Abstract
Background and aims
Southwest Australia is a biodiversity hotspot, with greatest plant species diversity on the most severely phosphorus (P)-impoverished soils. Here, non-mycorrhizal species with highly-effective carboxylate-releasing P-acquisition strategies coexist with mycorrhizal species that are less effective at accessing P on these soils. Non-mycorrhizal carboxylate-releasing species facilitate P acquisition of mycorrhizal neighbours that are better defended against pathogens. In the Southwest Australian Biodiversity Hotspot, there are also ‘cool spots’ of low-diversity tall mycorrhizal Eucalyptus communities on P-impoverished soils. These Eucalyptus trees obviously do not require facilitation of their P acquisition by carboxylate-releasing neighbours, because these are only a minor component of the low-diversity communities. We hypothesised that in low-diversity tall Eucalyptus forests, mycorrhizal species release carboxylates to acquire P. Thus, they would not depend on facilitation, and must be strong competitors. However, because they would not depend on external mycorrhizal hyphae to acquire P, they would also not be able to access soil organic nitrogen (N), for which they would need external hyphae.
Methods
Since carboxylates not only mobilise P, but also manganese (Mn), we used leaf Mn concentrations ([Mn]) in the natural habitat to proxy rhizosphere carboxylates. To verify this proxy, we also measured carboxylate exudation of targeted species with high leaf [Mn] using seedlings grown in low-P nutrient solutions.
Results
Using these complementary approaches, we confirmed our hypothesis that dominant Eucalyptus species in ‘cool spots’ release carboxylates. Since mineralisation of organic N is associated with fractionation of N, enriching organic N with 15N while nitrate is depleted in 15N, we measured the stable N isotope composition of leaf material. The results show that dominant Eucalyptus species did not access organic N, despite being ectomycorrhizal.
Conclusions
The low diversity of tall Eucalyptus forests in southwest Australia can be explained by dominant mycorrhizal species exhibiting a carboxylate-releasing strategy. The tall eucalypts are therefore strong competitors that do not require facilitation, but also do not access organic N.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Southwest Australia is a global biodiversity hotspot (Myers et al. 2000), one of only 36 in the world (Habel et al. 2019), with the greatest biodiversity associated with the most nutrient-depauperate soils that exhibit a very low availability of phosphorus (P) (Lambers 2014; Lambers et al. 2010). This trend of increasing plant diversity with decreasing soil P availability is not restricted to this biodiversity hotspot; it is a trend that is found globally (Huston 1994; Wardle et al. 2004; Zemunik et al. 2016).
A relatively large proportion of all plant species in severely P-impoverished habitats are non-mycorrhizal (Brundrett 2017; Zemunik et al. 2018; Zemunik et al. 2015). These non-mycorrhizal species use a range of carboxylate-releasing strategies to acquire P when the availability of soil P is extremely low (Lambers 2022). These non-mycorrhizal species co-occur with many mycorrhizal species, even though mycorrhizas are less effective than carboxylate-releasing roots when the availability of soil P is extremely low (Lambers et al. 2015a; Parfitt 1979; Treseder 2004). Yet, many mycorrhizal species are still present on the poorest soils (Lambers et al. 2008; Zemunik et al. 2018; Zemunik et al. 2015). This conundrum has been resolved by recent findings: Proteaceae that are most effective at acquiring P, based on a carboxylate-releasing P-mobilising strategy, are more sensitive to oomycete pathogens than co-occurring mycorrhizal species (Albornoz et al. 2017; Laliberté et al. 2015). However, mycorrhizal plants, while less effective at mobilising P from severely P-impoverished soils (Parfitt 1979; Treseder and Allen 2002), are protected against oomycete pathogens by their mycorrhizal partners (Albornoz et al. 2021b; Jung et al. 2012; Laliberté et al. 2015; Lambers et al. 2018). This protective role of mycorrhizal fungi in ecosystem functioning has been known for quite some time (Azcón-Aguilar and Barea 1997; Marx 1972; Wehner et al. 2010), and is increasingly acknowledged (Berdeni et al. 2018; Boutaj et al. 2020; Frew et al. 2021; Gonthier et al. 2019). Protection against oomycetes is also well documented (Cordier et al. 1998; Jung et al. 2012; Pozo et al. 2002; Veresoglou and Rillig 2012). The dominant species in Eucalyptus forests with a low plant diversity, E. patens and E. diversicolor, are both mycorrhizal species, being both arbuscular and ectomycorrhizal (Table 1).
Carboxylates released by roots not only mobilise P, but also manganese (Mn); since Mn uptake in roots is poorly controlled (Baxter et al. 2008), leaf Mn concentration ([Mn]) is a proxy for rhizosphere carboxylates (Lambers et al. 2021; Lambers et al. 2015b; Pang et al. 2018). Poaceae tend not to release large amounts of carboxylates, but typically release phytosiderophores (Ma 2005), which potentially also mobilise P (Zanin et al. 2017). In contrast, arbuscular and ectomycorrhizal hyphae intercept Mn (Bethlenfalvay and Franson 1989; Canton et al. 2016), so low leaf [Mn] indicates an effective mycorrhizal symbiosis. Since leaf [Mn] also depends on a range of other environmental factors (Lambers et al. 2021), either community-weighted average leaf [Mn] need to be known (Lambers et al. 2021), or appropriate reference species need to be included (Zhong et al. 2021). Positive reference species are those that are known to release large amounts of carboxylates, e.g., Banksia species (Proteaceae) (Lambers 2022; Zhong et al. 2021). Negative reference species comprise those that do not depend on carboxylates for their P acquisition, e.g., Xanthorrhoea species (Xanthorrhoeceae) (Zhong et al. 2021). In the event that no suitable negative reference species are available, young leaves may be used as an alternative, because Mn typically accumulates only in old leaves (Lambers et al. 2021; Shane and Lambers 2005b).
Within the Southwest Australian Biodiversity Hotspot, tall eucalypt forests form ‘cool spots’, characterised by low diversity of vascular plants at the community level and limited diversity of growth forms. For example, tall karri forests dominated by E. diversicolor, occupy a substantial area (190,000 ha) (Bradshaw 2015), whereas yarri (Swan River blackbutt) forests, dominated by E. patens, cover a somewhat smaller area and are far less studied (https://florabase.dpaw.wa.gov.au/browse/profile/5739). Both E. diversicolor and E. patens forests occur in recharge zones in high-rainfall areas with extraordinarily low soil P availability. Eucalyptus diversicolor occurs as tall (up to ~90 m) open forests in the high-rainfall region (> 900 mm annual rainfall) (Grove 1988; Hingston et al. 1979). Eucalyptus diversicolor forest soils contain “negligible concentrations of labile-P, low concentrations of total P and more P in organic forms than inorganic” (Adams 1992). Eucalyptus patens forests occur in recharge regions with >1100 mm rainfall (Macfarlane et al. 2010), with soils even less fertile than E. diversicolor forest soils.
Our main aim was to reveal why E. patens and E. diversicolor forests exhibit remarkably low plant diversity, compared with many other habitats in the Southwest Australian Biodiversity Hotspot (Gioia and Hopper 2017; Hopper and Gioia 2004; Lambers 2014). We showed previously that the mycorrhizal E. patens releases carboxylates (Lambers et al. 2021). Building on this, we hypothesised that some co-occurring species and E. diversicolor, whilst being protected against pathogens by their mycorrhizal partners, also release P-mobilising carboxylates. This would allow them to function like Proteaceae, which are well known to release P-mobilising carboxylates (Shane and Lambers 2005a) and not require facilitation by P-mobilising neighbours (Lambers et al. 2019). A second aim was to acquire more information on the nitrogen (N) nutrition of E. patens forest species including the understorey community. Ectomycorrhizal species would be expected to access organic N (Högberg 1990), but if they depend on carboxylates to acquire P, rather than on ectomycorrhizal symbioses, the ectomycorrhizal hyphae might not function to acquire organic N. We also aimed to assess the contribution of N fixation by Fabaceae and a cycad, Macrozamia riedlei to their N nutrition. Whilst N is not the key limiting nutrient in these P-impoverished habitats, N does need to be fixed, to compensate for losses of N due to denitrification, leaching and fire.
