Abstract
Background and aims
Plants have evolved an array of root traits associated with phosphorus (P) acquisition, including morphological and physiological traits. This study aimed to characterize the differences of various root traits in soybean (Glycine max) and explore their roles in P acquisition.
Methods
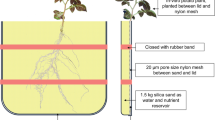
Root functional traits associated with P acquisition were characterized in 49 cultivated soybean landraces from the North China Plain grown in a glasshouse under low-P condition.
Results
We found a large variation in plant growth and all studied root traits. There was a significant correlation between total plant P content and root morphological traits in all 49 varieties. Hierarchical classification on principal components (HCPC) based on principal component analysis (PCA) indicated that soybean varieties could be grouped into three distinct clusters: total plant P content was positively correlated with some root morphological traits and seed P content in cluster 1 and showed positive correlations with root tissue density and rhizosheath carboxylates in cluster 2, while total P content showed no significant correlation with any root trait in cluster 3.
Conclusion
Root morphology and seed P content generally determined P acquisition of the present soybean varieties, but carboxylates also contributed to P uptake in some P-efficient varieties. We may be able to maximize soybean P acquisition via stacking root morphological and physiological traits, thus allowing plants to access different soil P pools.




Similar content being viewed by others
Data availability
This is not applicable to this manuscript.
Code availability
This is not applicable to this manuscript.
References
Adams ML, Norvell WA, Philpot WD, Peverly JH (2000) Spectral detection of micronutrient deficiency in ‘Bragg’soybean. Agron J 92:261–268
Bao SD (2000) Soil Agrochemical Analysis (in Chinese). China Agricultural Press, Beijing
Brown JC, Jones WE (1977) Fitting plants nutritionally to soils. I. soybeans 1. Agron J 69:399–404
Clarholm M, Skyllberg U, Rosling A (2015) Organic acid induced release of nutrients from metal-stabilized soil organic matter – the unbutton model. Soil Biol Biochem 84:168–176
Cong W-F, Suriyagoda LDB, Lambers H (2020) Tightening the phosphorus cycle through phosphorus-efficient crop genotypes. Trends Plant Sci 25:967–975
Delhaize E, James RA, Ryan PR (2012) Aluminium tolerance of root hairs underlies genotypic differences in rhizosheath size of wheat (Triticum aestivum) grown on acid soil. New Phytol 195:609–619
Ding W (2022) The promotion of legume nodulation in plant-soil-microbe systems under phosphorus-limited conditions. Plant Soil 476:251–262
Ding W, Cong W, Lambers H (2021) Plant phosphorus-acquisition and -use strategies affect soil carbon cycling. Trends Ecol Evol 36:899–906
Dong D, Peng X, Yan X (2004) Organic acid exudation induced by phosphorus deficiency and/or aluminium toxicity in two contrasting soybean genotypes. Physiol Plant 122:190–199
Hayes PE, Clode PL, Oliveira RS, Lambers H (2018) Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: an adaptation improving phosphorus-use efficiency. Plant Cell Environ 41:605–619
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Honvault N, Houben D, Nobile C, Firmin S, Lambers H, Faucon M-P (2021) Tradeoffs among phosphorus-acquisition root traits of crop species for agroecological intensification. Plant Soil 461:137–150
James RA, Weligama C, Verbyla K, Ryan PR, Rebetzke GJ, Rattey A, Richardson AE, Delhaize E (2016) Rhizosheaths on wheat grown in acid soils: phosphorus acquisition efficiency and genetic control. J Exp Bot 67:3709–3718
Jungk A (2009) Carl Sprengel—The founder of agricultural chemistry: A re-appraisal commemorating the 150th anniversary of his death. J Plant Nutr Soil Sci 172:633–636
Kafle A, Cope KR, Raths R, Krishna Yakha J, Subramanian S, Bücking H, Garcia K (2019) Harnessing soil microbes to improve plant phosphate efficiency in cropping systems. Agronomy 9:127
Kirkby EA (2023) Introduction, definition, and classification of nutrients. Elsevier, Marschner’s Mineral Nutrition of Plants
Krishnapriya V, Pandey R (2016) Root exudation index: screening organic acid exudation and phosphorus acquisition efficiency in soybean genotypes. Crop Pasture Sci 67:1096–1109
Lambers H, Cawthray GR, Giavalisco P, Kuo J, Laliberté E, Pearse SJ, Scheible W-R, Stitt M, Teste F, Turner BL (2012) Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus use efficiency. New Phytol 196:1098–1108
