Abstract
Background and aims
Cover crops have the potential to aid in adapting agricultural systems to climate change impacts through their ecosystem services, such as preventing soil erosion, remediating soil structure, and storing carbon belowground. Though roots are integral to these processes, there is a lack of cover crop root trait data. This study aims to characterize rooting behavior of several commercially available cover crops and assess how differences in root system architecture potentially impact their selection for ecosystem services.
Methods
Twenty-two cover crop cultivars across the grass, legume, and brassica families were grown in O’Fallon, Missouri, USA. Canopy cover was monitored throughout the growing season. Shoot and root biomass samples were collected and analyzed.
Results
Cereal rye and winter triticale were the most winter hardy cultivars and provided the highest percent canopy cover. Cereal rye and winter triticale also generated the highest amount of shoot and root biomass among treatments but diverged in their root system architectures. Winter triticale forms coarser roots and exhibited deeper rooting, which may be better suited for carbon sequestration. Rapeseed and Siberian kale have favorable C:N ratios for nutrient recycling, but rapeseed may invest more into lateral root formation and have a higher potential to “catch” excess nutrients.
Conclusion
Selection of cover crops for ecosystem services should account for root system architecture and their suitability for these ecosystem services. Differences in root traits among cultivars within the same family highlight the potential to breed cover crop root system architecture to further enhance ecosystem service efficacy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cover crops can help to mitigate and adapt to climate change impacts on agricultural lands through their ecosystem services. Agricultural practices such as tillage and overuse of synthetic fertilizers, pesticides, and herbicides are deleterious to soil health and contribute to the generation of greenhouse gases (Gomiero et al. 2011; Lal 2004; Yang et al. 2020). Fallow periods between cash crop growing seasons are another such practice. The unprotected, bare soil is susceptible to erosion during heavy rainfall and strong wind events, which are expected to increase in severity and frequency (Fischer and Knutti 2015; Li et al. 2021). It is estimated that 35% of cultivated land across the US Corn Belt has completely lost its carbon-rich A-horizon (Thaler et al. 2021). This soil loss is expected to decrease crop yields by 6% and lead to around $2.8 billion in annual economic losses (Thaler et al. 2021). Soil organic carbon can be lost through erosion as well as soil respiration during fallow periods (Olson et al. 2016; von Haden et al. 2019). Soil nitrogen from excess fertilizers can be lost via leaching during fallow periods or lost via soil respiration as nitrogen oxide gases that contribute 37% of total agricultural greenhouse gas production (Park et al. 2012; Paustian et al. 2016). By century end, soil respiration is projected to increase by 40% due to climate change (Nissan et al. 2023), which will have drastic impacts on soil health and agricultural greenhouse gas emission. To help mitigate these problems, cover crops can be planted during fallow periods between cash crop growing seasons to reduce erosion, “catch” excess fertilizer inputs, and act as a carbon sink to store carbon belowground in the form of plant biomass and rhizodeposits (Griffiths et al. 2022; Haruna et al. 2020; Kaspar and Singer 2011; Lal 2015; Poeplau and Don 2015; Thorup-Kristensen et al. 2003).
Roots play a vital role in the ability of plants to perform ecosystem functions and are the main conduit for delivering carbon to soils (Griffiths et al. 2022). Living roots are also a core principle of soil health (Bagnall et al. 2023; Canisares et al. 2023). Cover crop user’s report “increases overall soil health” and “increases soil organic matter” as the top two reasons for planting cover crops (CTIC 2016). Yet, cover crop rooting behaviors are rarely considered when selecting a cover crop. Root system architecture and interactions between roots and the soil microbiome have been shown to influence soil pore size distribution, microbiome composition, and soil carbon production (Bodner et al. 2014). Thus, consideration for cover crop root system architecture is important to how soil structure and chemistry will change. However, there is a lack of data on cover crop rooting behavior (Amsili and Kaye 2021; Bodner et al. 2019; Griffiths et al. 2022; Hudek et al. 2022; Kemper et al. 2020), and currently most cover crop breeding is based on aboveground plant traits. A deeper understanding of cover crop root traits and their impact on ecosystem services will better inform cover crop breeding and lead to the development of cultivars that more efficiently sequester carbon, remediate soil structure, or capture excess nutrients. Furthermore, this knowledge may help farmers select cover crops that better match their goals for improving soil health and increase adoption of cover crops in the face of climate change.
Cover crops are often selected for their functional traits and winter hardiness with limited consideration for root traits and carbon to nitrogen (C:N) ratio of shoot and root tissues, which have major influences on nutrient cycling and subsequent plant nutrition (Nevins et al. 2020). The most common cover crops include cereal rye, annual ryegrass, winter wheat, radish, oats, rapeseed, turnip, crimson clover, and hairy vetch according to national surveys (CTIC 2016; Wallander et al. 2021). This list of cover crops can be split into 3 functional groups: cool-season grasses, legumes, and brassicas. Grasses such as cereal rye, annual ryegrass, and winter wheat are thought to have fast-growing fibrous root systems that are beneficial for preventing soil erosion, sequestering carbon, and capturing excess nutrients throughout the soil profile. However, grasses have a high C:N ratio that may immobilize N and negatively impact the yield of the following cash crop (Adeli et al. 2019; Deines et al. 2023; Preza-Fontes et al. 2022; Qin et al. 2021). After cover crop termination, microbes decomposing high C:N ratio biomass consume soil available nitrogen. The microbes deplete and immobilize soil available nitrogen as microbial biomass. The lack of soil available N results in a lower N uptake during cash crop growth causing a loss in yield in plots covered in cover crop residue compared to no cover crop controls (Nevins et al. 2020). Legumes such as clover, hairy vetch, and winter pea produce root nodules that provide biological nitrogen fixation through rhizobia and increase mineralized nitrogen content reducing the need for chemical fertilizer before cash crop planting (Sainju et al. 2003, 2005). Though legumes have a more favorable C:N ratio, they do not produce as much biomass (Gentry et al. 2013). Brassicas such as radish, rapeseed, and turnip have thick taproots that can reduce soil compaction, and they can act as biofumigants to suppress soil-borne pathogens and pests (Dutta et al. 2019; Kirkegaard and Sarwar 1998; Williams and Weil 2004). Brassicas are more likely to winter kill than legumes and grasses which can impact the timing of plant litter decay and nutrient cycling (Ruark et al. 2018).
The aim of our study is to characterize root traits across commercially available cover crop cultivars and discuss their potential impact on ecosystem services such as soil structure remediation, carbon sequestration, and nutrient capture. We hypothesize that there are differences in root system architecture between cultivars and between species within each cover crop functional group (cool-season grass, legume, brassica). These differences in root system architecture influence their suitability for certain ecosystem services. We evaluated the performance of 22 commercially available cover crop cultivars selected from the 3 functional groups grown over the winter in O’Fallon, Missouri, USA. Table 1 shows the cover crop treatments assessed in this study and their seeding rates; for some of the 16 species in Table 1, there are multiple cultivars resulting in 22 total cover crop cultivars. Winter hardiness and plant growth were monitored throughout the growing season through periodic canopy imaging. Among the cultivars that survived the winter, 13 cover crop treatments were sampled to compare shoot and root biomass, vertical root distribution, and total carbon and nitrogen content. A better understanding of how these traits vary between cultivars and between species will help to inform future cover crop selection and breeding to aid in preventing soil erosion, sequestering carbon, and mineralizing nitrogen.
Materials and methods
Site
The field experiment was conducted at DDPSC Field Research Site at Planthaven Farms in O’Fallon, Missouri, USA (38°50’58.3"N, 90°41’06.2"W, 133 m elevation). The fields used in this experiment had previously been under commercial corn-soy rotation with soybeans planted in the most recent growing season prior to the winter cover crop experiment. The research site is classified as a USDA hardiness zone 6a, which corresponds to an average annual minimum winter temperature of -20.6 °C. Mean daily precipitation at the research station was 2.26 mm/day, and mean daily temperatures ranged from 25.6 °C in early October and late April to -10.6 °C in January. Monthly temperature and precipitation values for the growing season are reported against historical averages in Supplemental Fig. 1. December was warmer than average while January was colder than average. February and March were wetter than average. The soil series in these plots is classified by the USDA Web Soil Survey as a Hurst silt loam. Soil samples were taken before planting and sent to Ward laboratories (Kearney, NE, USA) for analysis. Soil organic matter (SOM), pH, nitrate-nitrogen concentration, and Mehlich 3 extractable nutrients are reported in Supplemental Table 1. The low soil pH likely results from nitrification from the biologically fixed N from the soybeans grown over the summer and may have been enhanced by tillage prior to sampling. A plow pan was observed around 12 cm below the soil surface as indicated by a soil color and texture change (Supplemental Fig. 2). Further details on soil color and texture changes are included in Supplemental Information. The planting area was also located near a creek and had a high water table as a result.