We tested our main hypothesis, first, by measuring leaf [Mn] of E. patens, E. diversicolor and co-occurring species, using leaf [Mn] as a proxy for rhizosphere carboxylate concentrations. Second, following this field study, we grew seedlings of a range of species for which we had acquired leaf [Mn] data in their natural habitat in low-P nutrient solutions in a glasshouse to measure their carboxylate release. To address our second aim, we measured leaf [N] and δ15N of E. patens forest species to disclose the contribution of N fixation by species of Fabaceae and a cycad, M. riedlei, as well as their dependence on organic N (Högberg 1990).
Materials and methods
Site descriptions
The three study sites comprise evergreen tall forests in southwest Australia, a region characterised by a Mediterranean climate, with hot dry summers (December to February) and cool wet winters (June to August). The E. patens site is located at Cypress Form, Waroona (32°47′35”S, 116°0′46″E), ~100 km south of Perth, with a mean annual rainfall of 992 mm (1990–2015), mean annual temperature of 21.9 °C, and mean monthly maxima from 15.1 °C (July) to 29.7 °C (January/ February) (Australian Bureau of Meteorology, http://www.bom.gov.au/climate/data/). This is where we sampled the vast majority of all vascular plant species present at the site. The two E. diversicolor sites occur on different soil types, in Torndirrup National Park (35°06′12”S, 117°53′57″E) and Porongurup National Park (34°41′28”S, 117°54′22″E), ~400 km south of Perth. The sites have a mean annual rainfall of 925 mm (1990–2015) and mean annual temperature of 19.5 °C and mean monthly maxima from 15.8 °C (July) to 22.9 °C (February) (Australian Bureau of Meteorology, http://www.bom.gov.au/climate/data/). These sites were included to allow a comparison of dominant Eucalyptus species in tall eucalypt forest in southwest Australia. At these sites we only sampled the focal species, and reference species where possible.
Soil samples
Moist surface soil was collected from five locations within each site (four samples combined at each location). Soil was sampled using an auger (diameter = 100 mm, depth = 150 mm). Samples were sieved (<2 mm), air-dried, and stored in zip-lock bags at 25 °C before chemical analyses. Soil pH was measured (in deionised water (DI) and 0.01 M CaCl2 in a 1:5 soil-to-solution ratio) using a pH and electrical conductivity (EC) probe (Orion 720a, Beverly, MA, USA) (Rayment and Lyons 2011). Total N was measured by the combustion method using a Leco analyser (FP628, St. Joseph, MI, USA). ‘Plant-available’ soil P was measured as resin-P using anion-exchange membranes (AEM) (Rayment and Lyons 2011), with soil samples shaken for 16 h in 30 ml deionised water and four anionic form AEM strips (10 × 40 mm; manufactured by BDH, Poole, UK). Phosphate was recovered by shaking strips with 30 ml 0.5 M HCl for 1 h. Soil organic P was determined by the ignition method (550 °C, for 1 h) and extraction in 1 M HCl (16 h, 1:50 soil to solution ratio) (Saunders and Williams 1955). The organic P concentration was calculated by subtracting the 1 M HCl-extracted P without ignition from the 1 M HCl-extracted P after ignition. Phosphate concentrations in extracts were determined by the malachite-green method (Rao et al. 1997), using a UV–VIS spectrophotometer (UV160A Shimadzu, Kyoto, Japan). Elements in soil were extracted with aqua regia (3:1 mixture of HNO3: HCl) digestion (1 h at 130 °C) and then determined by inductively coupled plasma optical emission spectrometry (ICP-OES; Model 5300DV spectrometer, Perkin Elmer, Shelton, CT, USA) (Rayment and Lyons 2011).
Selection of species
We collected 21 species at the E. patens site in October and November 2020, including a positive reference species (Banksia grandis) and a negative reference species (Xanthorrhoea preissii) (Fig. 1, Table 1). This comprised most species at the site, except for some understorey orchids, sundews, and sedges. We used X. preissii as a negative reference, because Xanthorrhoea species are mycorrhizal and do not release carboxylates; they invariably have a low leaf [Mn] (Hayes et al. 2014; Zhong et al. 2021).
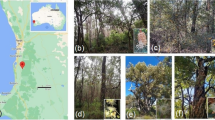
Distribution of (a) Eucalyptus diversicolor (karri) and (b) E. patens (yarri) in southwest Australia, including inset maps of Western Australia. Scale bars on main maps: 30 km; on insets: 200 km. Source: https://florabase.dpaw.wa.gov.au/. (c) Habitat and some of the studied species at the E. patens site in Waroona, located ~100 km south of Perth; (d) E. patens; (e) E. marginata; (f) Corymbia calophylla; (g) Banksia grandis; (h) Tremandra diffusa; (i) Bossiaea aquifolium. Photos: (c) Hans Lambers; (d) Kingsley Dixon; (e, f) Sophie Xiang (g) Hongtao Zhong; (h, i) Xue Meng Zhou
We also sampled species at the two E. diversicolor sites in December 2020 (Fig.1, Table 1). In Torndirrup National Park, there were Proteaceae (B. grandis and Adenanthos obovatus) located at somewhat greater distance of over 300 m from E. diversicolor, but there were no Xanthorrhoea species as negative reference. Therefore, we used young expanding leaves of Adenanthos obovatus as a negative reference, because young leaves tend to exhibit consistently low [Mn] while those in old leaves vary (Shane and Lambers 2005b).
In Porongurup National Park, E. diversicolor was the only Myrtaceae species, whereas no Proteaceae for a positive reference or any species for a negative reference were found within at least 1000 m. Thus, we selected young expanding leaves of E. diversicolor as possible reference material.
Leaf samples
Mature leaf total P and Mn concentrations were analysed from five to 10 plants per species. Oven-dried leaf samples (70 °C for two days) were ground in a ball-mill grinder (Geno/Grinder 2010, Spex SamplePrep, Metuchen, NJ, USA) using plastic vials and yttria-stabilised zirconia ceramic beads. Samples were then digested with hot concentrated HNO3:HClO4 (3:1) and analysed using ICP-OES (Model 5300DV, Perkin Elmer, Shelton, CT, USA).
Dry finely-ground leaf samples of selected species were analysed for N concentration ([N]) and 15N. Total [N] was measured by the combustion method using a Leco analyser (FP628, St. Joseph, MI, USA). Stable isotope 15N was measured using a Flash 1112 Elemental Analyser coupled via a continuous flow interface to Delta V Plus isotope ratio mass spectrometer (Thermo-Finnigan, Bremen, Germany) (Skrzypek and Debajyoti 2006). The natural stable isotope ratios were expressed as parts per thousand (‰).
The percentage of N derived from the atmosphere (%Ndfa) was calculated as: %Ndfa = 100 (average δ15N of the reference species - δ15N of N2-fixing species)/(average δ15N of reference species - ‘B’). We used a ‘B’ value of −1.4‰ to account for the isotopic fractionation within a plant fully reliant on N fixation following Png et al. (2017). This ‘B’ value is an average of several published shoot ‘B’ values of shrub and tree species and within the range of most species (between 0‰ and − 2‰) (Andrews et al. 2011). We re-designated values of %Ndfa that were > 100% as 100%, and values <0% as 0% (Unkovich et al. 2008). For each N2-fixing species, the reference plant species selected was M. stipoides, a co-occurring non-N2-fixing grass, which is presumably arbuscular mycorrhizal (Table 1). We aimed to avoid potential isotopic differences between the forms of soil N accessible by the different mycorrhizal types (Högberg 1990), but acknowledge that two of the sampled Fabaceae are both arbuscular mycorrhizal and ectomycorrhizal (Table 1). Only youngest fully expanded leaves from the same growing season were collected for analyses to reduce seasonal variation of leaf δ15N (Shearer and Kohl 1986).
