Abstract
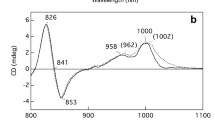
Low-frequency (90–435 cm−1) NIR-excitation (875–900 nm) resonance Raman (RR) studies are reported for the H(M202)G cavity mutant of bacterial photosynthetic reaction centers (RCs) from Rb. sphaeroides that was first described by Goldsmith et al. [(1996) Biochemistry 35: 2421–2428]. In this mutant, the His residue that axially ligates the Mg ion of the M-side bacteriochlorophyll (BChl) of the special pair primary donor (P) is replaced by a non-ligating Gly residue. Regardless, the Mg ion of PM in the H(M202)G RCs remains pentacoordinates and is presumably ligated by a water molecule, although this axial ligand has not been definitively identified. The low-frequency RR studies of the H(M202)G RCs are accompanied by studies of RCs exchanged with D2O and incubated with imidazole (Im). The RR studies of the cavity mutant RCs reveal the following: (1) The structure of PM in the H(M202)G RCs is different from that of the wild-type, consistent with an altered BChl core. (2) A water ligand for PM in the H(M202)G RCs is generally consistent with the low-frequency RR spectra. The Mg-OH2 stretching vibration is tentatively assigned to a band at 318 cm−1, a frequency higher than that of the Mg-His stretch of the native pigment (∼ ∼235 cm−1). (3) The BChl core structure of PM in the cavity mutant is rendered similar (but not identical) to that of the wild-type when the adventitious water axial ligand is replaced by Im. (4) Exchange with D2O results in more global structural changes, likely involving the protein, which in turn affect the structure of the BChls in P. (5) Assignment of the low-frequency vibrational spectrum of P is generally more complex than originally suggested.
Similar content being viewed by others
Abbreviations
- BChl:
-
bacteriochlorophyll
- BPh:
-
bacteriopheophytin
- DFT:
-
density functional theory
- LDAO:
-
N,N-dimethyldodecylamine-N-oxide
- P:
-
special pair dimer of BChls
- Q:
-
quinone
- RC:
-
reaction center
- RR:
-
resonance Raman
- tris:
-
tris[hydroxymethyl]aminomethane
References
Barrick D, (1994) Replacement of the proximal ligand of sperm whale myoglobin with free imidazole in the mutant his-93-gly Biochemistry 33:6546–6554
Becke AD, (1993) Density functional thermochemistry 3. The role of exact exchange J Chem Phys 98:5648–5652
Blankenship RE, Madigan MT, Bauer CE, (eds) (1995) Anoxygenic Photosynthetic Bacteria Kluwer Academic Publishers Dordrect, The Netherlands pp 503–708
Boxer SG, Goldstein RA, Lockhart DJ, Middendorf RT, Takiff LJ, (1989) Excited states, electron-transfer reactions, and intermediates in bacterial photosynthetic reaction centers Phys Chem 93:8280–8294
Bylina EJ, Youvan DC, (1988) Directed mutations affecting spectroscopic and electron-transfer properties of the primary donor in the photosynthetic reaction center Proc Natl Acad Sci USA 85:7226–7230
Chen L, Holten D, Bocian DF, Holten D, (2004) Effects of hydrogen bonding and structure of the accessory bacteriochlorophylls on charge separation in Rb. capsulatus reaction centers J Phys Chem B 108:10457–10464
Chen L, Kirmaier C, Holten D, Bocian DF, (2005) Resonance Raman characterization of Rhodobacter capsulatus reaction centers with lysine mutations near the accessory bacteriochlorophylls Photosynth Res 83:35–43
Cherepy NJ, Shreve AP, Moore LJ, Franzen S, Boxer SG, Mathies RA, (1994) Near-infrared resonance Raman spectroscopy of the special pair and the accessory bacteriochlorophylls in photosynthetic reaction centers J Phys Chem 98:6023–6029
Cherepy NJ, Holzwarth AR, Mathies RA, (1995) Near-infrared resonance Raman spectra of Chloroflexus aurantiacus photosynthetic reaction centers Biochemistry 34:5288–5293
Cherepy NJ, Shreve AP, Moore LJ, Boxer SG, Mathies RA, (1997a) Temperature dependence of the Qy resonance Raman spectra of bacteriochlorophylls, the primary electron donor, and bacteriopheophytins in the bacterial photosynthetic reaction center Biochemistry 36:8559–8566
