Abstract
Bioenergy crops have a secondary benefit if they increase soil organic C (SOC) stocks through capture and allocation below-ground. The effects of four genotypes of short-rotation coppice willow (Salix spp., ‘Terra Nova’ and ‘Tora’) and Miscanthus (M. × giganteus (‘Giganteus’) and M. sinensis (‘Sinensis’)) on roots, SOC and total nitrogen (TN) were quantified to test whether below-ground biomass controls SOC and TN dynamics. Soil cores were collected under (‘plant’) and between plants (‘gap’) in a field experiment on a temperate agricultural silty clay loam after 4 and 6 years’ management. Root density was greater under Miscanthus for plant (up to 15.5 kg m−3) compared with gap (up to 2.7 kg m−3), whereas willow had lower densities (up to 3.7 kg m−3). Over 2 years, SOC increased below 0.2 m depth from 7.1 to 8.5 kg m−3 and was greatest under Sinensis at 0–0.1 m depth (24.8 kg m−3). Miscanthus-derived SOC, based on stable isotope analysis, was greater under plant (11.6 kg m−3) than gap (3.1 kg m−3) for Sinensis. Estimated SOC stock change rates over the 2-year period to 1-m depth were 6.4 for Terra Nova, 7.4 for Tora, 3.1 for Giganteus and 8.8 Mg ha−1 year−1 for Sinensis. Rates of change of TN were much less. That SOC matched root mass down the profile, particularly under Miscanthus, indicated that perennial root systems are an important contributor. Willow and Miscanthus offer both biomass production and C sequestration when planted in arable soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been an increase in the use of dedicated biomass crops to exploit photosynthesis for bioenergy production over recent decades to address two pressing global concerns: C emission reduction and energy security [1, 2]. Two dedicated low-input bioenergy crops frequently planted in temperate regions, such as the UK, are willow (Salix spp.) in short rotation coppice (SRC) systems and species of the perennial grass genus Miscanthus [3]. Commercial willow plantations produce 9–12 Mg ha−1 year−1 of biomass in 2–4-year SRC harvest rotations typically [1, 4,5,6]. Miscanthus is an annually-harvested perennial rhizomatous grass originating from Asia which has C4 physiology and can produce biomass yields of 12–15 Mg ha−1 year−1 in the UK [1, 2, 6, 7]. In England in 2015, there were 2885 ha under SRC (willow and Populus), yielding 17–35 Gg of dry biomass, of which 15 Gg was used in power stations, and 6905 ha under Miscanthus, yielding 69–104 Gg of dry biomass, of which 33 Gg was used in power stations [6].
In addition to production of biomass, perennial bioenergy crops may have a secondary benefit if they increase stocks of soil organic C (SOC), and total N (TN) by association, through capture of atmospheric C and allocation to below-ground plant biomass [8]. Realising the potential for C sequestration over the lifetime of the stand, which is typically more than 15 years and up to 30 years for willow [9, 10], is likely as perennial bioenergy crops require no cultivation during their lifetime, aside from at planting, and hence disturbance to the soil and the root system is minimised. The roots may persist longer than under annual crops, which is important knowing that SOC is primarily derived from roots [8, 11]. The roots do need to turn over, however, to be incorporated into SOC, operationally defined as the < 2 mm soil size fraction, in order to contribute to sequestration. Perennial bioenergy crops allocate nutrients and C below ground to the root system during senescence in readiness for the following growing season [1, 12], and some crops extend their root systems deep into the subsoil, especially Miscanthus [8, 13, 14]. In their comprehensive review, Agostini et al. [15] found that root C stocks as an input were greater for willow (1.0 Mg ha−1 year−1) than Miscanthus (0.5 Mg ha−1 year−1), but that the latter had a greater mean residence time (1.3 years) than the former (1.8 years), based on the finer nature of willow roots and their fast turnover. The importance of root inputs to SOC is likely dependent on their physical (diameter, association with soil structure) and chemical (greater or lesser ‘labile’ compounds) characteristics.
The potential for C sequestration is site-specific in part, being dependent on local environmental and management factors, including previous land use which largely controls initial SOC and TN contents [2, 8, 16,17,18]. Whilst it may be desirable that bioenergy crops are concentrated on less-productive ‘marginal’ land [8, 16], land used for food production has also been planted with bioenergy crops, causing a conflict between food and bioenergy production on higher-quality soils [2]. This is important because whether such crops are established on degraded or fertile soils may control the potential for C sequestration [8]. Also important is the age of the stand, as others have reported an establishment phase as crop yields increase [19] where resident SOC turns over before full replacement by new SOC deriving from the bioenergy crop [16], particularly where the former land use was under perennial grass [18, 20, 21]. Therefore, a full assessment of the potential for bioenergy crops to sequester significant amounts of C is still far from certain [8, 15, 22, 23] and is reliant on monitoring the dynamics of SOC in well-designed field experiments. It has been estimated that sequestration under bioenergy crops needs to be at least 0.25 Mg SOC ha−1 year−1 for the system to be truly C-neutral [15, 24].
We sought to assess the effect of different species and genotypes of bioenergy crops on root production, SOC and TN in temperate agricultural soil. With detailed prior knowledge of species and genotypic differences in above-ground traits of such bioenergy crops, the main hypothesis was that differences would also be reflected in the below-ground biomass and that these, in turn, affect SOC and TN dynamics. An existing field experiment in the UK with established stands of different willow varieties (genotypes) and Miscanthus genotypes, the principal crops grown solely for bioenergy production in the UK, was used to test the hypothesis. Below-ground biomass and quantified SOC and TN were compared in the underlying soil on two successive occasions when the bioenergy crop stand was 4 and 6 years old to assess stocks and changes. In addition to bulk SOC, the natural abundance 13C isotope labelling of Miscanthus-derived SOC was used to estimate new C input in the establishment phase (inside 10 years).
Materials and Methods
Field Experiments and Treatments under Study
We focused on a field experiment established in 2009 at Rothamsted Research (Hertfordshire, UK) as part of the UK Biotechnology and Biological Sciences Research Council, Sustainable Bioenergy Centre [5]. Four genotypes each of willow and Miscanthus were planted in plots in a randomised block design with four replicates on a field previously under annual arable crops for at least 50 years [25]. Planting followed conventional commercial best practice [9, 10]: willows were planted as cuttings using the typical twin-row design at a planting density of 16,667 plants ha−1 and Miscanthus was planted in single rows at a planting density of 20,000 plants ha−1. Willows were cut back at the end of the establishment year in early 2010 and then subjected to a 2-year SRC regime thereafter, whereas Miscanthus was harvested annually. Harvesting and coppicing were done in January. Miscanthus plots received 100 kg N ha−1 every year in May and willow plots received 60 kg N ha−1 after each 2-year harvest (May 2010, 2012 and 2014), both as Nitram® (NH4NO3; 34.5% N), following typical guidelines during the establishment phase [9, 10]. Canopy traits were used to select two of the four willow varieties for investigation. ‘Terra Nova’ has short, ovate leaves, with a plant leaf area of 1.4 m2 per plant [5] and an average leaf area index of 1.93 [4], whereas ‘Tora’ has long, lanceolate leaves with a plant leaf area of 0.8 m2 per plant [5] and an average leaf area index of 1.26 [4]. Similarly, we chose two standard Miscanthus genotypes: M. × giganteus Greef et Deu ex Hodkinson & Renvoize is a non-tufted tall-growing genotype, and M. sinensis Andersson is a tufted genotype with a shorter stature and which produces many more, thinner stems. Details of the site, soils and experiment [26,27,28,29] are given in Table 1, and full details of characteristics of the genotypes are given elsewhere (Supplementary Material; [5, 23, 30]). For brevity, the genotypes are termed Terra Nova, Tora, Giganteus and Sinensis hereafter.