Carboxylate and phytosiderophore release
We selected five to 10 seedlings for each species that had relatively high leaf [Mn] (Fig. 2). Seedlings were grown in the glasshouses at the University of Western Australia and used to analyse root exudation rates of carboxylates (mainly oxalate, malate and citrate) or phytosiderophores (for M. stipoides only). Seedlings of Corymbia calophylla, E. diversicolor and Tremandra diffusa were purchased from Australian Native Nursery (Oakford, Western Australia), E. marginata was grown from seeds collected at Aroona Sanctuary, along Warren Beach Road and Warren River in Callcup, Western Australia, and plants of M. stipoides were collected at Cypress Form. All seedlings were grown in a low-P nutrient solution commonly used for growing southwest Australian native plants (Hayes et al. 2019).
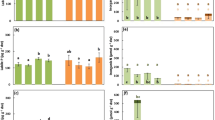
Leaf manganese concentrations ([Mn]) on a dry weight (DW) basis of a range of species co-occurring with Eucalyptus patens, yarri (Myrtaceae) at Cypress Form, Waroona. Banksia grandis (Proteaceae) was used as the positive reference, and Xanthorrhoea preissii (Xanthorrhoeaceae) as the negative reference. Data are means ± SE (n = 5–10). Different letters indicate statistically significant differences among species (P ≤ 0.05). Non-mycorrhizal species and the families they belong to are marked with*
After about 10 weeks in nutrient solution, root exudates were collected from excised whole or a part of root systems of seedlings, except for B. grandis, for which we used excised mature cluster roots. Exudates were collected in 3–10 ml of aerated 1/2 strength nutrient solution without P and Ca for 1.5 h between 9:00–11:00 h (Shane et al. 2003) and analysed for carboxylates using reverse phase liquid chromatography as described by Cawthray (2003), and for oxalate by Uloth et al. (2015). The carboxylates determined and their limits of detection (μM) were: citrate (5), malate (7), iso-citrate (10), oxalate (8), trans-aconitate (0.1), cis-aconitate (0.1), fumarate (0.06), maleate (0.05), succinate (15), and malonate (8) (Cawthray 2003; Uloth et al. 2015). Total carboxylates was the sum of all di- and tri-carboxylates, mainly citrate, malate, and oxalate. All carboxylate concentrations were expressed per unit root fresh weight.
Phytosiderophores were collected in the same way in 10 ml of 1/2 strength nutrient solution without P, Ca and Fe, over a period of three hours, and quantified by measuring Fe-solubilising capacity according to Ma et al. (2003).
Statistical analyses
Individual trees were considered as the unit of replication. Differences across species, leaf ages, or soil depths were analysed using one-way ANOVA. For each model, normality of the residuals was assessed using the Shapiro-Wilk test and heteroskedasticity assessed by visual inspection. Where appropriate (leaf [Mn], leaf P concentration ([P]), and root exudation rates; Figs. 2, 3, and 4) data were log10 transformed. Differences among species, leaf ages, or soil depths were defined using Tukey-Kramer HSD post-hoc tests. All data are presented as mean ± standard error (SE). All analyses were conducted in R 3.6.3 (R_Development_Core_Team 2019) using base R packages.
Leaf phosphorus concentrations ([P]) on a dry weight (DW) basis of a range of species co-occurring with Eucalyptus patens, yarri (Myrtaceae) at Cypress Form, Waroona. Data are means ± SE (n = 5–10). Different letters indicate statistically significant differences among species (P ≤ 0.05). Non-mycorrhizal species and the families they belong to are marked with*
Carboxylate exudation rates per unit root fresh mass (FM) of a range of species, for which leaf manganese concentrations ([Mn]) were available in their natural habitat which suggested carboxylates might play a role in their phosphorus (P) uptake. Seedlings of a range of species co-occurring with Eucalyptus patens, yarri (Myrtaceae) as well as Eucalyptus diversicolor, karri (Myrtaceae) were grown in an aerated low-P nutrient solution in a glasshouse for up to about 10 weeks. Root exudates were collected from excised whole or parts of root systems, except for Banksia grandis, for which we used excised mature cluster roots. (a) Rates of carboxylate exudation; (b) oxalate as a percentage of total carboxylates exuded. Data are means ± SE (n = 5). Different letters indicate statistically significant differences among species (P ≤ 0.05)
Results
Soil characteristics
Soils were acidic (pH (CaCl2) 4.1–4.8) at the E. patens sampling sites, and about neutral (pH (CaCl2) ~6.6) at the E. diversicolor locations (Tables 2 and 3). Soil total P concentrations were very low (20 to 50 mg P kg−1) at most locations, apart from the E. diversicolor location at Porongurup National Park (~140 mg kg−1). Readily-available soil P, measured as resin-P, was very low (<1 mg kg−1) across all sites, and organic-P was the major fraction of soil P at all sites, 65–95%.
Leaf [Mn] and [P] at the E. patens site
The two reference species exhibited the highest (B. grandis) and almost lowest (X. preissii) leaf [Mn], respectively (Fig. 2). Eucalyptus patens, which releases oxalate as the main carboxylate (Lambers et al. 2021), showed a relatively high leaf [Mn]. Species with the highest leaf [Mn] did not have the highest leaf [P] (Fig. 3). None of the Fabaceae exhibited a high leaf [P]. Microlaena stipoides, Pteridium esculentum and Diplolaena drummondii exhibited the highest leaf [P], ~0.90 mg g−1 dry weight (DW) (Fig. 3).
Leaf [Mn] of X. gracilis, M. riedlei and D. drummondii were indistinguishable from that of X. preissii, our negative reference. The low leaf [Mn] of Lepidosperma tetraquetrum was unexpected, since Cyperaceae, whether they produce dauciform roots or not (Güsewell and Schroth 2017) tend to release carboxylates. These species likely do not depend on carboxylates to acquire P.
Tremandra diffusa exhibited a high leaf [Mn] (Fig. 2), closest to that of B. grandis. In accordance with its high leaf [Mn], T. diffusa seedlings grown in nutrient solution released a similar amount of carboxylates as other investigated species from the same site with a relatively high leaf [Mn] (Fig. 4a). Bossiaea aquifolium also showed relatively high leaf [Mn] (Fig. 2) and released carboxylates in nutrient solution (Fig. 4a). Corymbia calophylla, M. stipoides, E. marginata and E. patens had similar leaf [Mn] (160–203 mg kg−1). As shown in Fig. 4a and in a previous paper for E. patens (Lambers et al. 2021), these Myrtaceae species released carboxylates. All other species had intermediate leaf [Mn] and likely did not release significant amounts of carboxylates.
Leaf [Mn] and [P] at the E. diversicolor sites
At Torndirrup National Park, B. grandis exhibited the highest leaf [Mn] (Fig. 5a), but the lowest leaf [P] and E. diversicolor had the highest leaf [P] (Fig. 5b). Using young leaves of A. obovatus as the negative reference showed that the co-occurring E. diversicolor had a similar leaf [Mn], but the soil pH under E. diversicolor was significantly higher than that under the negative reference species (pH 7.3 vs. pH 4.6) with similar soil [Mn] (Table 2). In the absence of negative reference species at the exact same location, we cannot draw a firm conclusion, but suggest E. diversicolor likely exuded carboxylates. This was confirmed using seedlings grown in nutrient solution (Fig. 4a).
