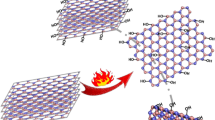
A chemical model for the formation of nanosized turbostratic boron nitride powder from urea is proposed. The mechanism is based on the interaction of metaborate acid O=B—OH with urea (NH2)2CO. When OH groups are replaced by NH2 groups, H2O and CO2 are released. Metaboryl amide O=B—NH2 formed in the replacement process is studied. It is found that dehydration of the compound releasing >B—N< radicals promotes the subsequent formation of turbostratic boron nitride.

Similar content being viewed by others
References
Jr. Thomas, N. E. Weston, and T. E. O’Connor, “Turbostratic boron nitride, thermal transformation to ordered-layer-lattice boron nitride,” J. Am. Chem. Soc., 84, 4619–4622 (1963).
T. S. Bartnitskaya and A. F. Alekseev, Study of Nitrides [in Russian], Naukova Dumka, Kyiv (1975), p. 267.
D. N. Poluboyarinov, E. P. Sadovskii, and I. G. Kuznetsova, Studying the Synthesis of Turbostratic Boron Nitride [in Russian], Silikaty, Moscow (1973), p. 279.
V. V. Vikulin, A. N. Rusanova, V. F. Kuznetsova, et al., “Dependence of the oxygen content of boron nitride on heat-treatment temperature and its effect on the structure and strength characteristics of this compound,” Powder Metall. Met. Ceram., 17, No. 9, 705–710 (1978).
M. Hubacek, M. Ueki, T. Sato, and V. Brozek, “High-temperature behavior of hexagonal boron nitride,” Thermochim. Acta, 283, 359–367 (1996).
W. Lei, D. Portehault, D. Liu, et al., “Porous boron nitride nanosheets for effective water cleaning,” Nat. Commun., 4, 1–7 (2013).
A. V. Kurdyumov, T. S. Bartnitskaya, V. I. Lyashenko, et al., “Structure formation patterns in carbamide synthesis of nanocrystalline graphite-like boron nitride,” Powder Metall. Met. Ceram., 44, No. 11–12, 590–597 (2005).
Production Methods, Properties, and Applications of Nitrides: Collected Papers [in Russian], Inst. Probl. Materialoved. AN USSR, Kyiv (1972), p. 381.
M. Hubacek and M. Ueki, “Chemical reactions in hexagonal boron-nitride system,” J. Solid State Chem., 123, No. 2, 215–222 (1996).
M. Hubacek, T. Sato, and T. Ishii, “A coexistence of boron nitride and boric oxide,” J. Solid State Chem., 109, 384–390 (1994).
V. I. Lyashenko, T. V. Tomila, V. F. Britun, and A. V. Ragulya, “Structurization of nanocrystalline boron nitride in synthesis from urea,” Nanostruct. Materialoved., No. 2, 24–31 (2012).
V. V. Garbuz, T. F. Lobunets, V. A. Petrova, et al., “Physicochemical characteristics of nitrogen sorption on high-porous powders of graphene-like boron nitride,” Powder Metall. Met. Ceram., 55, No. 7–8, 379–385 (2016).
V. V. Garbuz, V. A. Petrova, and A. V. Yakovlev, “The agreement phenomenon of the component analysis with dimensions of the graphenic-like carbon and boron nitride nanosized particles,” in: Carbon Nanomaterials in Clean Energy Hydrogen Systems II, NATO Science for Peace and Security Series C: Environmental Security (2011), Vol. 2, pp. 427–435.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 56, Nos. 7–8 (516), pp. 107–110, 2017.
Rights and permissions
About this article
Cite this article
Garbuz, V.V., Petrova, V.A., Suvorova, L.S. et al. Model of Reactions for the Synthesis of Turbostratic Boron Nitride Nanoparticles from Urea. Powder Metall Met Ceram 56, 445–447 (2017). https://doi.org/10.1007/s11106-017-9914-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-017-9914-4