Sample Collection and Preparation
We took intact soil cores (0.07 m diameter × 1 m length) using a steel core containing an inner sleeve which was driven into the soil using a hydraulic hammer and extracted using a tripod ratchet. As the crops were planted in rows (Table 1), two cores were collected from each plot containing the four genotypes: one in the twin row as close to the plant as possible (willow) or directly over the plant (Miscanthus), and another in the gap equidistant between rows (or twin rows for willow) of plants. This sampling regime was adopted to capture the spatial pattern associated with plants located at regular distances and are termed ‘plant’ and ‘gap’ locations hereafter. The cores were collected in the early summer when the stand age was 4 (June 2013) and 6 years (June 2015). In all, therefore, we collected 64 cores (4 genotypes × 2 locations (plant and gap) × 4 replicate plots (blocks) × 2 years).
All cores were wrapped in polythene to keep them intact and stored at − 18 °C immediately after collection. In the laboratory, each core was brought to room temperature and divided into five depth interval samples: 0–0.1, 0.1–0.2, 0.2–0.3, 0.3–0.5 and 0.5–1.0 m. Each depth interval sample was then further divided in half longitudinally and weighed fresh. For one of the half-interval samples, we washed the soil away gently to leave the > 2 mm fraction (stones, litter and rhizome) for mass (105 °C (stone) or 80 °C (plant) for 48 h) and volume adjustment, and the washed roots which were stored in water at 4 °C prior to analysis. The other half-interval sample was air-dried and crumbled to yield the same > 2 mm fraction for mass and volume adjustment, and the soil fraction (< 2 mm). A soil subsample was milled to < 350 μm with a Retsch PM 400 planetary ball mill (Retsch GmbH, Haan, Germany) for analysis and a separate subsample was oven-dried at 105 °C for 48 h to calculate water contents (air-dried and field-moist) and dry mass. We estimated the linear compaction introduced to the soil core during sampling through comparing the length of the soil core to the depth of the hole from where it had been taken, to fully ‘reconstruct’ the core in its original compaction-free field state. We encountered negligible compaction (mean = 1.1%) as the cores were taken when the soil was not in a plastic state.
To provide a baseline from which to compare, we made use of an existing data set collected before the field experiment was established. In July 2009, 16 soil samples were collected to a depth of 0.9 m using a 0.02-m-diameter hydraulic Norsk Hydro Soil Sampler (Norsk Hydro ASA, Oslo, Norway) from locations covering the area of the field experiment. The samples were divided into three depths (0–0.23, 0.23–0.60 and 0.60–0.90 m) to measure various soil properties, including SOC and TN concentration (see method below). In October 2009, small intact soil cores (0.077 m diameter × 0.05 m length) were collected from ten depths in the top 1 m at four random locations in the field to measure soil dry bulk density. These data were adjusted to the same three depths as above pro rata. The baseline data set is given in Table 1.
Root Analysis
We subjected washed roots to image analysis using the WinRHIZO 2008a program (Regent Instruments Inc., Québec, Canada) connected to an EPSON Expression 1600 3.4 (Epson America Inc., Long Beach, CA, USA) scanner. Roots were spread onto an A4 scanner bed, covered with a layer of water and scanned. The resulting binary image was analysed to determine the mean root diameter and the root length density (RLD; length per volume of soil). The roots were dried at 80 °C for 48 h to calculate their gravimetric concentration (per mass of soil) and the volumetric density (per volume of soil) through knowing the soil bulk density:
where rootdensity is the root density (kg m−3), rootconcentration is the root concentration (g kg−1) and ρ b is the soil dry bulk density (< 2 mm soil mass, total volume; Mg m−3).
Soil Analysis
Soil was analysed for total C and TN concentration using a Leco TruMac Combustion Analyser (LECO Corp., St Joseph, MI, USA). Inorganic C was analysed using a Skalar Primacs AIC Analyser (Skalar Analytical BV, Breda, Netherlands) and the difference with total C yielded the SOC concentration. Inorganic C was only a very minor component of total C (mean 0.13 g kg−1, n = 320) at the experimental site. Volumetric densities of SOC and TN were then calculated using the same approach as above (Eq. 1) with the same units (replacing ‘root’ with ‘SOC’ or ‘TN’). Following others [18, 31], we used an ‘equivalent soil mass’ approach to adjust the measured SOC and TN density of the initial baseline data to that based on the bulk density measured after 4 and 6 years, which did not differ significantly. For only the soils collected from Miscanthus plots, the 13C/12C stable isotope ratios were quantified on prepared carbonate-free soil [23] with an IsoPrime 100 Stable Isotope Ratio Mass Spectrometer (Isoprime Ltd., Cheadle Hulme, UK) coupled with a Vario MICRO Cube Elemental Analyser (Elementar Analysensysteme GmbH, Langenselbold, Germany). By convention, the 13C/12C ratio was expressed as a δ value (‰) relative to the international Vienna Pee Dee Belemnite (VPDB) standard. As Miscanthus has C4 physiology, we used the bulk soil δ13C value to estimate the SOC density derived from Miscanthus compared to older SOC deriving from the previous land use (all arable crops with C3 physiology):
where density-M indicates the Miscanthus-derived SOC density (kg m−3), δ13C M is the reference δ13C value representative of Miscanthus shoot and root plant material (−11.70 ‰), and δ13CC3 is the reference δ13C value representative of the soil at the study site under the previous C3 (cereal crops) plants (− 28.16‰) [23].
We estimated area-based SOC and TN stocks at the larger scale (field or plantation) by a three-stage calculation. Firstly, we calculated a revised density using Eq. 1 but with a mean bulk density for each genotype × location × depth treatment (this was more appropriate when upscaling rather than individual replicate bulk densities) and then multiplied this by the depth interval of interest (m), to give an area-based stock (kg m−2). Secondly, we combined intervals to make larger 0–0.3 m (‘topsoil’) and 0.3–1.0 m (‘subsoil’) depth stocks. Finally, we estimated the proportion of land that is most influenced by the plant, for which the plant stock is most representative, and the remainder, which is represented by the gap stock. For Terra Nova, Tora and Giganteus, stocks were similar for both plant and gap (see later), but nevertheless we used the minimum distance in the field between adjacent plants (0.50 m for willow in a row and 0.65 m for Giganteus between rows; Table 1), and assumed that each plant exerted a circular influence on the soil from its centre to the distance halfway to its nearest neighbour. Thus, we assumed the circular plant radius was 0.25 m for both willows and 0.33 m for Giganteus. For Sinensis, which had a well-defined tuft, the tuft circumference was measured on four representative plants on all four plots in August 2014 and October 2015 (representative of the stand at 4 and 6 years) and the mean radii were 0.17 m in 2014 and 0.18 m in 2015. Using these radii with the planting density of each genotype (Table 1), we were able to divide the total area into plant and gap and hence adjust the stock estimates pro rata. These stocks, and the estimated initial baseline stock, were analysed together with the rate of change between 0, 4 and 6 years, as expressed in conventional units (Mg ha−1).