Experimental design
Cover crops were planted on October 1, 2021, after a soybean growing season and terminated on April 27, 2022 (208 days). Before planting, the field site was disked, cultivated, and graded to prepare the seedbed and prevent flooding. Prior to sowing, plots were weeded using a hoe to remove emerging soybean plants; no other weeding was performed after planting. Cover crop seeds were obtained from Green Cover (Bladen, NE, USA). The 32 cover crop treatments were planted by hand broadcasting across 5 replicate blocks for a total of 160 plots in an alpha lattice design (Supplemental Table 2). Cover crop treatments reported in this study and their seeding rates are detailed in Table 1. Each plot measured 1.83 m by 1.83 m (6 ft by 6 ft) in size. Mechanical rolling was performed to improve seed-soil contact, and plots were irrigated for 2 weeks after planting to improve emergence. We note that some cover crop treatments suffered from poor germination and stand establishment, a common issue for broadcast-seeded cover crops (Brennan and Leap 2014). Germination, stand quality, and winter survival were scored for all 32 cover crop treatments and are reported in Supplemental Table 3.
Canopy imaging
Canopy images were collected with a Canon 5DS R camera mounted to a custom rig to center the image over each plot at a height of 6 ft. The camera was set to f/9.0 aperture, 1/200 sec. shutter speed, ISO100, pattern metering mode, and 16 mm focal length. Each image was 8688 by 5792 pixels in size with a resolution of 72 dpi. Images were collected every 3–4 weeks between 11am and 1pm CST every 3–4 weeks to minimize shadows in the images. Representative canopy images at harvest are shown in Supplemental Fig. 3. Cropped canopy images were processed using a custom OpenCV python code to segment green shoot tissue (Griffiths and Topp 2021). The percentage of pixels assigned as green shoot tissue is reported as canopy cover and is plotted across time to evaluate plant growth throughout the growing season.
Shoot and root sampling
Plant biomass samples were harvested between April 25, 2022 and April 27, 2022. Shoot and root biomass samples were collected from 13 of the 32 cover crop treatments which had moderate winter survival as evaluated from canopy images (Supplemental Table 3). Shoot biomass was collected from a representative 25.4 cm by 25.4 cm region within the plot using a quadrat and dried at 60 °C for at least 72 h before weighing. To minimize the effect of weeds, care was taken to avoid dense patches of weed within plots for evaluation of dominant cover crop presence. After drying, shoot material was powderized using a Wiley mill (Thomas Scientific, Swedesboro, NJ, USA) and passed through a 2 mm sieve.
Root biomass was estimated from soil cores sampled from 3 to 5 replicate plots using a Giddings coring rig (Giddings Machine Company, Windsor, CO USA). Each soil core was 7.62 cm in diameter and approximately 1 m in depth. The total number of soil cores per cover crop treatment varied between 3 and 10 cores and are reported in Supplementary Table 4. Cores were also carefully sampled from regions where the cover crop treatment dominated to minimize the effect of weeds. Each soil core was split into 4 depths: 0–15, 15–30, 30–60, and 60–90 cm in the field. Soil core segments were washed by hand within 2 days from sampling, and roots from each segment were cleaned and separated from particulate organic matter. Washed roots were stored in plastic bags and kept at 4 °C until flatbed scanning.
Carbon and nitrogen concentration measurements were conducted separately on both root and shoot tissue subsampled from the dried biomass. To ensure enough root tissue was collected for root C and N measurements, samples were taken from shovel-excavated soil monoliths from the same representative 25.4 cm by 25.4 cm region mentioned above to an approximate depth of 15 cm. Soil monoliths were washed, and roots cleaned before drying. A quarter of the dried mat of networked roots was ground by shaking in centrifuge tubes containing stainless steel ball bearing to produce powdered root tissues samples. Carbon and nitrogen content for root and shoot samples were determined from the powderized samples using an elemental analyzer system (vario ISOTOPE cube, Elementar, Langenselbold, Germany).
Washed roots from the soil cores were imaged using an Epson Expression 12000XL flatbed scanner equipped with a transparency unit (Epson America Inc, Los Alamitos, CA, USA). Roots were added to a transparent acrylic tray (420 mm × 300 mm) filled with a 10 mm layer of deoinized water and spread-out using forceps to avoid overlap between roots. Images were taken using the Epson Scan 2 software (Epson America Inc, Los Alamitos, CA, USA) at a resolution of 600 dpi as a JPG file with 99% quality. Total root length was computed from each scanned root sample using RhizoVision Explorer v2.0.2 (Seethepalli et al. 2021) based on diameter thresholds (in mm) of > 0.3 and < 0.3, respectively. The diameter ranges were chosen based on tests with representative root scans from all species used in the study for first order and tertiary roots. In the software, the threshold level was set to 200, filter non-root objects set to 1 mm2, and root pruning threshold set to 20 pixels. After scanning, the root material was placed in a paper envelope and dried at 60 °C for 3 d before root dry weight measurements.
Statistical methods
Statistical analysis was done in Python using packages SciPy (Virtanen et al. 2020), Statsmodels (Seabold and Perktold 2010), Numpy (Harris et al. 2020), Pandas (McKinney 2010; The pandas development team 2023), and Seaborn (Waskom 2021). 95% confidence intervals were calculated and are reported for each plant measurement. In addition, differences in plant traits between the different cover crop treatments were evaluated using a one-way ANOVA. The Tukey HSD test was applied to determine statistical significance in post hoc pairwise comparisons.
Results
Winter hardy grasses provide greater canopy cover throughout the growing season compared to legumes and brassicas
Cereal rye and winter triticale were the most winter hardy and had the highest amount of canopy cover among the cover crop treatments (Fig. 1A). Figure 1A reports canopy cover percent from October 2021 to April 2022; canopy cover was calculated as percentage green pixels from cover crop and weed shoots captured in cropped canopy images. As shown in Fig. 1A, green canopy cover provided a facile method for monitoring plant growth and frost damage over time. All cover crop treatments showed rapid growth between planting October 1st, 2021 and November 10th, 2021 (Fig. 1A), which corresponds to a steep increase in cumulative growing degree days (GDD) in Fig. 1B. Grasses, including cereal rye, winter triticale, black oat, and winter wheat, had canopy coverage ranging from 70 to 85%. The crimson and red clovers showed more moderate growth with ~60% canopy cover. Canopy cover in the brassica treatments ranged from 65 to 90% with Siberian kale showing the greatest coverage.
Cover crop growth throughout the winter growing season. A Comparison of percent canopy cover over time for select treatments. Red, blue and green lines denote grass, legume, and brassica families, respectively. Linestyles are used to distinguish species and cultivars within each family. Canopy cover includes cover crop and weed growth; percent canopy cover is overestimated for cover crops with no or moderate winter survivability. B Plot overlaying cumulative growing degree days (green) and daily precipitation totals in inches (blue) over the growing season. C Representative canopy images for African Cabbage and Cereal Rye cv. Rymin at three timepoints spread throughout the season (11/10/2021, 02/15/2022, and 04/19/2022)
As daily minimum temperatures dropped below freezing, frost damage resulted in a decrease in percentage canopy cover from mid-November to mid-February. The steepest decline in canopy cover matches with the flat cumulative GDD curve observed during this winter period. The percentage canopy cover hit a minimum on February 15th, 2022, and a comparison of green canopy cover at this date provided a representative measure of relative susceptibility to frost damage (Fig. 1a, Supplemental Table 5). Cereal rye and winter triticale had the highest canopy cover though the foliage was noticeably less green as shown in Fig. 1C. All four clover cultivars, winter barley, and winter wheat showed significant frost damage and moderate winter hardiness. Many plants in the black oat cv. ‘Cosaque’, Siberian kale, rapeseed cv. ‘Trophy’, and turnip cv. ‘Purple Top’ treatments were winter-killed; however, some plants survived. African cabbage suffered significant frost damage, and all plants died as can be seen in Fig. 1C. The radishes, collard, persian clover, and kale cv. Bayou also winter killed.