Lambers H, de Britto CP, Cawthray GR, Denton MD, Finnegan PM, Hayes PE, Oliveira RS, Power SC, Ranathunge K, Shen Q, Wang X, Zhong H (2022) Strategies to acquire and use phosphorus in phosphorus-impoverished and fire-prone environments. Plant Soil 476:133–160
Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90
Lambers H, Wright IJ, Guilherme Pereira C, Bellingham PJ, Bentley LP, Boonman A, Cernusak LA, Foulds W, Gleason SM, Gray EF, Hayes PE, Kooyman RM, Malhi Y, Richardson SJ, Shane MW, Staudinger C, Stock WD, Swarts ND, Turner BL, Turner J, Veneklaas EJ, Wasaki J, Westoby M, Xu Y (2021) Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil 461:43–61
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041–1049
Lynch JP, Ho MD, Phosphorus L (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269:45–56
Makarim AK, Cox FR (1983) Evaluation of the need for copper with several soil extractants. Agron J 75:493–496
Marschner H (1998) Role of root growth, arbuscular mycorrhiza, and root exudates for the efficiency in nutrient acquisition. Field Crops Res 56:203–207
Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E (2000) Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann Bot 85:909–919
Nian H, Ahn SJ, Yang ZM, Matsumoto H (2003) Effect of phosphorus deficiency on aluminium-induced citrate exudation in soybean (Glycine max). Physiol Plant 117:229–236
Ohki K (1976) Manganese deficiency and toxicity levels for ‘Bragg’ soybeans 1. Agron J 68:861–864
Ohki K (1977) Critical zinc levels related to early growth and development of determinate soybeans 1. Agron J 69:969–974
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US Department of Agriculture, Washington DC
Pan X, Li W, Zhang Q, Li Y, Liu M (2008) Assessment on phosphorus efficiency characteristics of soybean genotypes in phosphorus-deficient soils. Agric Sci Chin 7:958–969
Pang J, Bansal R, Zhao H, Bohuon E, Lambers H, Ryan MH, Ranathunge K, Siddique KHM (2018) The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529
Pang J, Ryan MH, Siddique KHM, Simpson RJ (2017) Unwrapping the rhizosheath. Plant Soil 418:129–139
Pang J, Ryan MH, Tibbett M, Cawthray GR, Siddique KHM, Bolland MDA, Denton MD, Lambers H (2010) Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant Soil 331:241–255
Plaxton W, Lambers H (2015) Annual Plant Reviews, Volume 48, Phosphorus Metabolism in Plants. John Wiley & Sons, Perth
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Raboy V (2001) Seeds for a better future: ‘low phytate’ grains help to overcome malnutrition and reduce pollution. Trends Plant Sci 6:458–462
Shen J, Rengel Z, Tang C, Zhang F (2003) Role of phosphorus nutrition in development of cluster roots and release of carboxylates in soil-grown Lupinus albus. Plant Soil 248:199–206
Song X, Li Y, Chang R, Guo P, Qiu L-J (2010) Population structure and genetic diversity of mini core collection of cultivated soybean (Glycine max) in China. Sci Agric Sin 43:2209–2219
Sulpice R, Ishihara H, Schlereth A, Cawthray GR, Encke B, GiavaliscoI P, Ivakov A, Arrivault S, Jost R, Krohn N, Kuo J, Laliberte E, Pearse SJ, Raven JA, Scheible W-R, Teste F, J. VE, Stitt M, Lambers H, (2014) Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species. Plant Cell Environ 37:1276–1298
Tantriani CW, Oikawa A, Tawaraya K (2023) Low phosphorus tolerance mechanisms in soybean cultivars grown in soil. J Soil Sci Plant Nutr 23:6331–6344
Vandamme E, Renkens M, Pypers P, Smolders E, Vanlauwe B, Merckx R (2013) Root hairs explain P uptake efficiency of soybean genotypes grown in a P-deficient Ferralsol. Plant Soil 369:269–282
Veneklaas EJ, Lambers H, Bragg J, Finnegan PM, Lovelock CE, Plaxton WC, Price CA, Scheible W-R, Shane MW, White PJ, Raven JA (2012) Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol 195:306–320
Wang L, Guan Y, Guan R, Li Y, Ma Y, Dong Z, Liu X, Zhang H, Zhang Y, Liu Z, Chang R, Xu H, Li L, Lin F, Luan W, Yan Z, Ning X, Zhu L, Cui Y, Piao R, Liu Y, Chen P, Qiu L (2006) Establishment of Chinese soybean Glycine max core collections with agronomic traits and SSR markers. Euphytica 151:215–223
Wang L, Liao H, Yan X, Zhuang B, Dong Y (2004) Genetic variability for root hair traits as related to phosphorus status in soybean. Plant Soil 261:77–84
Wang X, Pan Q, Chen F, Yan X, Liao H (2011) Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza 21:173–181
Wang X, Pang J, Wen Z, Gadot G, de Borda A, Siddique KHM, Lambers H (2021) Lower seed P content does not affect early growth in chickpea, provided starter P fertiliser is supplied. Plant Soil 463:113–24
Wang X, Yan X, Liao H (2010) Genetic improvement for phosphorus efficiency in soybean: a radical approach. Ann Bot 106:215–222