Statistical Analysis
All statistical analysis was performed using the GenStat (18th edition) program (VSN International Ltd., Hemel Hempstead, UK). We transformed the soil and root variates by log10 firstly to normalise the distribution of the residuals. We then analysed the variates using residual maximum likelihood (REML) for the following model structures:
where ‘genotype’, ‘depth’ and ‘age’ were self-explanatory, ‘location’ allocated data to either plant or gap, and ‘*’, ‘/’ and ‘×’ represent all cross-products, nesting and interactions of the factors, respectively. We introduced the variance structure above for all analyses as there was no independence of samples at different depths from each treatment in the strictest sense because a single soil core was taken and subdivided into depth samples. We may expect correlations between pairs of depths for any property to vary rather than be constant, depending on the distance between them. Therefore, we introduced an autoregressive variance structure into our REML models to incorporate this feature. For the SOC and TN stocks and their rate of change, neither data transformation nor the variance structure were required, ‘period’ (i.e. 0–4 and 4–6 years) replaced ‘age’ for the rates of change, and ‘location’ or ‘depth’ were removed when the data were composited into larger-scale spatial and full 1 m depth scales. We present the statistical analysis of all transformed data (where required) in a summary table but present the original data elsewhere for ease of displaying measured quantities. The Wald statistic produced during REML analysis assesses the contributions of individual terms in the fixed model and corresponds to the treatment sum of squares divided by the stratum mean square.
Results
Root Density
The full genotype × location × depth × age interaction was significant (P = 0.014) for root density (Table 2; Fig. 1). For Sinensis, root density was significantly greater (P < 0.05) for plant (6.6 ± 1.5 after 4 years and 15.5 ± 3.1 kg m−3 after 6 years; mean ± standard error) compared to gap (1.4 ± 0.4 after 4 years and 2.2 ± 0.4 kg m−3 after 6 years) at 0–0.1 m depths. The same was also true for Sinensis at 0.1–0.2 m depth (2.0 ± 0.2 after 4 years and 7.2 ± 2.5 kg m−3 after 6 years for plant, and 0.4 ± 0.1 after 4 years and 1.2 ± 0.2 kg m−3 after 6 years for gap) (P < 0.05). Root density was significantly greater under Giganteus at 0.1–0.2 m depth after 6 years for plant (4.4 ± 1.0 kg m−3) than gap (0.8 ± 0.2 kg m−3) (P < 0.05). Significant increases (P < 0.05) in root density between 4 and 6 years were found for Sinensis gap at 0.2–0.3 (0.2 ± 0.1 to 0.9 ± 0.2 kg m−3) and 0.3–0.5 m depths (0.1 ± 0.0 to 0.4 ± 0.1 kg m−3), and for Tora plant at 0.2–0.3 m depth (0.2 ± 0.1 to 1.1 ± 0.6 kg m−3). Significant genotype differences were apparent, but these were mainly restricted to upper layers, where density was often greater for Miscanthus (particularly Sinensis) than willow varieties. Root density decreased with depth for all genotypes.
Mean root density (n = 4) under willow (a Terra Nova and b Tora) and Miscanthus (c Giganteus and d Sinensis) genotypes, located either directly under the plant (P) or in the gap between adjacent plants (G), when the stand age was 4 and 6 years. With the data transformed (log10), the genotype × location × depth × age interaction was significant (P = 0.014) (see Table 2)
Root Diameter and RLD
Mean root diameter was significantly greater (P < 0.05) for Giganteus (0.55 ± 0.01 mm) and, at most depths, Sinensis (0.47 ± 0.02 mm) compared to both willow varieties (0.35 ± 0.02 mm) (Table 2; Fig. 2). Significant differences in root diameter between plant and gap were restricted to the 0–0.1 m depth for Sinensis and Tora. For Sinensis, roots in plant were coarser (0.51–0.81 mm) than in gap (0.34–0.43 mm), whereas for Tora roots in plant were finer (0.26–0.48 mm) than in gap (0.32–0.90 mm) (P < 0.05). Mean root diameter significantly increased between 4 and 6 years for all genotypes at all depths (P < 0.05) with few exceptions, and was generally greatest in the 0–0.1 m depth. The RLD was significantly greater (P < 0.05) for plant (3.9 ± 0.3 cm cm−3) compared to gap (3.1 ± 0.3 cm cm−3) averaged over all other factors and decreased significantly with depth (Table 2; Fig. 3). Giganteus had the smallest (0.2–9.9 cm cm−3) and Sinensis the greatest RLD (0.4–11.4 cm cm−3) at most depths. Only for Sinensis was there a significant increase (P < 0.05) in RLD between 4 and 6 years (2.7 ± 0.4 to 4.9 ± 0.6 cm cm−3).
Mean root diameter (n = 4) under willow (a Terra Nova and b Tora) and Miscanthus (c Giganteus and d Sinensis) genotypes, located either directly under the plant (P) or in the gap between adjacent plants (G), when the stand age was 4 and 6 years. With the data transformed (log10), the location × age (P = 0.041), genotype × location × depth (P = 0.014) and genotype × depth × age (P = 0.005) interactions were significant (see Table 2)
Mean root length density (n = 4) under willow (a Terra Nova and b Tora) and Miscanthus (c Giganteus and d Sinensis) genotypes, located either directly under the plant (P) or in the gap between adjacent plants (G), when the stand age was 4 and 6 years. With the data transformed (log10), the location factor (P = 0.008) and genotype × depth (P = 0.007) and genotype × age (P = 0.016) interactions were significant (see Table 2)
SOC and TN Density
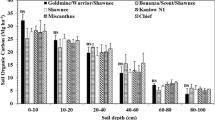
The SOC density increased significantly (P < 0.05) between 4 and 6 years at all depths below 0.2 m (from 3.6 ± 0.1 to 12.2 ± 0.7 kg m−3 after 4 years to 4.6 ± 0.1 to 14.3 ± 0.6 kg m−3 after 6 years, averaged over all other factors) (Table 2; Fig. 4). Genotype and location effects were not significant. The SOC density after 4 and 6 years was similar to the initial baseline (0 years) in the upper 0.5 m, though it was much greater under Sinensis and Tora after 6 years in the upper 0.2 m, but less than initial density below 0.5 m (Fig. 4). Miscanthus-derived SOC density was significantly greater (P < 0.05) under Sinensis plant (7.4 ± 2.1 to 11.6 ± 1.8 kg m−3) than Giganteus plant in the 0–0.1 m depth (2.9 ± 0.4 to 4.8 ± 0.5 kg m−3), and significantly greater under Giganteus gap (0.1 ± 0.0 to 0.5 ± 0.1 kg m−3) than Sinensis gap at 0.5–1.0 m depth (0.0 ± 0.0 to 0.5 ± 0.0 kg m−3) (Table 2; Fig. 5). Miscanthus-derived SOC density was also greater for plant than gap for Sinensis at most depths (P < 0.05), whereas differences between plant and gap were not significant for Giganteus. Averaged over both locations, Miscanthus-derived SOC density increased significantly (P < 0.05) between 4 and 6 years at all depths under both genotypes (by 0.4–3.2 kg m−3), with few exceptions.