Cherepy NJ, Shreve AP, Moore LJ, Boxer SG, Mathies RA, (1997b) Electron nuclear dynamics of the accessory bacteriochlorophylls in photosynthetic reaction centers J Phys Chem B 101:3250–3260
Czarnecki K, Schenck CC, Bocian DF, (1997a) Resonance Raman characterization of reaction centers in which bacteriochlorophyll replaces the photoactive bacteriopheophytin Biochemistry 36:14697–14704
Czarnecki K, Chynwat V, Erickson JP, Frank HA, Bocian DF, (1997b) Identification of the magnesium-histidine stretching vibration of the bacteriochlorophyll cofactors in photosynthetic reaction centers via 15N-labeling of the histidines J Am Chem Soc 119:2594–2595
Czarnecki K, Diers JR, Chynwat V, Erickson JP, Frank HA, Bocian DF, (1997c) Characterization of the strongly coupled, low-frequency vibrational modes of the special pair of photosynthetic reaction centers via isotopic labeling of the cofactors J Am Chem Soc 119:415–426
Czarnecki K, Cua A, Kirmaier C, Holten D, Bocian DF, (1999) Relationship between altered structure and photochemistry in mutant reaction centers in which bacteriochlorophyll replaces the photoactive bacteriopheophytin Biospectroscopy 5:346–357
Deisenhofer J and Norris JR (eds) (1993) The Photosynthetic Reaction Center, Vol II. Academic Press, San Diego, CA
Deisenhofer J, Epp O, Miki K, Huber R, Michel H, (1985) Structure of the protein subunits in the photosynthetic reaction center of Rhodopseudomonas viridis at 3 Å Resolution Nature 318:618–624
Deisenhofer J, Epp O, Sinning I, Michel H, (1995) Crystallographic refinement at 2.3-angstrom resolution and refined model of the photosynthetic reaction center from Rhodopseudomonas viridis J Mol Biol 246:429–457
Depillis GD, Decatur SM, Barrick D, Boxer SG, (1994) Functional cavities in proteins. A general method for proximal ligand substitution in myoglobin J Am Chem Soc 116:6981–6982
Eads DD, Moser C, Blackwood ME, Lin CY, Dutton L, Spiro TG, (2000) Selective enhancement of resonance Raman spectra of separate bacteriopheophytins in Rb. sphaeroides reaction centers Biopolymers 57:64–76
Elkabbani O, Chang CH, Tiede D, Norris J, Schiffer M, (1991) Comparison of reaction centers from Rhodobacter sphaeroides and Rhodopseudomonas viridis. Overall architecture and protein-pigment interactions Biochemistry 30:5361–5369
Ermler U, Fritzsch G, Buchanan SK, Michel H, (1994) Structure of the photosynthetic reaction center from Rhodobacter sphaeroides at 2.65-angstrom resolution Cofactors and protein-cofactor interactions Structure 2:925–936
Franzen S, Boxer SG, Dyer RB, Woodruff WH, (2000) Resonance Raman studies of heme axial ligation in H93G myoglobin J Phys Chem B 104:10359–10367
Frolov D, Gall A, Lutz M, Robert B, (2002) Structural asymmetry of bacterial reaction centers: A Q(y) resonant Raman study of the monomer bacteriochlorophylls J Phys Chem A 106:3605–3613
Goldsmith JO, King B, Boxer SG, (1996) Mg coordination by amino acid side chains is not required for assembly and function of the special pair in bacterial photosynthetic reaction centers Biochemistry 35:2421–2428
Hehre WJ, (2003) A Guide to Molecular Mechanics and Quantum Chemical Calculations Wavefunction Irvine, California
Kong J, White CA, Krylov AI, Sherrill CD, Adamson RD, Furlani TR, Lee MS, Lee AM, Gwaltney SR, Adams TR, Ochsenfeld C, Gilbert ATB, Kedziora GS, Rassolov VA, Maurice DR, Nair N, Shao Y, Besley NA, Maslen PE, Dombroski JP, Daschel H, Zhang W, Korambath PP, Baker J, Byrd EFC, Van Voorhis T, Oumi M, Hirata S, Hsu C-P, Ishikawa N, Florian J, Warshel A, Johnson BG, Gill PMW, Head-Gordon M, Pople JA, (2000) Q-chem 2.0: a high-performance ab initio electronic structure program package J Comput Chem 21:1532–1548
Laporte LL, Palaniappan V, Davis DG, Kirmaier C, Schenck CC, Holten D, Bocian DF, (1996) Influence of electronic asymmetry on the spectroscopic and photodynamic properties of the primary electron donor in the photosynthetic reaction center J Phys Chem 100:17696–17707