Leaf manganese concentrations ([Mn]) and phosphorus concentrations ([P]) of Eucalyptus diversicolor (Myrtaceae) collected at two locations: (a) [Mn] and (b) [P] in Torndirrup National Park, (c) [Mn] and (d) [P] in Porongurup National Park. In Torndirrup National Park, the reference plant species were not at the exact same location of E. diversicolor, because none were available there, and the two locations differed significantly in soil pH, which was higher under E. diversicolor, and hence its leaf [Mn] would be expected to be significantly lower. In Porongurup National Park, no reference plants were available at an acceptably short distance, and young leaves of E. diversicolor were used instead. Data are means ± SE (n = 5). Different letters indicate statistically significant differences among species (P ≤ 0.05)
At the low-diversity Porongurup National Park site, no suitable reference species occurred. We sampled young leaves of E. diversicolor, anticipating these might be suitable as negative reference (Shane and Lambers 2005b). Leaf [Mn] (Fig. 5c) was very high in mature leaves, compared with those at the other location, and significantly different from those in young leaves. The high leaf [Mn] suggests they released carboxylates. This was confirmed using seedlings grown in nutrient solution (Fig. 4a). Leaf [P] values were relatively high in mature leaves, and considerably higher in young leaves (Fig. 5d).
Carboxylate and phytosiderophore release
All species that exhibited relatively high leaf [Mn] released carboxylates (Figs. 2, 4a and 5c), including oxalate (Fig. 4b), except for the grass M. stipoides, which released phytosiderophores. Quantitative analysis of total released phytosiderophores showed a Fe-solubilising capacity of 0.50 ± 0.09 μg Fe g−1 root DW (n = 4), which corresponds with a phytosiderophore release rate of 8.7 ± 1.4 nmol DMA equivalent g−1 root DW (3 h)−1.
The fastest exudation rate was exhibited by E. diversicolor, in accordance with our hypothesis and contrary to the widely held view that this species (Bougher et al. 1990) and Eucalyptus species in general (Brundrett and Abbott 1991; Malajczuk et al. 1982) depend on mycorrhizal associations to acquire P.
Leaf N concentrations, N-isotope composition, and N derived from the atmosphere
Leaf [N] (Fig. 6a) was lowest in B. grandis, C. calophylla, E. marginata, and E. patens (10.2–11.3 mg g−1 DW), higher for the putatively N2-fixing M. riedlei (16.0 mg g−1 DW) and highest for the three Fabaceae (18.9–29.8 mg g−1 DW), with M. stipoides also showing a relatively high value (24.0 mg g−1 DW) (Fig. 6a). The δ15N values ranged from closest to zero for B. grandis to the most negative values for the eucalypts and M. stipoides (Fig. 6b), with the putatively N2-fixing species showing values in between. Using M. stipoides as a reference, we calculated the percentage N derived from atmospheric N2 fixation (%Ndfa) for the putatively N2-fixing species, and found values ranging from 73% in Acacia drummondii to 100% in Mirbelia dilatata (Fig. 6c).
(a) Leaf nitrogen concentrations ([N]), (b) leaf N isotope composition expressed as δ15N, and (c) N derived from atmospheric N2 fixation (Ndfa) for a range of species co-occurring with Eucalyptus patens (Myrtaceae) at Cypress Form, Waroona. Box-plots with medians, 25th and 75th percentiles. Whiskers extend to 1.5 times the interquartile range. Data presented beyond whiskers represent outliers. Data are means ± SE (n = 5–10). Different letters indicate statistically significant differences among species (P ≤ 0.05)
Discussion
We focused on P-acquisition strategies in ‘cool spots’ in a biodiversity hotspot, confirming our main hypothesis that common species in these habitats depend on a carboxylate-releasing P-mobilising strategy, while being mycorrhizal. Our results indicate that the tall eucalypt forest in southwest Australia, characterised by a low diversity of species, ‘cool spots’, on P-impoverished high-rainfall sites, function very differently from the hyperdiverse kwongan (or kwongkan; Hopper 2014) shrubland communities. We sampled most of the vascular plant species at the E. patens site; that number of 21 contrasted strongly with numbers of 40–50 per 100 m2 quadrats with a large variation among quadrats in kwongan shrublands on the sandplains in the Southwest Biodiversity Hotspot (Zemunik et al. 2016). In kwongan shrublands, Proteaceae species with very effective P-acquisition strategies are also very sensitive to oomycete pathogens (Albornoz et al. 2017; Laliberté et al. 2015). This trade-off has been offered as an explanation for coexistence of Proteaceae with mycorrhizal plants with less effective P-acquisition strategies but better protection against oomycete pathogens (Laliberté et al. 2015; Lambers et al. 2018). In contrast, the few dominant species in the ‘cool spots’ are mycorrhizal but depend on a carboxylate-releasing P-acquisition strategy, with the tall eucalypts dominating the canopy and other common species also being both mycorrhizal and carboxylate-releasing. We surmise that the degree of competition by dominant carboxylate-releasing mycorrhizal species for the extraordinarily low amounts of readily-available P in these environments explains the low species diversity and paucity of nitrogen-fixing species, whereby only a few understorey species exhibit carboxylate-releasing strategies to access P. The role of mycorrhizas in these high-rainfall systems is likely very important to boost species’ defence against pathogens (Albornoz et al. 2017; Laliberté et al. 2015), but given the very low availability of soil P in the studied habitats, mycorrhizas are unlikely to play a major role in inorganic P acquisition (Lambers et al. 2018; Parfitt 1979; Treseder and Allen 2002). The dominant species, being efficient at acquiring P, based on their carboxylate-releasing strategy, and protected against oomycete pathogens, because they are mycorrhizal, would not require facilitation of their P acquisition, as is common in kwongan (or kwongkan; Hopper 2014). Would it be likely that E. patens used both a carboxylate-releasing and mycorrhizal strategy to acquire P, possibly depending on space or time? We cannot exclude this, although our 15N results do not support this, as we discuss below. To further test this, we would need experiments done with a range of Myrtaceae species that naturally grow in kwongan (Albornoz et al. 2017). These authors grew Myrtaceae species in soil from their natural habitat inoculated with mycorrhizal fungi with different levels of mycorrhizal colonisation. Albornoz et al. (2017) found that the level of mycorrhizal colonisation did not affect the growth of Myrtaceae species, as long as there were no oomycete pathogens in the soil. These results are supported by the finding that in the natural kwongan habitat of these species, mycorrhizal fungi are commonly found inside the roots of mycorrhizal plants, but not in ingrowth soil cores outside the roots of these plants (Teste et al. 2016). We suggest that similar experiments are required in tall Eucalyptus forests to corroborate the present findings.
We used leaf [Mn] as a proxy for carboxylate-releasing P-acquisition strategies, based on the correlation between these two parameters in field (Lambers et al. 2021; Lambers et al. 2015b) and glasshouse studies (Pang et al. 2018; Wen et al. 2019). We then followed up with glasshouse studies to measure carboxylate exudation of seedlings grown in a low-P nutrient solution (Fig. 4). Our results lend further support for the use of leaf [Mn] as a proxy for rhizosphere carboxylate concentrations and confirm a recent contention that species in typical mycorrhizal clades may actually depend on carboxylate release to acquire P (Lambers et al. 2021). Likewise, our data show that one species in a typical non-mycorrhizal carboxylate-releasing clade (Cyperaceae) may actually not depend on carboxylate release to acquire P. Below, we discuss the wider implications of our findings.