After mid-February, the cumulative growing degree days curve increased as temperatures increased, and canopy cover also increased over the same time period. The transition from moderate regrowth to rapid spring growth as observed by percentage canopy cover is reflected in the increase in the slope of the cumulative GDD curve over the spring growing season. Winter hardy grasses, such as cereal rye and winter triticale, had the highest percent canopy cover. Less frost tolerant species showed significant spring regrowth; however, they did not reach the same percent canopy cover as observed in early November. For species that winter killed, the observed increase in canopy cover over the spring corresponded to weed growth within those plots as illustrated in Fig. 1C. The bottom left picture in Fig. 1C also showed the strong weed pressure in some parts of the field though weed pressure varied greatly. Weed growth over the growing season showed a similar trend of fall growth, winter die-back, and spring regrowth as shown in Supplemental Table 5.
Cover crop cultivars differ significantly in root system architecture
Winter hardy grasses produced the greatest shoot and root biomass among sampled treatments with some variation at the species and cultivar levels. Cover crop treatments with surviving plants and the most consistent stands were selected for further analysis by shoot and root biomass sampling (see Supplemental Table 3). In general, grasses had the highest shoot and root biomasses followed by the legumes and brassicas (Fig. 2A-B, Supplemental Table 6). Cereal rye produced the greatest shoot and root biomass by spring sample harvest (April 25th -27th, 2022) across all measured treatments. No significant difference in shoot or root biomass was observed between cereal rye cultivars (Elbon, Rymin, and Yankee). Winter barley had similar amounts of shoot biomass as cereal rye and winter triticale but generated significantly less root biomass. Consequently, winter barley had a significantly lower root:shoot ratio than some cereal rye and winter triticale cultivars (Fig. 2C, Supplemental Table 6). Winter triticale produced slightly less shoot biomass and similar amounts of root biomass. Winter wheat produced the least amount of shoot biomass among the grasses and moderate amounts of root biomass. Similar trends in shoot biomass, root biomass, and root:shoot ratio among the grasses (Supplemental Fig. 4) were seen in our previous field study in 2021 (Griffiths et al. 2022).
Cover crop shoot and root biomass in late spring. A Comparison of shoot biomass of some surviving cover crop treatments collected within representative 645.16 cm2 quadrat regions. Red, blue, and green bars denote the taxonomic families: grass, legume, and brassica, respectively. The brown outlined bar represents untreated/weed plots. Error bars indicate 95% confidence intervals. P-values from pairwise comparisons are reported in Supplemental Tables 7–9. B Comparison of total root biomass collected from 7.62 cm diameter by 1 m long soil cores that have been scaled to the same sampling area as the shoot samples. C Comparison of root to shoot ratios among sampled cover crop treatments
Within the legumes, hairy vetch had similar amounts of shoot biomass as cereal rye but significantly less root biomass than cereal rye cv. Rymin. In our 2021 study (Griffiths et al. 2022), hairy vetch was also found to produce much less root biomass than cereal rye (Supplemental Fig. 4). Among the clovers, crimson clover cv. Dixie had the most shoot biomass followed by red clover and crimson clover cv. Kentucky Pride, while red clover had the most root biomass followed by crimson clover cv. Kentucky Pride and crimson clover cv. Dixie. In Fig. 2C, the difference in biomass production between Kentucky Pride and Dixie is shown more clearly when comparing the root:shoot ratio. Kentucky Pride is a more recent crimson clover cultivar, and plant growth differences may result from breeding efforts. Siberian kale produced slightly more shoot biomass and more root biomass than rapeseed. Untreated or weed plots produced moderate amounts of shoot and root biomass highlighting the strong weed pressure present in the field when there is no cover crop for weed suppression.
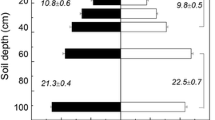
Root length density and specific root length varied significantly across cover crop treatments and may correlate to differences in root biomass among treatments at each soil depth. Root length density (RLD) measures root length per unit volume of soil and reports on the thoroughness of soil volume exploration by a root system (Freschet et al. 2021). On the other hand, specific root length (SRL) measures root length per dry root mass and can report on the investment in coarse versus fine roots. Thus, fine/thin roots have a high relative SRL while coarse/thick roots have a low relative SRL (Freschet et al. 2021). Root biomass samples collected from 1-meter soil cores were further separated by depth, and root traits were measured via flatbed scanning to assess differences in root system architecture across the sampled cover crop treatments. Figure 3 shows root biomass, root length density, and specific root length by soil depth for the top two species in each family for clarity; results for the other sampled cover crop treatments are included in Supplemental Information (Supplemental Figs. 5–6).
Cover crop rooting traits from select treatments at varying depths. A Comparison of total root biomass at 4 depths: 0–15, 15–30, 30–60, and 60–90 cm. Red, blue, and green bars represent grasses, legumes, and brassicas, respectively. Error bars indicate 95% confidence intervals. Mean values are shown next to some bars for clarity. P-values from pairwise comparisons are reported in Supplemental Tables 10–18. B Comparison of root length density. C Comparison of specific root length
Cereal rye and winter triticale have different root length density and specific root length values which correlate with their difference in root distribution. Cereal rye had the highest root biomass in the first 15 cm of soil with root biomass dropping off significantly below this depth likely due to a hard pan and changes in soil horizon (Fig. 3A, Supplemental Fig. 2). At depths below 15 cm, winter triticale had the greatest root biomass. Root length density and specific root length were higher for cereal rye cv. Rymin than winter triticale cv. SY TF 813 as shown in Fig. 3B-C. These differences suggest cereal rye invests in finer roots (Supplemental Fig. 7) to more thoroughly explore the soil volume. In contrast, winter triticale cv. SY TF 813 has more coarse roots as seen in Supplemental Fig. 8. In our 2021 study, winter triticale had similar RLD and SRL values as cereal rye (Supplemental Fig. 9) which likely is a result of the variety selected that season. Winter triticale cv. Montech has similar RLD and SRL values to cereal rye while winter triticale cv. SY TF 813 has values similar to winter wheat (Supplemental Figs. 5–6). This genotypic difference likely reflects the fact that winter triticale is a man-made cross between cereal rye and winter wheat.
Crimson clover and red clover had the next highest root biomass after the grasses within the top 15 cm of soil. There were no significant differences in root length density and specific root length suggesting similar root system architecture. Siberian kale and rapeseed had the lowest root biomass in the top 15 cm of soil. Rapeseed had a higher root length density and significantly higher specific root length than Siberian kale. Visual comparison of root scans suggest rapeseed forms more lateral roots and Siberian kale forms a thicker tap root (Supplemental Figs. 10–11). At depths below 15 cm, root biomass for clovers and brassicas decreased significantly with only a few short roots recovered at each soil depth as illustrated in Supplemental Figs. 10–13. Consequently, root length density dropped off significantly for all treatments below the hard pan at 15 cm though some relative trends between treatments continued at these lower soil depths. The specific root length of rapeseed decreased below 15 cm and was more similar to cereal rye. At the same time, the specific root length of Siberian kale increases when transitioning from 0 to 15 cm to 15–30 cm soil depths. This decrease likely corresponds to the lack of a thick storage tap root at depths below 15 cm as observed in root images (Supplemental Fig. 10). Significant root biomass was observed in the untreated or “weedy” field. The weeds had high root length density and specific root length consistent with thin, fibrous roots. Weed root biomass also drops off precipitously below 15 cm.