Mean soil organic C (SOC) density (n = 4) under willow (a Terra Nova and b Tora) and Miscanthus (c Giganteus and d Sinensis) genotypes, located either directly under the plant (P) or in the gap between adjacent plants (G), when the stand age was 4 and 6 years. With the data transformed (log10), the depth × age interaction was significant (P = 0.008), but neither genotype nor location factors were (see Table 2). The baseline data (0 years) for three depth intervals (adjusted for equivalent soil mass) is shown for comparison
Mean Miscanthus-derived soil organic C (SOC) density (n = 4) under Miscanthus (a Giganteus and b Sinensis) genotypes, located either directly under the plant (P) or in the gap between adjacent plants (G), when the stand age was 4 and 6 years. With the data transformed (log10), the genotype × location × depth (P = 0.024) and genotype × depth × age (P = 0.016) interactions were significant (see Table 2)
The TN density after 4 years was significantly greater (P < 0.05) for gap (2.2 ± 0.1 kg m−3) than plant (1.7 ± 0.1 kg m−3) at 0–0.2 m depths, but significantly less (P < 0.05) for gap (0.6 ± 0.0 kg m−3) than plant (0.8 ± 0.1 kg m−3) at 0.5–1.0 m depth (Table 2; Fig. 6). Averaged over all other factors, there was a significantly greater (P < 0.05) TN density associated with Sinensis and Terra Nova (1.4 ± 0.1 kg m−3) compared to Giganteus (1.2 ± 0.1 kg m−3). Compared to the initial baseline, only for Terra Nova and Sinensis was there a greater TN density recorded after 4 years at certain depths. There were no significant changes between 4 and 6 years. The SOC/TN ratio increased significantly (P < 0.05) from 4 to 6 years under all genotypes (by 1.2–1.6) except Giganteus, and at all depths (by 0.7–1.6) except 0–0.1 m (Fig. 7). Only at 0–0.2 m depths was there a significantly greater SOC/TN ratio for plant compared to gap (by 0.9–2.1). The SOC and TN densities and their ratio decreased significantly with depth (P < 0.05). The SOC/TN ratio after 4 and 6 years under bioenergy crops was greater than that in the baseline in the upper soil layers. The original gravimetric SOC and TN concentrations (g kg−1) are given elsewhere (Supplementary Material).
Mean soil total N (TN) density (n = 4) under willow (a Terra Nova and b Tora) and Miscanthus (c Giganteus and d Sinensis) genotypes, located either directly under the plant (P) or in the gap between adjacent plants (G), when the stand age was 4 and 6 years. With the data transformed (log10), the genotype factor (P = 0.032) and location × depth × age interaction (P = 0.025) were significant (see Table 2). The baseline data (0 years) for three depth intervals (adjusted for equivalent soil mass) is shown for comparison
Mean soil organic C/total N ratio (SOC:TN) (n = 4) under willow (a Terra Nova and b Tora) and Miscanthus (c Giganteus and d Sinensis) genotypes, located either directly under the plant (P) or in the gap between adjacent plants (G), when the stand age was 4 and 6 years. With the data transformed (log10), the location × depth (P < 0.001), genotype × age (P = 0.003) and depth × age (P < 0.001) interactions were significant (see Table 2). The baseline data (0 years) for three depth intervals (adjusted for equivalent soil mass) is shown for comparison
SOC and TN Stock
Stocks of SOC increased significantly (P < 0.05) in the 0.3–1.0 m depth between 4 and 6 years from 29 ± 2 to 38 ± 2 Mg ha−1, averaged over all crops, whereas TN stocks were unaffected by any factor (Table 3). Stocks of SOC in the full 1 m profile changed from 79 ± 4 to 81 ± 5 Mg ha−1 after 4 years to 87 ± 5 to 96 ± 4 Mg ha−1 after 6 years, and TN stocks in the full 1 m profile changed from 9.2 ± 0.9 to 11.0 ± 0.5 Mg ha−1 after 4 years to 9.4 ± 0.5 to 10.8 ± 0.4 Mg ha−1 after 6 years. Genotype-specific differences were not significant however. Miscanthus-derived SOC increased significantly (P < 0.05) from 3.4 ± 0.5 to 5.2 ± 0.6 Mg ha−1 after 4 years to 11.4 ± 0.8 to 14.2 ± 1.7 Mg ha−1 after 6 years. For both SOC and TN, stocks in the 0–0.3 m depth were greater and stocks in the 0.3–1.0 m depth were lower after 4 and 6 years compared to those estimated for the baseline. Between years 4 and 6, SOC increased to 1 m depth at 6.4 ± 1.9 for Terra Nova, 7.4 ± 2.5 for Tora, 3.1 ± 2.1 for Giganteus and 8.8 ± 3.4 Mg ha−1 year−1 for Sinensis, being greater significantly (P < 0.05) for the 0.3–1.0 m depth, averaged over genotypes. Stocks of TN increased slightly by 0.1 ± 0.3 Mg ha−1 year−1 except for Terra Nova where it declined by − 0.15 ± 0.2 Mg ha−1 year−1 over the same period. Generally, rates of change were greater for the 4- to 6-year period than the 0- to 4-year period. Location-based SOC and TN stocks and rates of change are given elsewhere (Supplementary Material).
Discussion
Bioenergy Crop Root Characteristics
Root mass was greater under Miscanthus compared to willow, although such differences were restricted to the upper 0.3 m. Root mass below 0.3 m was very small under all genotypes due in part to the high clay content of the subsoil (> 26%) where the ability of roots to penetrate was likely restricted to existing structures such as shrinkage cracks [32]. Only for Miscanthus was a spatial pattern between root mass and location, with respect to canopy structure, identified whereby distinct plant and gap zones down to 0.2 m depth developed, particularly under Sinensis. Sinensis is a tuft-forming genotype, especially during establishment, and its areal coverage in the gap does not increase as much as Giganteus with age [7, 23]. Only for Tora in the upper 0.1 m was there a suggestion of an effect of location with respect to willow root mass. We were not able to sample from directly over the centre of the willow plants; therefore, our plant sample under willow differs slightly from that under Miscanthus which may partly mask the lack of effect of location, although RLD was greater for plant compared to gap for both bioenergy crop species. Observed growth of other species (weeds) between willow plants may have provided a confounding source of roots that could not be distinguished using bulk measurements. Nevertheless, the results indicate that willow roots spread laterally as the stand matures [33].