Coexistence of P-acquisition strategies among sympatric species
Our findings provide evidence for a wide spectrum of P-acquisition strategies in tall eucalypt forests in southwest Australia (Fig. 2). At one end of the spectrum, there was B. grandis, our positive reference, which releases large amounts of carboxylates (Shane and Lambers 2005a), as is common in Proteaceae (Lambers 2022). At the other extreme were X. gracilis, X. preissii (our negative references) and M. riedlei, which grow very slowly between fires, but are among the first to resprout after a fire (Grove et al. 1980). These species likely depend on elevated soil P concentrations after a major disturbance such as fire (Lambers et al. 2022). In between the two extremes, some species released significant amounts of carboxylates, in accordance with their relatively high leaf [Mn]. Tremandra diffusa exhibited a leaf [Mn] closest to that of B. grandis, in agreement with data on the rainforest trees Elaeocarpus ferruginiflorus and E. ruminatus in the same family (Lambers et al. 2021). In agreement with its high leaf [Mn], seedlings of T. diffusa grown in nutrient solution released carboxylates (Fig. 4a). Bossiaea aquifolium, which formed a major understorey component, showed a high leaf [Mn] and released carboxylates, and likely also depends on a P-mining strategy.
One species that exhibited relatively high leaf [Mn] but did not release carboxylates, was M. stipoides. Its high leaf [Mn] is likely accounted for by its release of phytosiderophores, which is typical for Poaceae (Ma 2005). Grasses enhance the release of phytosiderophores when they are Fe-deficient, but M. stipoides in this case was grown with sufficient Fe. The present results suggest that M. stipoides up-regulated its phytosiderophore release in response to P deficiency. Phytosiderophores not only mobilise Fe, but also Mn (Zhang 1993), and they likely enhance P availability in soil, evidenced by findings for Fe-deficient Zea mays plants that accumulate more P than Fe-sufficient ones, in both roots and shoots (Zanin et al. 2017). Much inorganic P in soil is sorbed onto oxides and hydroxides of Fe, and phytosiderophores would therefore mobilise both Fe and P.
Some species with intermediate leaf [Mn] may depend on facilitation. This possibly includes Lygodactylus verticillatus (Fig. 2). Some species may access organic P in leaf litter, which may be accessed without involvement of carboxylates (Zhong et al. 2021). Since most of that leaf litter would have been made available by dominant P-mobilising species, we may consider this ‘indirect facilitation’ (Lambers et al. 2012a). We hypothesise that D. drummondii functions like this, whereas species with slightly higher leaf [Mn] are possibly ‘directly facilitated’, depending on P-mobilising carboxylates released by a neighbour. However, this contention requires further study.
Leaf [Mn] and [P]
Plants with the highest leaf [Mn] did not have the highest leaf [P] (Figs. 2, 3). This indicates that species with a high P-acquisition efficiency also exhibited a high P-use efficiency, which is well documented for Proteaceae (Lambers et al. 2012b; Sulpice et al. 2014) and co-occurring species (Guilherme Pereira et al. 2019). Interestingly, all species functioned at less than half of the leaf [P] that is considered adequate for crop plants, which is 2.00 mg g−1 leaf DW (Epstein and Bloom 2005) and also less than the global median leaf [P], which is 0.99 mg g−1 DW (Wright et al. 2004). Some of the low leaf [P] will be accounted for by the presence of sclerenchyma, but this does not offer a full explanation (Lambers et al. 2010). Leaves of the present species differed in their leaf dry matter content (Fig. S1a) but expressing leaf [P] on a FW basis did not substantially change the ranking (Figs. 3, S1b). Microlaena stipoides, P. esculentum and D. drummondii exhibited the highest leaf [P], about 0.90 mg g−1 DW (Fig. 3).
Leaf [N] and [P], and δ15N values
The leaf [P] and [N] of B. grandis were the same as recorded for two other populations of this species (Barrick 2003). Likewise, leaf [P] and [N] of C. calophylla and E. marginata were similar to or somewhat lower than published data (Hingston et al. 1980). For M. riedlei, the present leaf [N] and [P] data were in accordance with published data (Grove et al. 1980). We did not find data on leaf [N] and [P] in the literature for B. aquifolium, but published values for B. laidlawiana (regarded by some as B. aquifolium subsp. laidlawiana) growing in karri forest (Grove 1990) are similar to the ones for B. aquifolium we studied. We did not find pertinent data for the other species we studied in the literature.
Based on δ15N data (Fig. 6b), we conclude that 70–100% of all leaf N was acquired through symbiotic N2 fixation in M. riedlei and the three Fabaceae (Fig. 6c). These values are high compared with those for Fabaceae on severely P-impoverished Bassendean sands in Jurien Bay in southwest Australia, but similar to those on younger Spearwood sands, where the availability of both P and N is greater (Png et al. 2017). Since two of the Fabaceae appeared not to exhibit P-mobilising strategies, but possibly depended on facilitation, competition with the large eucalypts was likely severe. This strong competition with tall eucalypts likely explains why the dependence of the N2-fixing species on atmospheric N2 was very large, because legumes tend to increase their dependence on N2 fixation when neighbours compete with them for combined N (Li et al. 2009).
The high δ15N values for B. grandis suggest that this species accessed organic N, as shown for Hakea actites (Proteaceae) (Schmidt et al. 2003). Relatively high δ15N values are common for Proteaceae (Schmidt and Stewart 1997). Conversely, the very negative values for both M. stipoides and the eucalypts suggest that they predominantly accessed inorganic N. Being ectomycorrhizal (Brundrett and Abbott 1991), we might have expected the eucalypts to access organic N (Abuzinadah and Read 1986; Högberg 1990), but the stable isotope values do not support this expectation (Fig. 6b). We surmise that this is because in severely P-impoverished habitats, mycorrhizal fungi are largely restricted to roots, with very few hyphae extending into the soil (Teste et al. 2016). The leaf [Mn] data support this contention, because mycorrhizal hyphae (Bethlenfalvay and Franson 1989) including ectomycorrhizal hyphae (Canton et al. 2016) in soil tend to intercept Mn and cause low leaf [Mn].
Phosphorus acquisition in E. diversicolor
Grove (1990) reported high leaf [Mn] for unfertilised E. diversicolor of 857 mg kg−1 DW in comparison with 95 mg kg−1 DW for B. aquifolium subsp. laidlawiana (formerly B. laidlawiana) at the same location. Leaf [Mn] for E. diversicolor was 222 mg kg−1 DW in comparison with 70 mg kg−1 DW for C. calophylla at the same yellow podzolic soil site (Hingston et al. 1979), suggesting that E. diversicolor released carboxylates more vigorously than C. calophylla; our data on carboxylate exudation support this contention (Fig. 4a).
The present results suggest that E. diversicolor forests function like E. patens forests, with E. diversicolor releasing large amounts of carboxylates to acquire P, while its mycorrhizal associations protect it from pathogens. These traits make them independent of neighbours facilitating their P uptake, strong competitors, and dominant species in a low-diversity habitat.
Concluding remarks
The present results provide insights into some ‘cool spots’ in a biodiversity hotspot; some species in mycorrhizal families may actually depend on carboxylate release to acquire P in their natural habitat. They also indicate that the tall eucalypt forest in southwest Australia function differently from what we might believe, based on mycorrhizal surveys (Albornoz et al. 2021a; Brundrett and Abbott 1991). We do not know how common carboxylate-releasing strategies are amongst Myrtaceae and Elaeocarpaceae (Lambers et al. 2021), or if this trait is associated with high-rainfall locations in Australia.
Even in the severely P-impoverished habitats of the present study, a range of P-acquisition strategies co-exist, ranging from carboxylate release in species with high leaf [Mn] to direct facilitation by carboxylate-releasing neighbours in species with intermediate leaf [Mn]. Some species with low leaf [Mn] likely depended on indirect facilitation and accessed readily-available organic P in litter made available predominantly by the dominant P-mobilising species. Leaf [Mn] of Fabaceae indicates that they depended to a greater (B. aquifolium) or lesser (A. drummondii) extent on carboxylates, but they likely also accessed organic P (Houlton et al. 2008; Png et al. 2017). Our findings provide further incentive to revisit some of the species in ‘mycorrhizal families’ (Elaeocarpaceae, Myrtaceae) that have unusually high leaf [Mn] (Lambers et al. 2021). The present results show that significant carboxylate release associated with mobilisation of P and Mn, even without involvement of cluster roots, is more pervasive than commonly believed.