Winter hardy grasses generate the greatest amount of organic carbon and nitrogen
At the family level, the grasses generally had higher shoot and root C:N ratios than the legumes and brassicas consistent with values reported in the literature. Winter wheat had the highest shoot and root C:N ratios (31.0 ± 6.6 and 45.9 ± 3.4, respectively) across all sampled treatments (Fig. 4A-B). For cereal rye, winter barley, and winter triticale, shoot C:N ratios ranged from 23.1 to 29.0 while root C:N ratios ranged from 27.1 to 37.2. In all cases, the root C:N values were higher than the shoot C:N values for a given plant. Among the clovers, shoot C:N ratios ranged from 19.1 to 24.0, and root C:N ratios ranged from 24.4 to 34.6. These shoot and root C:N ratios for crimson clover and red clover are higher than values reported in other studies. Several factors may contribute to this difference. First, root nodulation and N fixation are sensitive to growing conditions such as soil pH (Roy et al. 2019; Streeter and Wong 1988); low soil pH may have resulted in reduced root nodulation and N uptake. Root nodules may also have been lost during the root washing process even though some root nodules were observed. Second, older roots from winter-killed plants within the plot may have begun to decay increasing the measured C:N ratio. Third, weed growth was observed within clover plots. Though care was taken to minimize sampling regions with heavy weed growth, some weeds were observed in shoot tissue samples. Weed plant tissue may raise the average C:N ratio in the sampled biomass. Siberian kale and rapeseed had similar shoot C:N ratios around 20.0, but rapeseed had a significantly higher root C:N ratio (28.9 ± 3.9) than Siberian kale (22.3 ± 0.7). Similarly, C:N values for the brassicas may be impacted by weeds present in the plots.
Carbon and nitrogen content analysis of shoot and root tissue in select cover crop treatments. A-B Comparison of shoot and root tissue C:N ratios. Error bars indicate 95% confidence intervals. P-values from pairwise comparisons are reported in Supplemental Tables 19–24. C-D Comparison of shoot and root carbon content. Values were calculated from the tissue carbon concentration multiplied by total shoot or root biomass as reported in Fig. 2. E-F Comparison of shoot and root nitrogen content. Values were calculated from the tissue nitrogen concentration multiplied by total shoot or root biomass as reported in Fig. 2
Winter hardy grasses contribute the largest amount of organic carbon and organic nitrogen in the form of shoot and root plant litter. The carbon concentration in shoot and root tissues for grass treatments were similar in value (Supplemental Fig. 14). In clovers and brassicas, the shoot C concentrations were also relatively similar in value between cultivars in each family. Clover shoot values were slightly lower compared to grass shoot C and brassica shoot C concentrations. Root C concentrations were relatively similar across all cultivars. As a result, the shoot C content and root C content were mostly driven by differences in shoot and root biomass. Cereal rye cultivars Elbon and Rymin produced significantly more shoot and root C content than the legumes and brassicas. Cereal rye cv. Elbon also produced the greatest shoot N content. Grasses generally produced greater shoot N content than legumes and brassicas as a result of their greater shoot biomass. Similarly, cereal rye and winter triticale root N content was significantly higher than crimson clover and rapeseed N content.
Discussion
Comparing shoot traits for soil erosion protection and weed suppression potential
The most winter hardy cover crop treatments may provide the greatest soil erosion protection and weed suppression due to their high canopy coverage and shoot biomass. The frequency of extreme precipitation events has increased since 1990 (Fischer and Knutti 2015; Li et al. 2021), and their continued increase will lead to significant soil erosion (Nearing et al. 2004). Rainfall, water infiltration, and water holding capacity greatly influence soil erosion. Cover crops can reduce sheet and interill erosion by acting as vegetative filters and reducing raindrop impact on soil surfaces (De Baets et al. 2011; Kaspar et al. 2001; Malik et al. 2000). Cover crops can also lower bulk density, create more extensive pore networks, and improve soil aggregation which increases soil water infiltration (Koudahe et al. 2022). Cereal rye and winter triticale exhibit rapid fall growth and maintain a high percent canopy cover throughout the growing season making it well-suited to mitigate soil erosion from heavy rainfall and strong wind events. Cereal rye and winter triticale also produce substantial shoot biomass to cover the soil and help prevent soil erosion upon cover crop termination and during cash crop establishment.
In addition to soil erosion protection, a high percent canopy cover can reduce light transmittance and soil temperature which can reduce weed seed germination and seedling emergence (Fernando and Shrestha 2023). Rapid fall cover crop growth may help to outcompete weeds for nutrients aiding in their suppression. Many of the brassicas exhibited rapid fall growth as well and had high canopy coverage comparable to the grasses. However, many of the brassicas winter-killed resulting in barren plots in early spring. Winter hardy grasses such as cereal rye and winter triticale may better suppress weeds throughout the growing season. Litter generated from cover crop shoot biomass can also act as a living mulch helping to suppress weeds during the cash crop growing season. A minimum of 5 Mg of shoot biomass/ha is needed to reduce weed biomass by 75% during the cash crop growing season (Nichols et al. 2020). All of the winter hardy grasses produced enough biomass to meet this threshold.
Canopy cover imaging provides a facile and quantitative method for assessing plant growth and frost tolerance throughout the growing season. Remote sensing technologies such as UAVs may enable breeders and researchers to easily assess and compare growth dynamics between cover crop treatments (Kümmerer et al. 2023; Trout et al. 2008). Predictive models of canopy cover from cumulative growing degree days may help to develop more comprehensive temporal models of cover crop impacts on soil erosion processes. However, GDD does not adequately account for frost damage as average daily temperatures below the base temperature are assumed to provide zero plant growth (McMaster and Wilhelm 1997). Further development of cumulative growing degree day models may also help to predict winter cover crop growth behavior in temperate climates.
Comparing root traits for carbon sequestration and soil structure remediation potential
Differences in root traits, such as the chemical composition of roots, rooting distribution, and rooting depth, can influence a plant’s capacity to sequester carbon and remediate soil structure (Bacq-Labreuil et al. 2019; Bodner et al. 2014; Hudek et al. 2022). However, more research is needed to characterize cover crop root dynamics such as those of cereal rye and winter triticale and their impact on soil organic carbon (SOC) stocks. In this study, the cover crop biomass partitioned to the root system ranged from 13 to 43% across the 13 cover crop cultivars. Yet, root biomass is rarely included in estimations of cover crop soil carbon and nitrogen contributions in cover crop studies. Cereal rye and winter triticale produced the greatest amount of root biomass and largest pool of plant carbon at all soil depths up to 1 m. As root biomass decays, CO2 gas is released into the atmosphere; plots with cover crops have been shown to release more CO2 than bare soil (Nilahyane et al. 2020). Biomass with a high C:N ratio decays more slowly (Jahanzad et al. 2016), which could increase particulate matter in the soil and decrease the rate of CO2 production from soil respiration. At deep soil depths, microbial activity is reduced (Thorup-Kristensen et al. 2003; White et al. 2017), which may further reduce rates of soil respiration. By this logic, grasses with high C:N ratios may be favorable. However, biomass with high C:N ratios can also reduce soil nitrogen availability during cash crop planting and decrease cash crop yields (Nevins et al. 2020; Preza-Fontes et al. 2022; Qin et al. 2021). A higher starter N application could help mitigate cash crop yield losses (Preza-Fontes et al. 2022). The timing of cover crop termination and cash crop planting can also help to shift cover crop nitrogen release dynamics relative to corn nitrogen demand (Nevins et al. 2020; Qin et al. 2021; Rosa et al. 2021). Future studies could incorporate both root and shoot analysis to further resolve the timing of nitrogen release from various cover crop species in relation to cash crop development and edaphic factors such as soil moisture, soil temperature, and microbial activity.
While cereal rye and winter triticale produced similar amounts of root biomass, their root length density and specific root length values differed significantly resulting in divergent root distributions. Cereal rye had a high root length density and specific root length corresponding to investment into fine roots rather than coarse roots. In contrast, winter triticale had a low root length density and lower specific root length indicating investment into thicker or more coarse roots. These differences in root thickness can be observed in the root scans shown in Supplemental Figs. 8–9. Fine roots may not easily penetrate compacted soil resulting in less root biomass at lower depths (Materechera et al. 1992). As a result, the thicker roots from winter triticale may penetrate deeper into the soil contributing to the difference in root distribution between winter triticale and cereal rye. We note that differences in root distribution by depth may also result from differences in root system architecture. Fine roots have also been shown to decompose significantly faster than coarse roots potentially due to their relatively larger surface area (Zhang and Wang 2015). As demonstrated in a recent switchgrass study, cultivars with higher proportions of fine roots accumulated more soil carbon (Adkins et al. 2016).
