:105–118
Klavsen SK, Maberly SC (2010) Effect of light and CO2 on inorganic carbon uptake in the invasive aquatic CAM-plant Crassula helmsii. Funct Plant Biol 37:1–11
Klavsen SK, Madsen TV (2008) Effect of leaf age on CAM activity in Littorella uniflora. Aqut Bot 89:50–56
Kluge M, Ting IP (1978) Crassulacean Acid Metabolism–analysis of an ecological adaptation. Springer-Verlag, Berlin
Lüttge U (2002) CO2-concentrating: consequences in Crassulacean acid metabolism. J Exp Bot 53:2131–2142
Lüttge U (2004) Ecophysiology of Crassulacean acid metabolism (CAM). Ann Bot 93:629–652
Maberly SC (1985) Photosynthesis by Fontinalis antipyretica. II. Assessment of environmental factors limiting photosynthesis and production. New Phytol 100:141–155
Maberly SC (1996) Diel, episodic and seasonal changes in pH and concentrations of inorganic carbon in a productive lake. Freshwat Biol 35:579–598
Maberly SC, Madsen TV (1998) Affinity for CO2 in relation to the ability of freshwater macrophytes to use HCO3 −. Fun Ecol 12:99–106
Maberly SC, Madsen TV (2002) Freshwater angiosperm carbon concentrating mechanisms: processes and patterns. Funct Plant Biol 29:393–405
Maberly SC, Spence DHN (1983) Photosynthetic inorganic carbon use by freshwater plants. J Ecol 71:705–724
Maberly SC, Spence DHN (1989) Photosynthesis and photorespiration in freshwater organisms: amphibious plants. Aquat Bot 34:267–286
Madsen TV (1987a) The effect of different growth conditions on dark and light carbon assimilation in Littorella uniflora. Physiol Plant 70:183–188
Madsen TV (1987b) Interactions between internal and external CO2 pools in the aquatic CAM plants Littorella uniflora (L.) Aschers and Isoetes lacustris L. New Phytol 106:35–50
Madsen TV (1987c) Sources of inorganic carbon aquired through CAM in Littorella uniflora (L.) Aschers. J Exp Bot 38:367–377
Madsen TV, Maberly SC (1991) Diurnal variation in light and carbon limitation of photosynthesis by two species of submerged freshwater macrophyte with a differential ability to use bicarbonate. Fresh Biol 26:175–187
Madsen TV, Maberly SC (2003) High internal resistance to CO2 uptake by submerged macrophytes that use HCO3 −: measurements in air, nitrogen and helium. Photos Res 77:183–190
Madsen TV, Sand-Jensen K (1991) Photosynthetic carbon assimilation in aquatic macrophytes. Aquat Bot 41:5–40
Madsen TV, Sand-Jensen K (2006) Aquatic plants. In: Sand-Jensen K, Friberg N, Murphy J (eds) Running waters. Ministry of the Environment, Denmark
Madsen TV, Olesen B, Bagger J (2002) Carbon acquisition and carbon dynamcis by aquatic isoetids. Aquat Bot 73:351–371
Nielsen LT, Borum J (2008) Why the free floating macrophyte Stratiotes aloides mainly grows in highly CO2-supersaturated waters. Aquat Bot 89:379–384
Nielsen SL, Garcia E, Sand-Jensen K (1991) Landplants of amphibious Littorella uniflora (L.) Aschers. maintain utilization of CO2 from the sediment. Oecologia 88:258–262
Nimmo HG (2000) The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci 5:75–80
Osmond CB (1978) Crassulacean acid metabolism: a curiosity in context. Ann Rev Plant Physiol 29:379–414
Osmond CB, Ramus J, Levavasseur G, Franklin LA, Henley WJ (1993) Fluorescence quenching during photosynthesis and photoinhibition of Ulva rotundata Blid. Planta 190:97–106
Prins HBA, De Guia MB (1986) Carbon source for the water soldier, Stratiotes aloides L. Aquat Bot 26:225–234
Prins HBA, Elzenga JTM (1989) Bicarbonate utilization: function and mechanism. Aquat Bot 34:59–83
Rattray MR, Webb DR, Brown JMA (1992) Light effects on crassulacean acid metabolim in the submerged aquatic plant Isoetes kirkii A. Braun. N Z J Mar Freshwat Res 26:465–470
Raven JA (1995) Photosynthesis in aquatic plants. In: Schulze ED, Coldwell MM (eds) Ecophysiology of photosynthesis. Springer-Verlag, Berlin, pp 299–318
Raven JA, Spicer RA (1996) The evolution of Crassulacean Acid Metabolism. In: Winter K, Smith JAC (eds) Crassulacean Acid Metabolism–biochemistry, ecophysiology and evolution. Springer-Verlag, Berlin