References
Abuzinadah RA, Read DJ (1986) The role of proteins in the nitrogen nutrition of ectomycorrhizal plants. V. Nitrogen transfer in birch (Betula pendula) grown in association with mycorrhizal and non-mycorrhizal fungi. New Phytol 103:481–493. https://doi.org/10.1111/j.1469-8137.1989.tb00309.x
Adams MA (1992) Phosphatase activity and phosphorus fractions in karri (Eucalyptus diversicolor F. Muell.) forest soils. Biol Fert Soils 14:200–204. https://doi.org/10.1007/BF00346061
Albornoz FE, Burgess TI, Lambers H, Etchells H, Laliberté E (2017) Native soil-borne pathogens equalize differences in competitive ability between plants of contrasting nutrient-acquisition strategies. J Ecol 105:549–557. https://doi.org/10.1111/1365-2745.12638
Albornoz FE, Dixon KW, Lambers H (2021a) Revisiting mycorrhizal dogmas: are mycorrhizas really functioning as they are widely believed to do? Soil Ecol Lett 3:73–82. https://doi.org/10.1007/s42832-020-0070-2
Albornoz FE, Shane MW, Lambers H (2021b) Contrasting phosphorus sensitivity of two Australian native monocots adapted to different habitats. Plant Soil 461:151–162. https://doi.org/10.1007/s11104-020-04760-5
Andrews M, James EK, Sprent JI, Boddey RM, Gross E, dos Reis FB (2011) Nitrogen fixation in legumes and actinorhizal plants in natural ecosystems: values obtained using 15N natural abundance. Plant Ecol Divers 4:131–140. https://doi.org/10.1080/17550874.2011.644343
Azcón-Aguilar C, Barea JM (1997) Arbuscular mycorrhizas and biological control of soil-borne plant pathogens – an overview of the mechanisms involved. Mycorrhiza 6:457–464. https://doi.org/10.1007/s005720050147
Barrick KA (2003) Comparison of the nutrient ecology of coastal Banksia grandis elfinwood (windswept shrub-like form) and low trees, cape Leeuwin-Naturaliste National Park, Western Australia. Austral Ecol 28:252–262. https://doi.org/10.1046/j.1442-9993.2003.01272.x
Baxter I R, Vitek O, Lahner B, Muthukumar B, Borghi M, Morrissey J, ... Salt D E (2008) The leaf ionome as a multivariable system to detect a plant's physiological status. Proc Natl Acad Sci U S A 105, 12081-12086. 10.1073pnas.0804175105
Berdeni D, Cotton TEA, Daniell TJ, Bidartondo MI, Cameron DD, Evans KL (2018) The effects of arbuscular mycorrhizal fungal colonisation on nutrient status, growth, productivity, and canker resistance of apple (Malus pumila). Front Microbiol 9:1461. https://doi.org/10.3389/fmicb.2018.01461
Bethlenfalvay GJ, Franson RL (1989) Manganese toxicity alleviated by mycorrhizae in soybean. J Plant Nutr 12:953–970. https://doi.org/10.1080/01904168909364006
Bougher NL, Grove TS, Malajczuk N (1990) Growth and phosphorus acquisition of karri (Eucalyptus diversicolor F. Muell.) seedlings inoculated with ectomycorrhizal fungi in relation to phosphorus supply. New Phytol 114:77–85. https://doi.org/10.1111/j.1469-8137.1990.tb00376.x
Boutaj H, Meddich A, Chakhchar A, Wahbi S, El Alaoui-Talibi Z, Douira A et al (2020) Arbuscular mycorrhizal fungi improve mineral nutrition and tolerance of olive tree to Verticillium wilt. Arch Phytopathol Plant Protect 53:673–689. https://doi.org/10.1080/03235408.2020.1792603
Bradshaw FJ (2015) Reference material for karri forest silviculture. Department of Parks and Wildlife, Perth
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77. https://doi.org/10.1007/s11104-008-9877-9
Brundrett MC (2017) Global diversity and importance of mycorrhizal and nonmycorrhizal plants. In: Tedersoo L (ed) Biogeography of mycorrhizal Symbiosis. Springer International Publishing, Cham, pp 533–556
Brundrett MC, Abbott LK (1991) Roots of jarrah forest plants. I. Mycorrhizal associations of shrubs and herbaceous plants. Aust J Bot 39:445–457. https://doi.org/10.1071/BT9910445
Canton GC, Bertolazi AA, Cogo AJD, Eutrópio FJ, Melo J, de Souza SB et al (2016) Biochemical and ecophysiological responses to manganese stress by ectomycorrhizal fungus Pisolithus tinctorius and in association with Eucalyptus grandis. Mycorrhiza 26:475–487. https://doi.org/10.1007/s00572-016-0686-3
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromotog A 1011:233–240. https://doi.org/10.1016/S0021-9673(03)01129-4
Cordier C, Pozo MJ, Barea JM, Gianinazzi S, Gianinazzi-Pearson V (1998) Cell defense responses associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. Mol Plant-Microbe Interact 11:1017–1028
Epstein E, Bloom AJ (2005) Mineral nutrition of plants: principles and perspectives. Sinauer, Sunderland
Frew A, Antunes PM, Cameron DD, Hartley SE, Johnson SN, Rillig MC, Bennett AE (2021) Plant herbivore protection by arbuscular mycorrhizas: a role for fungal diversity? New Phytol 233:1022–1031. https://doi.org/10.1111/nph.17781
Gardner JH, Malajczuk N (1988) Recolonisation of rehabilitated bauxite mine sites in western Australia by mycorrhizal fungi. For Ecol Manag 24:27–42. https://doi.org/10.1016/0378-1127(88)90022-9
Gioia P, Hopper SD (2017) A new phytogeographic map for the southwest Australian floristic region after an exceptional decade of collection and discovery. Bot J Linn Soc 184:1–15. https://doi.org/10.1093/botlinnean/box010
Gonthier P, Giordano L, Zampieri E, Lione G, Vizzini A, Colpaert JV, Balestrini R (2019) An ectomycorrhizal symbiosis differently affects host susceptibility to two congeneric fungal pathogens. Fungal Ecol 39:250–256. https://doi.org/10.1016/j.funeco.2018.12.008
Grove TS (1988) Growth responses of trees and understorey to applied nitrogen and phosphorus in karri (Eucalyptus diversicolor) forest. For Ecol Manag 23:87–103. https://doi.org/10.1016/0378-1127(88)90076-X
Grove TS (1990) Twig and foliar nutrient concentrations in relation to nitrogen and phosphorus supply in a eucalypt (Eucalyptus diversicolor F. Muell.) and an understorey legume (Bossiaea laidlawiana Tovey and Morris). Plant Soil 126:265–275. https://doi.org/10.1007/BF00012829
Grove TS, O'connell AM, Malajczuk N (1980) Effects of fire on the growth, nutrient content and rate of nitrogen fixation of the cycad Macrozamia riedlei. Aust J Bot 28:271–281. https://doi.org/10.1071/BT9800271
Guilherme Pereira C, Hayes PE, O'Sullivan O, Weerasinghe L, Clode PL, Atkin OK, Lambers H (2019) Trait convergence in photosynthetic nutrient-use efficiency along a 2-million year dune chronosequence in a global biodiversity hotspot. J Ecol 107:2006–2023. https://doi.org/10.1111/1365-2745.13158
Güsewell S, Schroth MH (2017) How functional is a trait? Phosphorus mobilization through root exudates differs little between Carex species with and without specialized dauciform roots. New Phytol 215:1438–1450. https://doi.org/10.1111/nph.14674
Habel JC, Rasche L, Schneider UA, Engler JO, Schmid E, Rödder D et al (2019) Final countdown for biodiversity hotspots. Conserv Lett 12:e12668. https://doi.org/10.1111/conl.12668
Halliday J, Pate JS (1976) Symbiotic nitrogen fixation by coralloid roots of the cycad Macrozamia riedlei: physiological characteristics and ecological significance. Funct Plant Biol 3:349–358. https://doi.org/10.1071/PP9760349
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410. https://doi.org/10.1111/1365-2745.12196
Hayes PE, Clode PL, Guilherme Pereira C, Lambers H (2019) Calcium modulates leaf cell-specific phosphorus allocation in Proteaceae from South-Western Australia. J Exp Bot 70:3995–4009. https://doi.org/10.1093/jxb/erz156