Root mass and RLD increased substantially over the 2-year period in the upper 0.2 m between measurements under Giganteus and, especially, Sinensis. Continual growth of roots under Miscanthus was expected as the stand was still maturing during this period [34], with each new shoot developing its own root system. Root biomass may also increase for Miscanthus during periods of water stress [1]. Although both 2014 and 2015 recorded greater-than-average (1981–2010) annual temperature and rainfall (+ 1.5 °C and + 191 mm in 2014; + 0.7 °C and + 48 mm in 2015), in March and April prior to sampling in 2015, rainfall had been up to 50% lower than average [29]. The root system of willows, particularly under the 2-year SRC regime, may have developed to the full extent prior to the first measurement after 4 years such that subsequent root growth was balanced by turnover, as observed by others [35]. Total above-ground yield for both willow varieties decreased from about 25 Mg ha−1 in 2012–2013 to 22 Mg ha−1 in the 2014–2015 SRC growth cycle, whereas biennial production increased under Giganteus (26 to 34 Mg ha−1) and Sinensis (18 to 20 Mg ha−1) over the same period. This would appear to support the differences in root biomass described above. Willow roots were finer than Miscanthus roots which may increase their turnover rate [15], although mean root diameter under all genotypes increased in size over time, and RLD did not vary much over the measurement period. Despite measurable differences in some above-ground traits of willows [5], these did not appear to be manifest in differences in the root traits we measured in this study.
Our estimates of root biomass are comparable with other studies. Ferchaud et al. [22] reported root biomass of 4.1 Mg ha−1, assuming a C content of 43% [15], under a 5-year stand of Giganteus in northern France under similar climatic conditions, similar to our measurement (2.9–7.1 Mg ha−1) when root density is expressed as a stock. The rhizome biomass of 18.1 Mg ha−1 given by Ferchaud et al. [22] is within the wide range of that calculated for the 4-year-old Giganteus herein (6.4 in gap and 109.3 Mg ha−1 in plant). The rhizome itself may accumulate more than 1 Mg C ha−1 year−1 under established Miscanthus stands, providing a larger store of C than the root system (< 1 Mg C ha−1 year−1), according to Agostini et al. [15].
SOC and TN under Bioenergy Crops
We found that SOC increased over time under all genotypes below, though not above, 0.2 m depth. Differences in topsoil SOC gravimetric concentration (g kg−1; Supplementary Material) were not manifest in differences in volumetric density due to bulk density (which was significantly lower (P < 0.05) under plant at 0–0.1 m, averaged over all genotypes), as observed previously [23]. Concentrations of SOC matched patterns in root concentration and density, where regressions explained 0.58 (concentration) and 0.55 (density) of the proportion of the variance (Supplementary Material). This was most apparent for Miscanthus where there was strong evidence linking Miscanthus-derived SOC to root mass, with regressions explaining up to 0.79 proportion of the variance (Supplementary Material), though not obviously RLD. This supports the hypothesis that roots were the main source of SOC under bioenergy crops [8], assumed to be facilitated by the reallocation of photosynthate to the root and rhizome at senescence. Similar mechanisms occur with respect to the stools of willow [1, 33].
Whilst there may be some inputs to the soil from leaf litter under willow, this may be recycled back into the plant rapidly as established stands effectively provide their own nutrient needs through internal cycling [1]. Rubino et al. [36] quantified significant incorporation of 13C-labelled poplar litter into the topsoil horizon in Italy. Significant input from Miscanthus leaves is unlikely, however [18], despite a potential input of up to 7 Mg C ha−1 year−1 [37,38,39]. Even for late-harvest Miscanthus, leaf fall remains largely undecomposed [22], partly because of its reduced quality arising from the translocation of N from senescing leaves to the rhizome at the end of the growing season [40]. This supports below-ground biomass as the primary source of SOC.
Above-ground yields over the 2012–2016 period were greatest for Giganteus (15 Mg ha−1 year−1) and least for Sinensis (9 Mg ha−1 year−1), with the willow varieties being intermediate (12 Mg ha−1 year−1), yet this was not reflected in root biomass or SOC. As TN did not change significantly over time, the SOC/TN ratio increased, particularly in the top 0.2 m under Sinensis. This may reflect the high C/N ratio of the source material: the C/N ratio of Miscanthus roots can exceed 40 (data not presented) suggesting that microbial N mining may control the decomposition of organic C, thereby increasing potential C sequestration rates, and the maintenance of poor TN contents in this N-limited system [41].
Inputs of C to the soil may be important when willow is coppiced as increased root turnover may follow harvesting of above-ground biomass [2]. Comparable SOC sequestration rates of 3.4 Mg ha−1 year−1 in the upper 1 m under SRC willow in the UK [42] and up to 6.7–10.2 Mg ha−1 year−1 in the upper 0.6 m of soil under SRC willow and poplar in Belgium were recorded recently [43]. Others have reported comparable SOC sequestration rates below willow stands of 1.0 Mg ha−1 year−1 in the upper 0.1 m over a 6-year period in Italy [44] and 1.5–1.7 Mg ha−1 year−1 in the upper 0.9 m over an 11-year period in Germany [45]. Lesser accumulations of 0.2–0.3 Mg ha−1 year−1 were reported at 0–0.25 m depth under a 12-year willow stand in Germany [46], whilst others have reported losses of SOC under willow [47], particularly on former grassland [18, 48]. On average, a lower mean annual SOC sequestration rate under willow of 0.6 Mg ha−1 year−1 has been reported [15].
Determining the stable 13C isotope signature of SOC from Miscanthus stands confirms that fresh Miscanthus-derived C was progressively added to the soil since planting in 2009. Giganteus had a more extensive effect initially on increasing SOC stocks, but Sinensis became more effective at increasing SOC stocks. Equivalent Miscanthus-derived SOC accumulation rates in the 0–0.3 m depth during the first 4 years were 1.1 for Giganteus and 0.8 Mg ha−1 year−1 for Sinensis. In an older stand of Miscanthus at the same site, Richter et al. [23] reported accumulations of 1.2 under Giganteus and 0.7 Mg ha−1 year−1 under Sinensis over 14 years. Our rates are comparable to those measured by others for topsoil under Giganteus in France (0.4–0.7 Mg ha−1 year−1; [22]), Ireland (0.6 Mg ha−1 year−1; [12, 34]), Germany (0.7 Mg ha−1 year−1; [45]), the USA (1.1 Mg ha−1 year−1; [49]), Italy (1.2 Mg ha−1 year−1; [50]), the UK (1.4 Mg ha−1 year−1; [20]) and various sites in Europe (0.4–1.0 Mg ha−1 year−1; [17]), and similar to recent global estimates of sequestration under Miscanthus of around 1.2 Mg ha−1 year−1 [15, 51].