Rooting behavior of cereal rye and winter triticale potentially make them efficient at remediating soil structure as well. Though the thick tap roots of brassicas are thought to be ideal for reducing soil compaction (Chen et al. 2014; Williams and Weil 2004), Siberian kale had a low root length density value suggesting it does not invest heavily in soil exploration. Cereal rye invested more in soil exploration as seen in the significantly higher root length density. The greater soil exploration produces a larger area of impact and potentially creates a larger pore network which could improve soil water infiltration. In contrast, winter triticale did not explore the soil as efficiently but did exhibit deeper rooting. Thus, winter triticale may be better suited to creating pore networks deeper in the soil than other treatments and may allow the following cash crop to grow deeper roots along these pores (Zhou et al. 2020). Data suggests coarse roots increase macropore formation while fine roots influence micropore formation (Bodner et al. 2014). Thus, cereal rye will likely form more micropores than winter triticale. The resulting pore size distributions would differ, which could result in different hydraulic conductivities (Aravena et al. 2011; Larsbo et al. 2014). Consideration of root traits during cover crop selection will likely enhance soil carbon sequestration and soil structure remediation efforts and highlights the potential for root trait breeding to enhance these ecosystem services.
Comparing root traits for excess nutrient capture and release
Rapeseed may be more ideal for excess nutrient capture in the fall and nutrient release upon termination in the spring when compared to other treatments in our study. Cover crops can significantly decrease N leaching without significantly increasing direct N2O emissions resulting in a lower net greenhouse gas balance (Abdalla et al. 2019). Root traits such as root length density, root thickness, and rooting depth could impact nutrient capture in the fall and nutrient release in the spring. Higher root length density in nutrient rich patches is thought to increase N capture in invasive grasses (James et al. 2010). Deeper rooting is a key component of the hypothetical ideotype ‘steep, cheap, and deep’ to improve N acquisition (Lynch 2013). Rapeseed had a moderate root length density and the highest specific root length, which suggests it forms many thin lateral roots as seen in Supplemental Fig. 11. The large number of fine lateral roots may point to its ability to efficiently scavenge and mineralize nutrients in the soil. In addition, the shoot and root C:N ratios of rapeseed are 20.9 ± 1.6 and 28.9 ± 3.9, respectively, which is close to the ideal C:N ratio of 24:1 for controlled nutrient release and uptake by the following cash crop (Hunter et al. 2019; Ibewiro et al. 2000). Rapeseed, like many of the brassicas, are not as winter hardy as the grasses resulting in lower shoot and root biomass, although this relationship could vary across latitudinal clines. In our study, some plants in the rapeseed treatment plots may not have reached the optimal rosette size before first frost and were winter-killed which may release N too early before cash crop planting. Timing of rapeseed planting before first frost influences winter survivability (Page et al. 2021), and an increase in winterkilled plants would result in an early partial release of nutrients. (Page et al. 2021)
Cereal rye produces a higher amount of root biomass especially at deeper depths and efficiently explores the soil through its network of fine roots. However, its high C:N ratio may impede plant litter degradation and nutrient release (Grünwald et al. 2000; Hunter et al. 2019). In addition, microbes involved in the decomposition process may readily seek starter nitrogen and may increase the amount of fertilizer required at cash crop planting (Adeli et al. 2019; Deines et al. 2023; Preza-Fontes et al. 2022; Qin et al. 2021). This impact on soil nitrogen may be reduced for legume cash crops such as soybean. In fact, yield impacts from cereal rye on soybeans seem to be minimal (Adeli et al. 2019; Hunter et al. 2019; Qin et al. 2021). Therefore, breeding efforts for cover crops selected for nutrient recycling should account for root system architecture, root and shoot C:N ratios, and winter hardiness.
Assessing the potential for root-centered cover crop breeding and selection
Overall, differences in root traits at the cultivar and species levels within grasses and brassicas highlight the potential for root system architecture breeding of cover crops to enhance ecosystem services. Cereal rye cultivars did not show a significant difference in shoot and root traits in the environment tested. However, we observe differences in shoot and root growth in winter triticale. Elite cultivars are already highly productive, which may limit the potential to further improve biomass-related plant traits (Finney et al. 2016). The introduction of favorable alleles for genes known to control rooting depth in agronomic environments, such as Deeper Rooting 1 (DRO1) and Enhanced Gravitropism 1 (EGT1) (Fusi et al. 2022; Uga et al. 2013), may provide a route to generating deeper-rooted varieties and/or alter root:shoot ratios. Winter triticale is a man-made hybrid between cereal rye and winter wheat, which have distinct root system architectures. Further breeding of winter triticale may provide a quicker route towards manipulating root traits while preserving winter hardiness. In the Brassicaceae family, Brassica oleracea and Brassica napus have high morphological diversity (Soengas et al. 2008; Thomas et al. 2016; Yu et al. 2013). For example, Siberian kale and rapeseed trophy are distinct cultivars of Brassica napus and exhibit distinct root phenotypes. Rapeseed trophy produced much more fine lateral roots compared to Siberian kale which produced a thicker storage taproot. The morphological diversity in brassicas may facilitate breeding efforts to enhance specific ecosystem services in brassicas. Winter barley produces comparable quantities of shoot biomass to cereal rye. However, winter barley produced significantly lower root biomass highlighting the need to incorporate belowground plant traits in future breeding. Surprisingly, the lower frost tolerance of winter triticale cv. SY TF 81 compared to winter triticale Montech Forage FX 1001 did not result in significantly lower root biomass in our study. However, crimson clover cv. Kentucky Pride produced more root biomass than crimson clover cv. Dixie, which is known to have a lower winter survival rate (Herget 2020). Therefore, improving winter hardiness in some species may provide enhancements to root biomass and root-related ecosystem services. As we experience more extreme cold due to climate change (Cohen et al. 2021), winter hardiness may play an increased role in winter cover crop selection and breeding to improve ecosystem services (Poffenbarger et al. 2023).
Conclusion
Cover crops have the potential to sequester carbon, capture excess nutrients, and improve soil structure, which can help current agricultural systems adapt to climate change impacts and help mitigate greenhouse gas emissions that contribute to climate change. Despite their role in the ability for a cover crop to perform a given ecosystem service, our understanding of cover crop root system architecture is limited. In our study, we surveyed several cover crop cultivars across multiple species and families to characterize their rooting behavior. Our results provide a broad assessment of cover crop performance in Missouri and within the USDA hardiness zone 6a. We also observe significant differences in cover crop root traits at the cultivar and species levels which may impact their root distribution within the soil. These differences in rooting behavior may subsequently affect a cover crop’s potential to perform particular ecosystem services such as carbon sequestration and nutrient capture. Future studies that measure how root system architecture influences soil structure, soil microbiomes, and soil organic matter pools over several years will be critical. We also demonstrate that simple green canopy cover measurements can capture plant productivity over the growing season and provide a quantitative comparison of frost tolerance. Cumulative growing degree days are shown to follow plant growth behavior throughout the growing season and has potential as a predictive model of plant growth stage in cover crops. Finally, root trait differences among cover crops in our study highlight the potential for future cover crop breeding efforts that engineer root system architecture to enhance ecosystem services. Elite varieties with optimized root systems may help reduce the use of chemical fertilizers, reduce crop loss due to extreme weather events, and improve crop yield while sequestering carbon and reducing greenhouse gas emissions. Such improvements could improve the economics of incorporating cover crops into commercial agriculture and increase their adoption.
Data availability
The datasets supporting the results of this study are available online at https://doi.org/10.17605/OSF.IO/8DGF7.