Hingston FJ, Dimmock GM, Turton AG (1980) Nutrient distribution in a jarrah (Eucalyptus marginata Donn ex Sm.) ecosystem in south-West Western Australia. For Ecol Manag 3:183–207. https://doi.org/10.1016/0378-1127(80)90015-8
Hingston FJ, Turton AG, Dimmock GM (1979) Nutrient distribution in karri (Eucalyptus diversicolor F. muell.) ecosystems in southwest western Australia. For Ecol Manag 2:133–158. https://doi.org/10.1016/0378-1127(79)90042-2
Högberg P (1990) 15N natural abundance as a possible marker of the ectomycorrhizal habit of trees in mixed African woodlands. New Phytol 115, 483–486. 10.1111/j.1469-8137.1990.tb00474.x
Hopper SD (2014) Sandplain and Kwongkan: historical spellings, meanings, synonyms, geography and definition. In: Lambers H (ed) Plant life on the sandplains in Southwest Australia, a global biodiversity hotspot. UWA Publishing, Crawley, pp 23–33
Hopper SD, Gioia P (2004) The southwest Australian floristic region: evolution and conservation of a global hotspot of biodiversity. Annu Rev Ecol Evol Syst 35:623–650. https://doi.org/10.1146/annurev.ecolsys.35.112202.130201
Houlton BZ, Wang Y-P, Vitousek PM, Field CB (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–330. https://doi.org/10.1038/nature07028
Huston MA (1994) Biological diversity. Cambridge University Press, Cambridge
Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ (2012) Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol 38:651–664. https://doi.org/10.1007/s10886-012-0134-6
Laliberté E, Lambers H, Burgess TI, Wright SJ (2015) Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytol 206:507–521. https://doi.org/10.1111/nph.13203
Lambers H (ed) (2014) Plant life on the sandplains in Southwest Australia, a global biodiversity hotspot. University of Western Australia Publishing, Crawley
Lambers H (2022) Phosphorus acquisition and utilization in plants. Annu Rev Plant Biol 73:17–42. https://doi.org/10.1146/annurev-arplant-102720-125738
Lambers H, Albornoz F, Kotula L, Laliberté E, Ranathunge K, Teste FP, Zemunik G (2018) How belowground interactions contribute to the coexistence of mycorrhizal and non-mycorrhizal species in severely phosphorus-impoverished hyperdiverse ecosystems. Plant Soil 424:11–34. https://doi.org/10.1007/s11104-017-3427-2
Lambers H, Albornoz FE, Arruda AJ, Barker T, Finnegan PM, Gille C et al (2019) Nutrient-acquisition strategies. In: Lambers H (ed) A Jewel in the crown of a global biodiversity hotspot. Kwongan Foundation and the Western Australian Naturalists' Club Inc, Perth, pp 227–248
Lambers H, Bishop JG, Hopper SD, Laliberté E, Zúñiga-Feest A (2012a) Phosphorus-mobilization ecosystem engineering: the roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Ann Bot 110:329–348. https://doi.org/10.1093/aob/mcs130
Lambers H, Brundrett MC, Raven JA, Hopper SD (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334:11–31. https://doi.org/10.1007/s11104-010-0444-9
Lambers H, Cawthray GR, Giavalisco P, Kuo J, Laliberté E, Pearse SJ et al (2012b) Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol 196:1098–1108. https://doi.org/10.1111/j.1469-8137.2012.04285.x
Lambers H, Clode PL, Hawkins H-J, Laliberté E, Oliveira RS, Reddell P et al (2015a) Metabolic adaptations of the non-mycotrophic Proteaceae to soil with a low phosphorus availability. In: Plaxton WC, Lambers H (eds) Annual plant reviews, volume 48, phosphorus metabolism in plants. John Wiley & Sons, Chicester, pp 289–336
Lambers H, de Britto CP, Cawthray GR, Denton MD, Finnegan PM, Hayes PE et al (2022) Strategies to acquire and use phosphorus in phosphorus-impoverished and fire-prone environments. Plant Soil, in press. https://doi.org/10.1007/s11104-022-05464-8
Lambers H, Guilherme Pereira C, Wright IJ, Bellingham PJ, Bentley LP, Boonman A et al (2021) Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil 461:43–61. https://doi.org/10.1007/s11104-020-04690-2
Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL (2015b) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90. https://doi.org/10.1016/j.tplants.2014.10.007
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103. https://doi.org/10.1016/j.tree.2007.10.008
Li Y-Y, Yu C-B, Cheng X, Li C-J, Sun J-H, Zhang F-S et al (2009) Intercropping alleviates the inhibitory effect of N fertilization on nodulation and symbiotic N2 fixation of faba bean. Plant Soil 323:295–308. https://doi.org/10.1007/s11104-009-9938-8
Ma JF (2005) Plant root responses to three abundant soil minerals: silicon, aluminum and iron. Crit Rev Plant Sci 24:267–281. https://doi.org/10.1080/07352680500196017
Ma JF, Ueno H, Ueno D, Rombolà AD, Iwashita T (2003) Characterization of phytosiderophore secretion under Fe deficiency stress in Festuca rubra. Plant Soil 256:131–137. https://doi.org/10.1023/A:1026285813248
Macfarlane C, Bond C, White DA, Grigg AH, Ogden GN, Silberstein R (2010) Transpiration and hydraulic traits of old and regrowth eucalypt forest in southwestern Australia. For Ecol Manag 260:96–105. https://doi.org/10.1016/j.foreco.2010.04.005
Malajczuk N, Molina R, Trappe JM (1982) Ectomycorrhiza formation in Eucalyptus. New Phytol 91:467–482. https://doi.org/10.1111/j.1469-8137.1982.tb03325.x
Marx DH (1972) Ectomycorrhizae as biological deterrents to pathogenic root infections. Annu Rev Phytopathol 10:429–454
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Pang J, Ruchi B, Zhao H, Bansal R, Bohuon E, Lambers H et al (2018) The carboxylate-releasing phosphorus-mobilising strategy could be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529. https://doi.org/10.1111/nph.15200
Parfitt RL (1979) The availability of P from phosphate-goethite bridging complexes. Desorption and uptake by ryegrass. Plant Soil 53:55–65. https://doi.org/10.1007/BF02181879
Png GK, Turner BL, Albornoz FE, Hayes PE, Lambers H, Laliberté E (2017) Greater root phosphatase activity in nitrogen-fixing rhizobial but not actinorhizal plants with declining phosphorus availability. J Ecol 105:1246–1255. https://doi.org/10.1111/1365-2745.12758
Pozo MJ, Cordier C, Dumas-Gaudot E, Gianinazzi S, Barea JM, Azcón-Aguilar C (2002) Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J Exp Bot 53:525–534. https://doi.org/10.1093/jexbot/53.368.525
R_Development_Core_Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rao AS, Reddy KS, Takkar PN (1997) Malachite green method compared to ascorbic acid for estimating small amounts of phosphorus in water, 0.01 M calcium chloride, and Olsen soil extracts. Comm Soil Sci Plant Anal 28:589–601. https://doi.org/10.1080/00103629709369813
Rayment GE, Lyons DJ (2011) Soil chemical methods - Australasia. CSIRO Publishing, Collingwood
Saunders WMH, Williams EG (1955) Observatioms on the determination of total organic phosphorus in soils. J Soil Sci 6:254–267. https://doi.org/10.1111/j.1365-2389.1955.tb00849.x
Schmidt S, Mason M, Sangtiean T, Stewart GR (2003) Do cluster roots of Hakea actities (Proteaceae) acquire complex organic nitrogen? Plant Soil 248:157–165. https://doi.org/10.1023/A:1022352415728
Schmidt S, Stewart GR (1997) Waterlogging and fire impacts on nitrogen availability and utilization in a subtropical wet heathland (wallum). Plant Cell Environ 20:1231–1241. https://doi.org/10.1046/j.1365-3040.1997.d01-20.x