Data on SOC changes below 0.3 m are limited, but our estimated Miscanthus-derived SOC rates of up to 0.2 Mg ha−1 year−1 over the initial 4 years for both genotypes are similar to those reported in a global review of Giganteus planted following arable cropping (0.1 Mg ha−1 year−1; [51]) and studies in Italy (0.5 Mg ha−1 year−1; [44]) and the UK (0.1 Mg ha−1 year−1; [23]). For the following 2 years, we report considerably greater Miscanthus-derived SOC accumulation rates of 1.5 (Giganteus) and 3.3 Mg ha−1 year−1 (Sinensis) in the 0–0.3 m depth and 1.6 (Giganteus) and 2.1 Mg ha−1 year−1 (Sinensis) in the 0.3–1.0 m depth. These rates are still comparable, however, to others reported in the literature. Rates of up to 6.8 for a 4–5-year-old stand and 8.8 Mg ha−1 year−1 for an 8–9-year-old stand were recorded in the upper 0.25 m under Giganteus in Germany [52], and rates of up to 4 Mg ha−1 year−1 in the topsoil were reported for grass bioenergy crops in general [8].
Increases in SOC stock observed under Miscanthus were not wholly explained by Miscanthus-derived inputs, suggesting an input of C to the system from another (non-C4) source. On occasions, we observed other plants to be present on the plot, despite weed control, though we could not quantify the areal extent. Other factors include trends in atmospheric CO2 [53, 54], and variations in plant input signatures relating to physiological stress [55, 56], which all affect the δ13C determinations used to estimate Miscanthus-derived inputs. The former would be unlikely to have been significant given the short period between measurements, but the latter may have been important. In the 0–0.3 m depth of gap, however, we did estimate a greater accumulation rate of Miscanthus-derived SOC than total SOC, which would indicate turnover of existing C3 plant-derived SOC. Preferential processing of inherent SOC leading to persistence of Miscanthus-derived SOC and an overall balance in SOC have been observed previously [22, 57], and have led to a caveat that effective sequestration may be marginal [18, 23].
Temporal changes in soil TN were largely insignificant, but interesting differences were observed between genotypes. There were marginal increases in TN in topsoil for plant (up to 0.13 Mg ha−1 year−1) and marginal decreases in gap (down to − 0.31 Mg ha−1 year−1), but the opposite in the 0.3–1.0 m depth (down to − 0.38 for plant and up to 0.41 Mg TN ha−1 year−1 for gap). We suggest that processes such as uptake of N by lateral roots in the gap, loss through leaching down the profile into the subsoil (where not directly mediated by the plant) and loss through denitrification (waterlogging has been observed at the field site in very wet periods) may have been responsible. Increased TN for plant probably derived from the same residues associated with SOC increases, albeit at much lower rates due to the relatively large C/N ratio of the plant inputs.
We estimate that by year 6, SOC was sequestered at a rate of 3 to 9 Mg SOC ha−1 year−1 under all crops in the upper 1 m, following an apparent loss of SOC in the first 4 years specific to the 0.3–1.0 m depth. That SOC may be lost in the establishment phase of bioenergy crop stands is normal, but we must be cautious with the baseline data. Stocks in 2009 prior to establishment were calculated from separate samples collected on separate occasions for SOC concentration and bulk density. The bulk density values in 2009 (1.47–1.61 Mg m−3) were greater than average bulk densities in 2013 and 2015 (1.22–1.41 Mg m−3). It was likely that measured bulk density was greater in 2009 in part because samples were collected in smaller cores presumably with less chance of including > 2 mm stones that are otherwise accounted for and removed. Additionally, the depth intervals in 2009 did not correspond to those in 2013 and 2015 which required adjustment pro rata. Nevertheless, we report an increase in SOC accumulation rates under all bioenergy crops with time. After 6 years, approximately 14% of SOC (11 to 14 Mg ha−1) had derived from Miscanthus. Soil TN was a different matter, and we estimated losses of up to − 0.15 Mg TN ha−1 year−1 under Terra Nova but marginal increases of up to 1.0 Mg TN ha−1 year−1 under the other crops. We may presume that high N requirements of willows [58] were met in part by soil TN reserves in addition to fertiliser.
Previous land use is important for a full evaluation of sequestration potential [8, 16]. In their review of global datasets, Qin et al. [51] reported greater accumulation rates of SOC where willow and Miscanthus were planted on former arable land, compared to grassland where some net losses were recorded. This has been supported by paired-site studies in the UK [18]. Former grassland soils have been observed to recover such lost SOC in the medium term however [12, 42]. Undoubtedly, some SOC was lost following initial cultivation of the field site, but after the first few SRC cycles of willow, or the first few harvests of Miscanthus, we may expect the SOC stock to increase such that the initial losses are regained, particularly as the site was previously under arable cropping. We therefore expect C to have been sequestered in the soils under bioenergy crops, compared to the previous arable land use, over the lifetime of the plantation [8].
We observed soil SOC accumulation rates exceeding by far a proposed C-neutrality threshold of 0.25 Mg ha−1 year−1 [15, 24]. Indeed, if our rates of 3.09–8.84 Mg C ha−1 year−1 are converted firstly to CO2 equivalents (11.3–32.4 Mg ha−1 year−1), we may then use the 100-year global warming potential factor of 298 to calculate the equivalent N2O emission threshold that should not be exceeded to maintain overall greenhouse gas mitigation. This estimate of 38–109 kg N2O ha−1 year−1 is far greater than current estimates of emissions under both arable (0.9–11.0 kg N2O ha−1 year−1) and grassland agriculture (1.6–22.0 kg N2O ha−1 year−1) in the UK [59]. Sequestration in the subsoil may be especially important as such SOC may become protected against further processing [60].
Although our SOC accumulation rates are greater than others (e.g. [17, 48]), they are not unprecedently so (e.g. [43, 52]), and the rates of change relative to initial SOC stocks are comparable elsewhere. Poeplau et al. [61] calculated accumulations of SOC of 10 to 50% within 10 years from arable to grass and arable to woodland land use conversions, which covers the kinds of proportional increases we found here (up to 12% increase in the 0–0.3 m depth and up to 43% increase in the 0.3–1.0 m depth from 4 to 6 years). The subsoil may also be affected by land use change [61, 62] and accumulation rates may be greater where the subsoil SOC stock was low initially [17]. Berhongaray et al. [43] also calculated greater sequestration rates under SRC willow and poplar at 0.3–0.6 m depth compared to 0–0.3 m depth. Not all studies sample to the same depths as this present study and hence may miss SOC changes at depth. We ascribe our greater accumulation rates under both bioenergy crops to the younger age of the stand and the low initial SOC status of the soil which had been under long-term arable management prior.
Conclusions
Genotypes of willow and Miscanthus sequestered SOC in an underlying temperate agricultural silty clay loam soil with the root system being the most likely source. Soil TN was little-affected and hence addition of fresh residues to the soil increased the SOC/TN under all genotypes. This may lead to persistence of the new residues, although isotopic evidence for turnover of the inherent SOC in preference to fresh Miscanthus-derived SOC was limited, perhaps due to inputs from undergrowth species. Both bioenergy crops offer the double benefit of biomass production and C sequestration when planted in arable soil initially low in SOC. Future studies will continue to monitor changes in SOC and TN to assess the dynamics reported here and will exploit isotopic and biochemical approaches to focus on turnover rates of above- and below-ground plant constituents over the full life cycle of bioenergy crop stands.