Weemstra M, Mommer L, Visser EJW, van Ruijven J, Kuyper TW, Mohren GMJ, Sterck FJC (2016) Towards a multidimensional root trait framework: a tree root review. New Phytol 211:1159–1169
Wen Z, Li H, Shen J, Rengel Z (2017) Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant Soil 416:377–389
Wen Z, Li H, Shen Q, Tang X, Xiong C, Li H, Pang J, Ryan MH, Lambers H, Shen J (2019) Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol 223:882–895
Wen Z, Pang J, Tueux G, Liu Y, Shen J, Ryan MH, Lambers H, Siddique KHM (2020) Contrasting patterns in biomass allocation, root morphology and mycorrhizal symbiosis for phosphorus acquisition among 20 chickpea genotypes with different amounts of rhizosheath carboxylates. Funct Ecol 34:1311–1324
White PJ, Veneklaas EJ (2012) Nature and nurture: the importance of seed phosphorus content. Plant Soil 357:1–8
Wu D, Yu Q, Lu C, Hengsdijk H (2006) Quantifying production potentials of winter wheat in the North China Plain. Eur J Agron 24:226–235
Xin Y, Tao F (2020) Developing climate-smart agricultural systems in the North China Plain. Agric, Ecosyst Environ 291:106791
Yang ZM, Sivaguru M, Horst WJ, Matsumoto H (2000) Aluminium tolerance is achieved by exudation of citric acid from roots of soybean (Glycine max). Physiol Plant 110:72–77
Zhang D, Zhang C, Tang X, Li H, Zhang F, Rengel Z, Whalley WR, Davies WJ, Shen J (2016) Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol 209:823–831
Zhao J, Fu J, Liao H, He Y, Nian H, Hu Y, Qiu L, Dong Y, Yan X (2004) Characterization of root architecture in an applied core collection for phosphorus efficiency of soybean germplasm. Chin Sci Bull 49:1611–1620
Zhou T, Du Y, Ahmed S, Liu T, Ren M, Liu W, Yang W (2016) Genotypic differences in phosphorus efficiency and the performance of physiological characteristics in response to low phosphorus stress of soybean in southwest of China. Front Plant Sci 7:1776
Zhou XM, Ranathunge K, Cambridge ML, Dixon KW, Hayes PE, Nikolic M, Shen Q, Zhong H, Lambers H (2022) A cool spot in a biodiversity hotspot: why do tall Eucalyptus forests in Southwest Australia exhibit low diversity? Plant Soil 476:669–688
Acknowledgements
This research was supported by National Natural Science Foundation of China (32072676, 32271610, 32102468), and the Australian Research Council (LP200100341). Wenli Ding was supported by the International Postdoctoral Exchange Fellowship Program (Talent-Introduction Program YJ20200203), which is co-funded by the Office of China Postdoc Council and China Agricultural University, and by China Postdoctoral Science Foundation (2021M700165). We thank Advanced Discipline Construction in Beijing (Agriculture Green Development) and Introducing Talents of Discipline to Universities (Plant-soil interactions innovative research platform 1031-00100701) to support this research. In addition, we thank Lijuan Qiu and Yongzhe Gu for providing soybean seeds as well as related information which was funded by the Ministry of Science and Technology, Platform of National Crop Germplasm Resources of China (NICGR-2019-04), Ministry of Agriculture and Rural Affairs: Crop Germplasm Resources Protection Platform (2019NWB036-05). We thank Zhihui Wen for the internal review. We also thank two anonymous reviewers for their highly valuable comments.
Funding
The study was financially supported by National Natural Science Foundation of China (32271610, 32072676, 32102468), International Postdoctoral Exchange Fellowship Program (Talent-Introduction Program YJ20200203), China Postdoctoral Science Foundation (2021M700165), the Australian Research Council (LP200100341), Advanced Discipline Construction in Beijing (Agriculture Green Development) and Introducing Talents of Discipline to Universities (Plant-soil interactions innovative research platform 1031–00100701).
Author information
Authors and Affiliations
Contributions
Wenfeng Jiao, Wen-Feng Cong, Jiayin Pang and Hans Lambers designed the study. Wenli Ding, Wenfeng Jiao and Boyu Zheng performed the experiment and collected the data. Wenli Ding analysed the data. Wenli Ding, Jiayin Pang, Wen-Feng Cong and Hans Lambers wrote this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Peter J. Gregory.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, W., Jiao, W., Pang, J. et al. Clusters of cultivated soybean landraces from the North China Plain coordinate root morphology and rhizosheath carboxylates enhancing phosphorus acquisition. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06717-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06717-4