References
Abdalla M, Hastings A, Cheng K, Yue Q, Chadwick D, Espenberg M, Truu J, Rees RM, Smith P (2019) A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob Change Biol 25:2530–2543. https://doi.org/10.1111/gcb.14644
Adeli A, Brooks JP, Read JJ, Feng G, Miles D, Shankle MW, Jenkins JN (2019) Corn and soybean grain yield responses to soil amendments and cover crop in upland soils. J Plant Nutr 42:2484–2497. https://doi.org/10.1080/01904167.2019.1655046
Adkins J, Jastrow JD, Morris GP, Six J, de Graaff M-A (2016) Effects of switchgrass cultivars and intraspecific differences in root structure on soil carbon inputs and accumulation. Geoderma 262:147–154. https://doi.org/10.1016/j.geoderma.2015.08.019
Amsili JP, Kaye JP (2021) Root traits of cover crops and carbon inputs in an organic grain rotation. Renew Agric Food Syst 36:182–191. https://doi.org/10.1017/S1742170520000216
Aravena JE, Berli M, Ghezzehei TA, Tyler SW (2011) Effects of root-induced compaction on rhizosphere hydraulic properties - X-ray microtomography imaging and numerical simulations. Environ Sci Technol 45:425–431. https://doi.org/10.1021/es102566j
Bacq-Labreuil A, Crawford J, Mooney SJ, Neal AL, Ritz K (2019) Cover crop species have contrasting influence upon soil structural genesis and microbial community phenotype. Sci Rep 9:7473. https://doi.org/10.1038/s41598-019-43937-6
Bagnall DK, Rieke EL, Morgan CLS, Liptzin DL, Cappellazzi SB, Honeycutt CW (2023) A minimum suite of soil health indicators for north American agriculture. Soil Secur 10:100084. https://doi.org/10.1016/j.soisec.2023.100084
Bodner G, Leitner D, Kaul HP (2014) Coarse and fine root plants affect pore size distributions differently. Plant Soil 380:133–151. https://doi.org/10.1007/s11104-014-2079-8
Bodner G, Loiskandl W, Hartl W, Erhart E, Sobotik M (2019) Characterization of cover crop rooting types from integration of rhizobox imaging and root atlas information. Plants 8:514
Brennan EB, Leap JE (2014) A comparison of drill and broadcast methods for establishing cover crops on beds. HortScience Horts 49:441–447. https://doi.org/10.21273/hortsci.49.4.441
Canisares LP, Banet T, Rinehart B, McNear D, Poffenbarger H (2023) Litter quality and living roots affected the formation of new mineral-associated organic carbon but did not affect total mineral-associated organic carbon in a short-term incubation. Geoderma 430:116302. https://doi.org/10.1016/j.geoderma.2022.116302
Chen G, Weil RR, Hill RL (2014) Effects of compaction and cover crops on soil least limiting water range and air permeability. Soil Tillage Res 136:61–69. https://doi.org/10.1016/j.still.2013.09.004
Cohen J, Agel L, Barlow M, Garfinkel CI, White I (2021) Linking arctic variability and change with extreme winter weather in the United States. Science 373:1116–1121. https://doi.org/10.1126/science.abi9167
CTIC (2016) Annual Report 2015–2016 Cover Crop Survey. Conversation Technology Information Center, the North Central Region Sustainable Agriculture Research and Education Program, and the American Seet Trade Association, West Lafayette, IN. https://acsess.onlinelibrary.wiley.com/doi/full/10.2136/sssaj2017.05.0139?casa_token=xC85FeBtRfIAAAAA%3Ajz5yfNt5USwCqBixXRWKNTb4D0mE-5yXPGQx3dGu2sB_CkRpehIZMNvqNel-5FumVU-rjG0A41TgLg and https://www.cambridge.org/core/journals/weed-science/article/abs/are-cover-crop-mixtures-better-at-suppressingweeds-than-cover-crop-monocultures/5C2C825277C04C7CE629A21AEC2DEFCC
De Baets S, Poesen J, Meersmans J, Serlet L (2011) Cover crops and their erosion-reducing effects during concentrated flow erosion. CATENA 85:237–244. https://doi.org/10.1016/j.catena.2011.01.009
Deines JM, Guan K, Lopez B, Zhou Q, White CS, Wang S, Lobell DB (2023) Recent cover crop adoption is associated with small maize and soybean yield losses in the United States. Glob Change Biol 29:794–807. https://doi.org/10.1111/gcb.16489
Dutta TK, Khan MR, Phani V (2019) Plant-parasitic nematode management via biofumigation using brassica and non-brassica plants: current status and future prospects. Curr Plant Biology 17:17–32. https://doi.org/10.1016/j.cpb.2019.02.001
Fernando M, Shrestha A (2023) The potential of cover crops for weed management: a sole tool or component of an integrated weed management system? Plants 12:752
Finney DM, White CM, Kaye JP (2016) Biomass Production and Carbon/Nitrogen Ratio Influence Ecosystem Services from Cover Crop mixtures. Agron J 108:39–52. https://doi.org/10.2134/agronj15.0182
Fischer EM, Knutti R (2015) Anthropogenic contribution to global occurrence of heavy-precipitation and high-temperature extremes. Nat Clim Change 5:560–564. https://doi.org/10.1038/nclimate2617
Freschet GT, Pagès L, Iversen CM, Comas LH, Rewald B, Roumet C, Klimešová J, Zadworny M, Poorter H, Postma JA, Adams TS, Bagniewska-Zadworna A, Bengough AG, Blancaflor EB, Brunner I, Cornelissen JHC, Garnier E, Gessler A, Hobbie SE, Meier IC, Mommer L, Picon-Cochard C, Rose L, Ryser P, Scherer-Lorenzen M, Soudzilovskaia NA, Stokes A, Sun T, Valverde-Barrantes OJ, Weemstra M, Weigelt A, Wurzburger N, York LM, Batterman SA, Gomes de Moraes M, Janeček Š, Lambers H, Salmon V, Tharayil N, McCormack ML (2021) A starting guide to root ecology: strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol 232:973–1122. https://doi.org/10.1111/nph.17572
Fusi R, Rosignoli S, Lou H, Sangiorgi G, Bovina R, Pattem JK, Borkar AN, Lombardi M, Forestan C, Milner SG, Davis JL, Lale A, Kirschner GK, Swarup R, Tassinari A, Pandey BK, York LM, Atkinson BS, Sturrock CJ, Mooney SJ, Hochholdinger F, Tucker MR, Himmelbach A, Stein N, Mascher M, Nagel KA, De Gara L, Simmonds J, Uauy C, Tuberosa R, Lynch JP, Yakubov GE, Bennett MJ, Bhosale R, Salvi S (2022) Root angle is controlled by EGT1 in cereal crops employing an antigravitropic mechanism. Proc Natl Acad Sci 119:e2201350119. https://doi.org/10.1073/pnas.2201350119
Gentry LE, Snapp SS, Price RF, Gentry LF (2013) Apparent red clover nitrogen credit to corn: evaluating cover crop introduction. Agron J 105:1658–1664. https://doi.org/10.2134/agronj2013.0089
Gomiero T, Pimentel D, Paoletti MG (2011) Environmental impact of different agricultural management practices: conventional vs. organic agriculture. CRC Crit Rev Plant Sci 30:95–124. https://doi.org/10.1080/07352689.2011.554355
Griffiths M, Delory BM, Jawahir V, Wong KM, Bagnall GC, Dowd TG, Nusinow DA, Miller AJ, Topp CN (2022) Optimisation of root traits to provide enhanced ecosystem services in agricultural systems: a focus on cover crops. Plant Cell Environ 45:751–770. https://doi.org/10.1111/pce.14247
Griffiths M, Topp CN (2021) Images, data, and statistical analysis scripts for review article on cover crop roots. https://doi.org/10.5281/zenodo.5039308
Grünwald NJ, Hu S, van Bruggen AHC (2000) Short-term cover crop decomposition in organic and conventional soils: characterization of soil C, N, microbial and plant pathogen dynamics. Eur J Plant Pathol 106:37–50. https://doi.org/10.1023/A:1008720731062
Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, Wieser E, Taylor J, Berg S, Smith NJ, Kern R, Picus M, Hoyer S, van Kerkwijk MH, Brett M, Haldane A, del Río JF, Wiebe M, Peterson P, Gérard-Marchant P, Sheppard K, Reddy T, Weckesser W, Abbasi H, Gohlke C, Oliphant TE (2020) Array programming with NumPy. Nature 585:357–362. https://doi.org/10.1038/s41586-020-2649-2