Lathrop EJP, Friesner RA, (1994) Simulation of optical spectra from the reaction center of Rhodobacter sphaeroides – Effects of an internal charge-separated state of the special pair J Phys Chem 98:3056–3066
Lee CT, Yang WT, Parr RG, (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density Phys Rev B 37:785–789
McRee DE, Jensen GM, Fitzgerald MM, Siegel HA, Goodin DB, (1994) Construction of a bisaquo heme enzyme and binding by exogenous ligands Proc Natl Acad Sci USA 91:12847–12851
Michel-Beyerle ME, (eds)fs (1996) The Reaction Center of Photosynthetic Bacteria Springer Berlin-Heidelberg
Newmyer SL, Sun J, Loehr TM, deMontellano PRO, (1996) Rescue of the horseradish peroxidase His-170->Ala mutant activity by imidazole: importance of proximal ligand tethering Biochemistry 35:12788–12795
Palaniappan V, Aldema MA, Frank HA, Bocian DF, (1992) Qy-excitation resonance Raman-scattering from the special pair in Rhodobacter sphaeroides reaction centers Implications for primary charge separation Biochemistry 31:11050–11058
Palaniappan V, Martin PC, Chynwat V, Frank HA, Bocian DF, (1993) Comprehensive resonance Raman study of photosynthetic reaction centers from Rhodobacter sphaeroides. Implications for pigment structure and pigment-protein interactions J Am Chem Soc 115:12035–12049
Palaniappan V, Schenck CC, Bocian DF, (1995) Low-frequency near-infrared-excitation resonance Raman spectra of (M)H202l mutant reaction centers from Rhodobacter sphaeroides. Implications for the structural, vbronic, and electronic properties of the bacteriochlorin cofactors J Phys Chem 99:17049–17058
Parson WW, Warshel A, (1987) Spectroscopic properties of photosynthetic reaction centers. 2. Application of the theory to Rhodopseudomonas viridis J Am Chem Soc 109:6152–6163
Schenck CC, Gaul D, Steffen M, Boxer SG, McDowell L, Kirmaier C, Holten D, (1990) Site directed mutation affecting primary photochemistry in reaction centers: effects of dissymmetry in the special pair In: Michel-Beyerle ME, (eds) Reaction Centers of Photosynthetic Bacteria Springer Verlag Berlin pp 229–238
Scott AP, Radom L, (1996) Harmonic vibrational frequencies: an evaluation of Hartree-Fock, Moller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors J Phys Chem 100:16502–16513
Sherer POJ, Fischer S, (1991) Interpretation of optical reaction center spectra. In: Scheer H, (eds) Chlorophylls CRC Press Boca Raton, FL pp 855–902
Sun J, Loehr TM, Wilks A, Demontellano PRO, (1994) Identification of histidine-25 as the heme ligand in human liver heme oxygenase Biochemistry 33:13734–13740
Sun J, Fitzgerald MM, Goodin DB, Loehr TM, (1997) Solution and crystal structures of the H175G mutant of cytochrome c peroxidase: A resonance Raman study J Am Chem Soc 119:2064–2065
Thompson MA, Zerner MC, Fajer J, (1991) A theoretical examination of the electronic structure and excited states of the bacteriochlorophyll b dimer from Rhodopseudomonas viridis J Phys Chem 95:5693–5700
Yeates TO, Komiya H, Chirino A, Rees DC, Allen JP, Feher G, (1988) Structure of the reaction center from Rhodobacter sphaeroides R-26 and 2.4.1. Protein-cofactor (bacteriochlorophyll, bacteriopheophytin, and carotenoid) interactions .4 Proc Natl Acad Sci USA 85:7993–7997
Yu NT, Srivastava RB, (1980) Resonance Raman spectroscopy of heme-proteins with intensified vidicon detectors. Studies of low-frequency modes and excitation profiles in cytochrome c and hemoglobin J Raman Spectrosc 9:166–171
Acknowledgements
We thank Professor S. G. Boxer for providing the cultures of the cavity mutants and P. Dolan and A. deWinter for preparation of the RCs. This work was supported by Grants GM-39781 (D.F.B.) and GM-30353 (H.A.F.) from the National Institute of General Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Czarnecki, K., Chen, L., Diers, J.R. et al. Low-frequency resonance Raman studies of the H(M202)G cavity mutant of bacterial photosynthetic reaction centers. Photosynth Res 88, 31–41 (2006). https://doi.org/10.1007/s11120-005-9019-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-005-9019-7