References
Karp A, Shield I (2008) Bioenergy from plants and the sustainable yield challenge. New Phytol 179(1):15–32. https://doi.org/10.1111/j.1469-8137.2008.02432.x
Don A, Osborne B, Hastings A, Skiba U, Carter MS, Drewer J, Flessa H, Freibauer A, Hyvonen N, Jones MB, Lanigan GJ, Mander U, Monti A, Djomo SN, Valentine J, Walter K, Zegada-Lizarazu W, Zenone T (2012) Land-use change to bioenergy production in Europe: implications for the greenhouse gas balance and soil carbon. Glob Change Biol Bioenergy 4(4):372–391. https://doi.org/10.1111/j.1757-1707.2011.01116.x
Lovett A, Sünnenberg G, Dockerty T (2014) The availability of land for perennial energy crops in Great Britain. Glob Change Biol Bioenergy 6(2):99–107. https://doi.org/10.1111/gcbb.12147
Caslin B, Finnan J, McCracken A (eds) (2012) Willow varietal identification guide. Teagasc, Carlow
Cunniff J, Purdy SJ, Barraclough TJP, Castle M, Maddison AL, Jones LE, Shield IF, Gregory AS, Karp A (2015) High yielding biomass genotypes of willow (Salix spp.) show differences in below ground biomass allocation. Biomass Bioenergy 80:114–127. https://doi.org/10.1016/j.biombioe.2015.04.020
DEFRA (2016) Crops grown for bioenergy in England and the UK: 2008–2015. UK Government Department for Environment, Food and Rural Affairs. https://www.gov.uk/government/statistics/area-of-crops-grown-for-bioenergy-in-england-and-the-uk-2008-2015. Accessed 1 March 2018
Clifton-Brown JC, Lewandowski I, Andersson B, Basch G, Christian DG, Kjeldsen JB, Jørgensen U, Mortensen JV, Riche AB, Schwarz KU, Tayebi K, Teixeira F (2001) Performance of 15 Miscanthus genotypes at five sites in Europe. Agron J 93(5):1013–1019. https://doi.org/10.2134/agronj2001.9351013x
Blanco-Canqui H (2016) Growing dedicated energy crops on marginal lands and ecosystem services. Soil Sci Soc Am J 80(4):845–858. https://doi.org/10.2136/sssaj2016.03.0080
DEFRA (2001) Planting and growing Miscanthus. Best practice guidelines for applicants to Defra’s energy crops scheme. UK Government Department for Environment, Food and Rural Affairs, London
DEFRA (2002) Growing short rotation coppice. Best practice guidelines for applicants to Defra’s energy crops scheme. UK Government Department for Environment, Food and Rural Affairs, London
Rasse DP, Rumpel C, Dignac M-F (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269(1–2):341–356. https://doi.org/10.1007/s11104-004-0907-y
Zimmermann J, Dauber J, Jones MB (2012) Soil carbon sequestration during the establishment phase of Miscanthus × giganteus: a regional-scale study on commercial farms using 13C natural abundance. Glob Chang Biol Bioenergy 4(4):453–461. https://doi.org/10.1111/j.1757-1707.2011.01117.x
Monti A, Zatta A (2009) Root distribution and soil moisture retrieval in perennial and annual energy crops in northern Italy. Agric Ecosyst Environ 132(3–4):252–259. https://doi.org/10.1016/j.agee.2009.04.007
Neukirchen D, Himken M, Lammel J, Czyionka-Krause U, Olfs H-W (1999) Spatial and temporal distribution of the root system and root nutrient content of an established Miscanthus crop. Eur J Agron 11(3–4):301–309. https://doi.org/10.1016/s1161-0301(99)00031-3
Agostini F, Gregory AS, Richter GM (2015) Carbon sequestration by perennial energy crops: is the jury still out? Bioenergy Res 8(3):1057–1080. https://doi.org/10.1007/s12155-014-9571-0
McCalmont JP, Hastings A, McNamara NP, Richter GM, Robson P, Donnison IS, Clifton-Brown J (2017) Environmental costs and benefits of growing Miscanthus for bioenergy in the UK. Glob Chang Biol Bioenergy 9:489–507. https://doi.org/10.1111/gcbb.12294
Poeplau C, Don A (2014) Soil carbon changes under Miscanthus driven by C4 accumulation and C3 decomposition—toward a default sequestration function. Glob Chang Biol Bioenergy 6(4):327–338. https://doi.org/10.1111/gcbb.12043
Rowe RL, Keith AM, Elias D, Dondini M, Smith P, Oxley J, McNamara NP (2016) Initial soil C and land-use history determine soil C sequestration under perennial bioenergy crops. Glob Chang Biol Bioenergy 8(6):1046–1060. https://doi.org/10.1111/gcbb.12311
Christian DG, Riche AB, Yates NE (2008) Growth, yield and mineral content of Miscanthus × giganteus grown as a biofuel for 14 successive harvests. Ind Crop Prod 28(3):320–327. https://doi.org/10.1016/j.indcrop.2008.02.009
Zatta A, Clifton-Brown J, Robson P, Hastings A, Monti A (2014) Land use change from C3 grassland to C4 Miscanthus: effects on soil carbon content and estimated mitigation benefit after six years. Glob Chang Biol Bioenergy 6(4):360–370. https://doi.org/10.1111/gcbb.12054
Zimmermann J, Dondini M, Jones MB (2013) Assessing the impacts of the establishment of Miscanthus on soil organic carbon on two contrasting land-use types in Ireland. Eur J Soil Sci 64(6):747–756. https://doi.org/10.1111/ejss.12087
Ferchaud F, Vitte G, Mary B (2016) Changes in soil carbon stocks under perennial and annual bioenergy crops. Glob Chang Biol Bioenergy 8(2):290–306. https://doi.org/10.1111/gcbb.12249
Richter GM, Agostini F, Redmile-Gordon M, White R, Goulding KWT (2015) Sequestration of C in soils under Miscanthus can be marginal and is affected by genotype-specific root distribution. Agric Ecosyst Environ 200:169–177. https://doi.org/10.1016/j.agee.2014.11.011
Mann MK, Spath PL (1997) Life cycle assessment of a biomass gasification combined-cycle system. Report no. NREL/TP-430-23076. National Renewable Energy Laboratory, Golden
Johnston AE, Poulton PR, McEwan J (1981) The soils of Rothamsted farm. The carbon and nitrogen of the soils and the effect of changes in crop rotation and manuring on soil pH, P, K and Mg. Rothamsted Experimental Station. Report for 1980. Part 2. Lawes Agricultural Trust, Harpenden, pp 5–20
Avery BW (1980) Soil classification for England and Wales (higher categories). Technical monograph 14. Soil Survey of England and Wales, Harpenden, UK
Avery BW, Catt JA (1995) The soil at Rothamsted. Lawes Agricultural Trust, Harpenden