Haruna SI, Anderson SH, Udawatta RP, Gantzer CJ, Phillips NC, Cui S, Gao Y (2020) Improving soil physical properties through the use of cover crops: a review. Agrosyst Geosci Environ 3:e20105. https://doi.org/10.1002/agg2.20105
Herget M (2020) Evaluation of Selected Cool Season Cover Crop Varieties in Northeast Missouri. USDA Natural Resources Conservation Service and/or Elsberry Plant Materials Center, Elsberry, MO. https://www.nrcs.usda.gov/plantmaterials/mopmcsr13641.pdf
Hudek C, Putinica C, Otten W, De Baets S (2022) Functional root trait-based classification of cover crops to improve soil physical properties. Eur J Soil Sci 73:e13147. https://doi.org/10.1111/ejss.13147
Hunter MC, Schipanski ME, Burgess MH, LaChance JC, Bradley BA, Barbercheck ME, Kaye JP, Mortensen DA (2019) Cover crop mixture effects on maize, soybean, and wheat yield in rotation. Agric Environ Lett 4:180051. https://doi.org/10.2134/ael2018.10.0051
Ibewiro B, Sanginga N, Vanlauwe B, Merckx R (2000) Nitrogen contributions from decomposing cover crop residues to maize in a tropical derived savanna. Nutr Cycl Agrosyst 57:131–140. https://doi.org/10.1023/A:1009846203062
Jahanzad E, Barker AV, Hashemi M, Eaton T, Sadeghpour A, Weis SA (2016) Nitrogen release dynamics and decomposition of buried and surface cover crop residues. Agron J 108:1735–1741. https://doi.org/10.2134/agronj2016.01.0001
James JJ, Ziegenhagen L, Aanderud ZT (2010) Exploitation of nutrient-rich soil patches by invasive annual and native perennial grasses. Invasive Plant Science and Management 3:169–177. https://doi.org/10.1614/IPSM-D-09-00033.1
Kaspar TC, Radke JK, Laflen JM (2001) Small grain cover crops and wheel traffic effects on infiltration, runoff, and erosion. J Soil Water Conserv 56:160–164
Kaspar TC, Singer JW (2011) The Use of Cover Crops to Manage Soil. In: Hatfield JL, Sauer TJ (eds) Soil Management: Building a Stable Base for Agriculture. https://doi.org/10.2136/2011.soilmanagement.c21
Kemper R, Bublitz TA, Müller P, Kautz T, Döring TF, Athmann M (2020) Vertical root distribution of different cover crops determined with the profile wall method. Agriculture 10:503
Kirkegaard JA, Sarwar M (1998) Biofumigation potential of brassicas. Plant Soil 201:71–89. https://doi.org/10.1023/A:1004364713152
Koudahe K, Allen SC, Djaman K (2022) Critical review of the impact of cover crops on soil properties. Int Soil Water Conserv Res 10:343–354. https://doi.org/10.1016/j.iswcr.2022.03.003
Kümmerer R, Noack PO, Bauer B (2023) Using high-resolution UAV imaging to measure canopy height of diverse cover crops and predict biomass. Remote Sens 15:1520
Lal R (2004) Carbon emission from farm operations. Environ Int 30:981–990. https://doi.org/10.1016/j.envint.2004.03.005
Lal R (2015) Soil carbon sequestration and aggregation by cover cropping. J Soil Water Conserv 70:329–339. https://doi.org/10.2489/jswc.70.6.329
Larsbo M, Koestel J, Jarvis N (2014) Relations between macropore network characteristics and the degree of preferential solute transport. Hydrol Earth Syst Sci 18:5255–5269. https://doi.org/10.5194/hess-18-5255-2014
Li C, Zwiers F, Zhang X, Li G, Sun Y, Wehner M (2021) Changes in annual extremes of daily temperature and precipitation in CMIP6 models. J Clim 34:3441–3460. https://doi.org/10.1175/jcli-d-19-1013.1
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Botany 112:347–357. https://doi.org/10.1093/aob/mcs293
Malik RK, Green TH, Brown GF, Mays D (2000) Use of cover crops in short rotation hardwood plantations to control erosion. Biomass Bioenergy 18:479–487. https://doi.org/10.1016/S0961-9534(00)00016-7
Materechera SA, Alston AM, Kirby JM, Dexter AR (1992) Influence of root diameter on the penetration of seminal roots into a compacted subsoil. Plant Soil 144:297–303. https://doi.org/10.1007/BF00012888
McKinney W (2010) Data structures for statistical computing in python. In: van der Walt S, Millman J (eds) Proceedings of the 9th Python in Science Conference, pp 56–61. https://doi.org/10.25080/Majora-92bf1922-00a
McMaster GS, Wilhelm WW (1997) Growing degree-days: one equation, two interpretations. Agric for Meteorol 87:291–300. https://doi.org/10.1016/S0168-1923(97)00027-0
Nearing M, Pruski FF, O’Neal MR (2004) Expected climate change impacts on soil erosion rates: a review. J Soil Water Conserv 59:43–50
Nevins CJ, Lacey C, Armstrong S (2020) The synchrony of cover crop decomposition, enzyme activity, and nitrogen availability in a corn agroecosystem in the Midwest United States. Soil Tillage Res 197:104518. https://doi.org/10.1016/j.still.2019.104518
Nichols V, Martinez-Feria R, Weisberger D, Carlson S, Basso B, Basche A (2020) Cover crops and weed suppression in the U.S. Midwest: a meta-analysis and modeling study. Agric Environ Lett 5:e20022. https://doi.org/10.1002/ael2.20022
Nilahyane A, Ghimire R, Thapa VR, Sainju UM (2020) Cover crop effects on soil carbon dioxide emissions in a semiarid cropping system. Agrosyst Geosci Environ 3:e20012. https://doi.org/10.1002/agg2.20012
Nissan A, Alcolombri U, Peleg N, Galili N, Jimenez-Martinez J, Molnar P, Holzner M (2023) Global warming accelerates soil heterotrophic respiration. Nat Commun 14:3452. https://doi.org/10.1038/s41467-023-38981-w
Olson KR, Al-Kaisi M, Lal R, Cihacek L (2016) Impact of soil erosion on soil organic carbon stocks. J Soil Water Conserv 71:61A-67A. https://doi.org/10.2489/jswc.71.3.61A
Page ER, Meloche S, Moran M, Caldbeck B, Barthet V (2021) Effect of seeding date on winter canola (Brassica napus L.) yield and oil quality in southern Ontario. Can J Plant Sci 101:490–499. https://doi.org/10.1139/cjps-2020-0220
Park S, Croteau P, Boering KA, Etheridge DM, Ferretti D, Fraser PJ, Kim KR, Krummel PB, Langenfelds RL, van Ommen TD, Steele LP, Trudinger CM (2012) Trends and seasonal cycles in the isotopic composition of nitrous oxide since 1940. Nat Geosci 5:261–265. https://doi.org/10.1038/ngeo1421
Paustian K, Lehmann J, Ogle S, Reay D, Robertson GP, Smith P (2016) Climate-smart soils. Nature 532:49–57. https://doi.org/10.1038/nature17174
Poeplau C, Don A (2015) Carbon sequestration in agricultural soils via cultivation of cover crops – A meta-analysis. Agric Ecosyst Environ 200:33–41. https://doi.org/10.1016/j.agee.2014.10.024
Poffenbarger H, Castellano M, Egli D, Jaconi A, Moore V (2023) Contributions of plant breeding to soil carbon storage: retrospect and prospects. Crop Sci 63:990–1018. https://doi.org/10.1002/csc2.20920
Preza-Fontes G, Miller H, Camberato J, Roth R, Armstrong S (2022) Corn yield response to starter nitrogen rates following a cereal rye cover crop. Crop Forage Turfgrass Manag 8:e20187. https://doi.org/10.1002/cft2.20187
Qin Z, Guan K, Zhou W, Peng B, Villamil MB, Jin Z, Tang J, Grant R, Gentry L, Margenot AJ, Bollero G, Li Z (2021) Assessing the impacts of cover crops on maize and soybean yield in the U.S. midwestern agroecosystems. Field Crop Res 273:108264. https://doi.org/10.1016/j.fcr.2021.108264
Rosa AT, Creech CF, Elmore RW, Rudnick DR, Lindquist JL, Fudolig M, Butts L, Werle R (2021) Implications of cover crop planting and termination timing on rainfed maize production in semi-arid cropping systems. Field Crop Res 271:108251. https://doi.org/10.1016/j.fcr.2021.108251