Shane MW, De Vos M, De Roock S, Cawthray GR, Lambers H (2003) Effects of external phosphorus supply on internal phosphorus concentration and the initiation, growth and exudation of cluster roots in Hakea prostrata r.Br. Plant Soil 248:209–219. https://doi.org/10.1023/A:1022320416038
Shane MW, Lambers H (2005a) Cluster roots: a curiosity in context. Plant Soil 274:101–125. https://doi.org/10.1007/s11104-004-2725-7
Shane MW, Lambers H (2005b) Manganese accumulation in leaves of Hakea prostrata (Proteaceae) and the significance of cluster roots for micronutrient uptake as dependent on phosphorus supply. Physiol Plant 124:441–450. https://doi.org/10.1111/j.1399-3054.2005.00527.x
Shearer G, Kohl D (1986) N2-fixation in field settings: estimations based on natural 15N abundance. Funct Plant Biol 13:699–756. https://doi.org/10.1071/PP9860699
Skrzypek G, Debajyoti P (2006) δ13C analyses of calcium carbonate: comparison between the GasBench and elemental analyzer techniques. Rap Comm Mass Spectrom 20:2915–2920. https://doi.org/10.1002/rcm.2688
Sulpice R, Ishihara H, Schlereth A, Cawthray GR, Encke B, Giavalisco P et al (2014) Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species. Plant Cell Environ 37:1276–1298. https://doi.org/10.1111/pce.12240
Teste FP, Laliberté E, Lambers H, Auer Y, Kramer S, Kandeler E (2016) Mycorrhizal fungal biomass and scavenging declines in phosphorus-impoverished soils during ecosystem retrogression. Soil Biol Biochem 92:119–132. https://doi.org/10.1016/j.soilbio.2015.09.021
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355. https://doi.org/10.1111/j.1469-8137.2004.01159.x
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515. https://doi.org/10.1046/j.1469-8137.2002.00470.x
Uloth MB, You MP, Cawthray G, Barbetti MJ (2015) Temperature adaptation in isolates of Sclerotinia sclerotiorum affects their ability to infect Brassica carinata. Plant Pathol 64:1140–1148. https://doi.org/10.1111/ppa.12338
Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey B, Giller K et al (2008) Measuring plant-associated nitrogen fixation in agricultural systems. Australian Centre for International Agricultural Research, Canberra
Veresoglou SD, Rillig MC (2012) Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol Lett 8:214–217. https://doi.org/10.1098/rsbl.2011.0874
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363. https://doi.org/10.1007/2Fs00572-005-0033-6
Wardle DA, Walker LR, Bardgett RD (2004) Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305:509–513. https://doi.org/10.1126/science.1098778
Wehner J, Antunes PM, Powell JR, Mazukatow J, Rillig MC (2010) Plant pathogen protection by arbuscular mycorrhizas: a role for fungal diversity? Pedobiologia 53:197–201. https://doi.org/10.1016/j.pedobi.2009.10.002
Wen Z, Li H, Shen Q, Tang X, Xiong C, Li H et al (2019) Trade-offs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol 223:882–895. https://doi.org/10.1111/nph.15833
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F et al (2004) The worldwide leaf economics spectrum. Nature 428:821–827. https://doi.org/10.1038/nature02403
Zanin L, Venuti S, Zamboni A, Varanini Z, Tomasi N, Pinton R (2017) Transcriptional and physiological analyses of Fe deficiency response in maize reveal the presence of strategy I components and Fe/P interactions. BMC Genomics 18:154. https://doi.org/10.1186/s12864-016-3478-4
Zemunik G, Lambers H, Turner BL, Laliberté E, Oliveira RS (2018) High abundance of non-mycorrhizal plant species in severely phosphorus-impoverished Brazilian Campos rupestres. Plant Soil 424:255–271. https://doi.org/10.1007/s11104-017-3503-7
Zemunik G, Turner BL, Lambers H, Laliberté E (2015) Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat Plants 1:15050. https://doi.org/10.1038/nplants.2015.50
Zemunik G, Turner BL, Lambers H, Laliberté E (2016) Increasing plant species diversity and extreme species turnover accompany declining soil fertility along a long-term chronosequence in a biodiversity hotspot. J Ecol 104:792–805. https://doi.org/10.1111/1365-2745.12546
Zhang FS (1993) Mobilisation of iron and manganese by plant-borne and synthetic metal chelators. Plant Soil 155:111–114. https://doi.org/10.1007/bf00024996
Zhong H, Zhou J, Azmi A, Arruda A J, Doolette A L, Smernik R J and Lambers H (2021) Xylomelum occidentale (Proteaceae) accesses relatively mobile soil organic phosphorus without releasing carboxylates. J Ecol 109, 246–259. https://doi.org/10.1111/1365-2745.13468
Acknowledgements
Funding was provided by a Future Fellowship (FT170100195) from the Australian Research Council (ARC) to KR, Australian Research Council Discovery Grants (DP130100005; DP200101013) to HL, and (DP220100494) to HL and KR. HL also acknowledges support from the Deputy Vice Chancellor (Research) at the University of Western Australia towards field trips and a living allowance for XMZ. QS was supported by a scholarship from the China Scholarship Council (CSC) and a top-up scholarship from the Kwongan Foundation. We thank Shu Tong Liu and Toby Bird for helping with hydroponic experiments, induction to lab instruments and help in daily life for XMZ. We thank Jiayin Pang for teaching how to grow healthy plants in hydroponics and suggestion for data statistics, Michael Smirk for chemical analyses of plant and soil nutrients, Gregory Cawthray for carboxylate analyses, Douglas Ford for plant isotope analyses, Kevin Thiele for identification of Hibbertia sylvestris, Aiwu Jiang for critically reading our manuscript before submission, and two anonymous Reviewers for their constructive comments.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Xue Meng Zhou was responsible for most of the field and glasshouse work and chemical and statistical analyses and contributed to the writing; Kosala Ranathunge was responsible for some of the glasshouse work and statistical analyses and contributed to the writing; Marion L. Cambridge was responsible for some of the field work and contributed to the writing; Kingsley W. Dixon was responsible for some of the field work and contributed to the writing; Patrick E. Hayes was responsible for some of the field work and statistical analyses and contributed to the writing; Miroslav Nikolic was responsible for the analysis of phytosiderophores and contributed to the writing; Qi Shen was responsible for some of the field work and contributed to the writing; Hongtao Zhong was responsible for some of the field work and soil analyses and contributed to the writing; Hans Lambers conceived the study, contributed to field work, wrote the first draft of the manuscript and took care of several revisions.
Corresponding author
Additional information
Responsible Editor: Tim S. George.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 423 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, X.M., Ranathunge, K., Cambridge, M.L. et al. A cool spot in a biodiversity hotspot: why do tall Eucalyptus forests in Southwest Australia exhibit low diversity?. Plant Soil 476, 669–688 (2022). https://doi.org/10.1007/s11104-022-05559-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05559-2