Raven JA, Handley LL, MacFarlane JJ, McInroy S, McKenzie L, Richards JH, Samuelsson G (1988) The role of CO2 uptake by roots and CAM in acquisition of inorganic C by plants of the isoetid life-form: a review, with new data on Eriocaulon decangulare L. New Phytol 108:125–148
Raven JA, Cockell CS, De La Rocha CL (2008) The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos T Roy Soc B 363:2641–2650
Reiskind JB, Madsen TV, Van Ginkel LC, Bowes G (1997) Evidence that inducible C4-type photosynthesis is a chloroplastic CO2-concentrating mechanism in Hydrilla, a submerged monocot. Plant Cell Environ 20:211–220
Richardson K, Griffiths H, Reed ML, Raven JA, Griffiths NM (1984) Inorganic carbon assimilation in the isoetids, Isoetes lacustris L. and Lobelia dortmanna L. Oecologia 61:115–121
Robe WE, Griffiths H (1990) Photosynthesis of Littorella uniflora grown under two PAR regimes: C3 and CAM gas exchange and the regulation of internal CO2 and O2 concentrations. Oecologia 85:128–136
Robe WE, Griffiths H (1994) The impact of NO -3 loading on the freshwater macrophyte Littorella uniflora: N utilization strategy in a slow-growing species from oligotrophic habitats. Oecologia 100:368–378
Robe WE, Griffiths H (2000) Physiological and photosynthetic plasticity in the amphibious, freshwater plant, Littorella uniflora, during the transition from aquatic to dry terrestrial environments. Plant Cell Environ 23:1041–1054
Sage RF, Kubien DS (2003) Quo vadis C4? An ecophysiological perspective on global climate change and the future of C4 plants. Photos Res 77:209–225
Salvucci ME, Bowes G (1981) Induction of reduced photorespiratory activity in submerged and amphibious aquatic macrophytes. Plant Physiol 67:335–340
Sand-Jensen K, Søndergaard M (1997) Plants and environmental conditions in Danish Lobelia-lakes. In: Sand-Jensen K, Pedersen O (eds) Freshwater Biology. Priorities and Development in Danish Research. Gad, København, pp 54–73
Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC (2010) Evolution of the crassulacean acid metabolism continuum. Funct Plant Biol 37:995–1010
Smith WG, Boston HL, Adams MS (1985) A preliminary study of the source and fate of carbon acquired though CAM in Littorealla uniflora var. americana (Fern.) Gl. J Fresh Ecol 3:203–209
Ueno O, Samejima M, Muto S, Miyachi S (1988) Photosynthetic characteristics of an amphibious plant, Eleocharis vivipara: expression of C3 and C4 modes in contrasting environments. Proc Natl Acad Sci USA 85:6733–6737
Vadstrup M, Madsen TV (1995) Growth limitation of submerged aquatic macrophytes by inorganic carbon. Fresh Biol 34:411–419
Van TK, Haller WT, Bowes G (1976) Comparison of photosynthetic characteristics of three submereged aquatic plants. Plant Physiol 58:761–768
Vestergaard O, Sand-Jensen K (2000) Alkalinity and trophic state regulate aquatic plant distribution in Danish lakes. Aquat Bot 67:85–107
White A, Reiskind JB, Bowes G (1996) Dissolved inorganic carbon influences the photosynthetic responses of Hydrilla to photoinhibitory conditions. Aquat Bot 53:3–13
Winter K, Holtum JAM (2002) How closely do the δ13C values of Crassulacean Acid Metabolism plants reflect the proportion of CO2 fixed day and night? Plant Physiol 129:1843–1851
Winter K, Smith JAC (1996) An introduction to Crassulacean Acid Metabolism. Biochemical Principles and Ecological Diversity. In: Smith JAC, Winter K (eds) Crassulacean acid metabolism—biochemistry, ecophysiology and evolution. Springer-Verlag, Berlin, pp 1–13
Acknowledgments
This work was supported by a grant to Signe Koch Klavsen from the Danisch Research Council for independent research: Natural Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klavsen, S.K., Madsen, T.V. & Maberly, S.C. Crassulacean acid metabolism in the context of other carbon-concentrating mechanisms in freshwater plants: a review. Photosynth Res 109, 269–279 (2011). https://doi.org/10.1007/s11120-011-9630-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-011-9630-8