Roy S, Liu W, Nandety RS, Crook A, Mysore KS, Pislariu CI, Frugoli J, Dickstein R, Udvardi MK (2019) Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 32:15–41. https://doi.org/10.1105/tpc.19.00279
Ruark MD, Chawner MM, Ballweg MJ, Proost RT, Arriaga FJ, Stute JK (2018) Does cover crop radish supply nitrogen to corn? Agron J 110:1513–1522. https://doi.org/10.2134/agronj2017.06.0352
Sainju UM, Whitehead WF, Singh BP (2003) Cover crops and nitrogen fertilization effects on soil aggregation and carbon and nitrogen pools. Can J Soil Sci 83:155–165. https://doi.org/10.4141/s02-056
Sainju UM, Whitehead WF, Singh BP (2005) Biculture legume–cereal cover crops for enhanced biomass yield and carbon and nitrogen. Agron J 97:1403–1412. https://doi.org/10.2134/agronj2004.0274
Seabold S, Perktold J (2010) Statsmodels: Econometric and statistical modeling with python. In: 9th Python in Science Conference. https://www.statsmodels.org/v0.10.2/
Seethepalli A, Dhakal K, Griffiths M, Guo H, Freschet GT, York LM (2021) RhizoVision Explorer: open-source software for root image analysis and measurement standardization. AoB PLANTS 13:plab056. https://doi.org/10.1093/aobpla/plab056
Soengas P, Cartea ME, Velasco P, Padilla G, Ordás A (2008) Morphologic and agronomic diversity of Brassica napus crops. J Am Soc Hortic Sci J Amer Soc Hort Sci 133:48–54. https://doi.org/10.21273/JASHS.133.1.48
Streeter J, Wong PP (1988) Inhibition of legume nodule formation and N2 fixation by nitrate. CRC Crit Rev Plant Sci 7:1–23. https://doi.org/10.1080/07352688809382257
Thaler EA, Larsen IJ, Yu Q (2021) The extent of soil loss across the US Corn Belt. Proc Natl Acad Sci 118:e1922375118. https://doi.org/10.1073/pnas.1922375118
The pandas development team (2023) pandas-dev/pandas: Pandas (v2.1.3). https://doi.org/10.5281/zenodo.10107975
Thomas CL, Alcock TD, Graham NS, Hayden R, Matterson S, Wilson L, Young SD, Dupuy LX, White PJ, Hammond JP, Danku JMC, Salt DE, Sweeney A, Bancroft I, Broadley MR (2016) Root morphology and seed and leaf ionomic traits in a Brassica napus L. diversity panel show wide phenotypic variation and are characteristic of crop habit. BMC Plant Biol 16:214. https://doi.org/10.1186/s12870-016-0902-5
Thorup-Kristensen K, Magid J, Jensen LS (2003) Catch crops and green manures as biological tools in nitrogen management in temperate zones. Advances in Agronomy. Academic
Trout TJ, Johnson LF, Gartung J (2008) Remote sensing of canopy cover in horticultural crops. HortScience Horts 43:333–337. https://doi.org/10.21273/hortsci.43.2.333
Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, Inoue H, Takehisa H, Motoyama R, Nagamura Y, Wu J, Matsumoto T, Takai T, Okuno K, Yano M (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45:1097–1102. https://doi.org/10.1038/ng.2725
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J, van der Walt SJ, Brett M, Wilson J, Millman KJ, Mayorov N, Nelson ARJ, Jones E, Kern R, Larson E, Carey CJ, Polat İ, Feng Y, Moore EW, VanderPlas J, Laxalde D, Perktold J, Cimrman R, Henriksen I, Quintero EA, Harris CR, Archibald AM, Ribeiro AH, Pedregosa F, van Mulbregt P, Vijaykumar A, Bardelli AP, Rothberg A, Hilboll A, Kloeckner A, Scopatz A, Lee A, Rokem A, Woods CN, Fulton C, Masson C, Häggström C, Fitzgerald C, Nicholson DA, Hagen DR, Pasechnik DV, Olivetti E, Martin E, Wieser E, Silva F, Lenders F, Wilhelm F, Young G, Price GA, Ingold G-L, Allen GE, Lee GR, Audren H, Probst I, Dietrich JP, Silterra J, Webber JT, Slavič J, Nothman J, Buchner J, Kulick J, Schönberger JL, de Cardoso M, Reimer JV, Harrington J, Rodríguez J, Nunez-Iglesias JLC, Kuczynski J, Tritz J, Thoma K, Newville M, Kümmerer M, Bolingbroke M, Tartre M, Pak M, Smith M, Nowaczyk NJ, Shebanov N, Pavlyk N, Brodtkorb O, Lee PA, McGibbon P, Feldbauer RT, Lewis R, Tygier S, Sievert S, Vigna S, Peterson S, More S, Pudlik S, Oshima T, Pingel T, Robitaille TJ, Spura TP, Jones T, Cera TR, Leslie T, Zito T, Krauss T, Upadhyay T, Halchenko U, Vázquez-Baeza YO, SciPy Y (2020) SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods 17:261–272. https://doi.org/10.1038/s41592-019-0686-2
von Haden AC, Marín-Spiotta E, Jackson RD, Kucharik CJ (2019) Soil microclimates influence annual carbon loss via heterotrophic soil respiration in maize and switchgrass bioenergy cropping systems. Agric For Meteorol 279:107731. https://doi.org/10.1016/j.agrformet.2019.107731
Wallander S, Smith D, Bowman M, Claassen R (2021) Cover Crop trends, programs, and practices in the United States. USDA, Economic Research Service. https://doi.org/10.22004/ag.econ.309562
Waskom ML (2021) Seaborn: statistical data visualization. J Open Source Softw 6:3021
White CM, DuPont ST, Hautau M, Hartman D, Finney DM, Bradley B, LaChance JC, Kaye JP (2017) Managing the trade off between nitrogen supply and retention with cover crop mixtures. Agric Ecosyst Environ 237:121–133. https://doi.org/10.1016/j.agee.2016.12.016
Williams SM, Weil RR (2004) Crop cover root channels may alleviate soil compaction effects on soybean crop. Soil Sci Soc Am J 68:1403–1409. https://doi.org/10.2136/sssaj2004.1403
Yang T, Siddique KHM, Liu K (2020) Cropping systems in agriculture and their impact on soil health-A review. Glob Ecol Conserv 23:e01118. https://doi.org/10.1016/j.gecco.2020.e01118
Yu J, Zhao M, Wang X, Tong C, Huang S, Tehrim S, Liu Y, Hua W, Liu S (2013) Bolbase: a comprehensive genomics database for Brassica oleracea. BMC Genomics 14:664. https://doi.org/10.1186/1471-2164-14-664
Zhang X, Wang W (2015) The decomposition of fine and coarse roots: their global patterns and controlling factors. Sci Rep 5:9940. https://doi.org/10.1038/srep09940
Zhou H, Whalley WR, Hawkesford MJ, Ashton RW, Atkinson B, Atkinson JA, Sturrock CJ, Bennett MJ, Mooney SJ (2020) The interaction between wheat roots and soil pores in structured field soil. J Exp Bot 72:747–756. https://doi.org/10.1093/jxb/eraa475
Acknowledgements
The authors would like to thank Elisa Morales, Dhinesh Thiruppathi, Clara Lebow, Nida Mutan, Michelle Cho, August Thies, Sumeet Mankar, Cody Bagnall, and Keith Duncan for sample collection and processing. We would also like to thank Rob Meyers and Green Cover for providing seeds used in this study. We also thank Nelson Curran and Eli Isele for their help planting the cover crops, collecting samples, and terminating the plants at the DDPSC Field Research Site.
Funding
This research was funded by the Subterranean Influences on Nitrogen and Carbon Cycling Center at the Donald Danforth Plant Science Center.
Author information
Authors and Affiliations
Contributions
KMW, MG, and CNT conceptualized and designed the experiment. KMW, MG, AEL, AJ, AM, and MAS collected and processed samples. KMW and MG performed the analysis. KMW drafted the manuscript and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Responsible Editor: Yinglong Chen.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 6.68 MB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wong, K.M., Griffiths, M., Moran, A. et al. Cover crop cultivars and species differ in root traits potentially impacting their selection for ecosystem services. Plant Soil 500, 279–296 (2024). https://doi.org/10.1007/s11104-023-06431-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06431-7