IUSS-ISRIC-FAO (2006) World Reference Base for soil resources 2006. World soil resources report 103. International Union of Soil Sciences—International Soil Reference and Information Centre—Food and Agriculture Organization of the United Nations. FAO, Rome, Italy
Rothamsted Research (2017) The Electronic Rothamsted Archive: Meteorological data. http://www.era.rothamsted.ac.uk/. Accessed 1 March 2018
Macalpine WJ, Shield IF, Trybush SO, Hayes C, Karp A (2008) Overcoming barriers to crossing in willow (Salix spp.) breeding. Asp Appl Biol 90:173–180
Poulton PR, Pye E, Hargreaves PR, Jenkinson DS (2003) Accumulation of carbon and nitrogen by old arable land reverting to woodland. Glob Chang Biol 9(6):942–955. https://doi.org/10.1046/j.1365-2486.2003.00633.x
Gao W, Hodgkinson L, Jin K, Watts CW, Ashton RW, Shen J, Ren T, Dodd IC, Binley A, Phillips AL, Hedden P, Hawkesford MJ, Whalley WR (2016) Deep roots and soil structure. Plant Cell Environ 39(8):1662–1668. https://doi.org/10.1111/pce.12684
Karp A, Hanley SJ, Trybush SO, Macalpine W, Pei M, Shield I (2011) Genetic improvement of willow for bioenergy and biofuels. J Integr Plant Biol 53(2):151–165. https://doi.org/10.1111/j.1744-7909.2010.01015.x
Clifton-Brown JC, Breuer J, Jones MB (2007) Carbon mitigation by the energy crop, Miscanthus. Glob Chang Biol 13(11):2296–2307. https://doi.org/10.1111/j.1365-2486.2007.01438.x
Rytter RM (2001) Biomass production and allocation, including fine-root turnover, and annual N uptake in lysimeter-grown basket willows. For Ecol Manag 140(2–3):177–192. https://doi.org/10.1016/s0378-1127(00)00319-4
Rubino M, Dungait JAJ, Evershed RP, Bertolini T, De Angelis P, D'Onofrio A, Lagomarsino A, Lubritto C, Merola A, Terrasi F, Cotrufo MF (2010) Carbon input belowground is the major C flux contributing to leaf litter mass loss: evidences from a 13C labelled-leaf litter experiment. Soil Biol Biochem 42(7):1009–1016. https://doi.org/10.1016/j.soilbio.2010.02.018
Amougou N, Bertrand I, Machet J-M, Recous S (2011) Quality and decomposition in soil of rhizome, root and senescent leaf from Miscanthus × giganteus, as affected by harvest date and N fertilization. Plant Soil 338(1–2):83–97. https://doi.org/10.1007/s11104-010-0443-x
Beuch S, Boelcke B, Belau L (2000) Effect of the organic residues of Miscanthus × giganteus on the soil organic matter level of arable soils. J Agron Crop Sci 184(2):111–119. https://doi.org/10.1046/j.1439-037x.2000.00367.x
Kahle P, Belau L, Boelcke B (2002) Effects of 10 years of Miscanthus cultivation on different properties of mineral soil in north-east Germany. J Agron Crop Sci 188(1):43–50. https://doi.org/10.1046/j.1439-037x.2002.00530.x
Robertson AD, Whitaker J, Morrison R, Davies CA, Smith P, McNamara NP (2017) A Miscanthus plantation can be carbon neutral without increasing soil carbon stocks. Glob Chang Biol Bioenergy 9:645–661. https://doi.org/10.1111/gcbb.12397
Chen RR, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin XG, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Chang Biol 20(7):2356–2367. https://doi.org/10.1111/gcb.12475
Harris ZM, Alberti G, Viger M, Jenkins JR, Rowe R, McNamara NP, Taylor G (2017) Land-use change to bioenergy: grassland to short rotation coppice willow has an improved carbon balance. Glob Chang Biol Bioenergy 9:469–484. https://doi.org/10.1111/gcbb.12347
Berhongaray G, Verlinden MS, Broeckx LS, Janssens IA, Ceulemans R (2017) Soil carbon and belowground carbon balance of a short-rotation coppice: assessments from three different approaches. Glob Chang Biol Bioenergy 9(2):299–313. https://doi.org/10.1111/gcbb.12369
Chimento C, Almagro M, Amaducci S (2016) Carbon sequestration potential in perennial bioenergy crops: the importance of organic matter inputs and its physical protection. Glob Chang Biol Bioenergy 8(1):111–121. https://doi.org/10.1111/gcbb.12232
Gauder M, Billen N, Zikeli S, Laub M, Graeff-Honninger S, Claupein W (2016) Soil carbon stocks in different bioenergy cropping systems including subsoil. Soil Tillage Res 155:308–317. https://doi.org/10.1016/j.still.2015.09.005
Hellebrand HJ, Straehle M, Scholz V, Kern J (2010) Soil carbon, soil nitrate, and soil emissions of nitrous oxide during cultivation of energy crops. Nutr Cycl Agroecosyst 87(2):175–186. https://doi.org/10.1007/s10705-009-9326-z
Pacaldo RS, Volk TA, Briggs RD (2013) Greenhouse gas potentials of shrub willow biomass crops based on below- and aboveground biomass inventory along a 19-year chronosequence. Bioenergy Res 6(1):252–262. https://doi.org/10.1007/s12155-012-9250-y
Walter K, Don A, Flessa H (2015) No general soil carbon sequestration under central European short rotation coppices. Glob Chang Biol Bioenergy 7(4):727–740. https://doi.org/10.1111/gcbb.12177
Das A, Lal R, Somireddy U, Bonin C, Verma S, Rimal BK (2016) Changes in soil quality and carbon storage under biofuel crops in Central Ohio. Soil Res 54(4):371–382. https://doi.org/10.1071/sr14353
Gioacchini P, Cattaneo F, Barbanti L, Montecchio D, Ciavatta C, Marzadori C (2016) Carbon sequestration and distribution in soil aggregate fractions under Miscanthus and giant reed in the Mediterranean area. Soil Tillage Res 163:235–242. https://doi.org/10.1016/j.still.2016.06.009
Qin ZC, Dunn JB, Kwon HY, Mueller S, Wander MM (2016) Soil carbon sequestration and land use change associated with biofuel production: empirical evidence. Glob Chang Biol Bioenergy 8(1):66–80. https://doi.org/10.1111/gcbb.12237
Kahle P, Beuch S, Boelcke B, Leinweber P, Schulten HR (2001) Cropping of Miscanthus in Central Europe: biomass production and influence on nutrients and soil organic matter. Eur J Agron 15(3):171–184. https://doi.org/10.1016/s1161-0301(01)00102-2
Randerson JT, Field CB, Fung IY, Tans PP (1999) Increases in early season ecosystem uptake explain recent changes in the seasonal cycle of atmospheric CO2 at high northern latitudes. Geophys Res Lett 26(17):2765–2768. https://doi.org/10.1029/1999gl900500
Zhao FJ, Spiro B, McGrath SP (2001) Trends in 13C/12C ratios and C isotope discrimination of wheat since 1845. Oecologia 128(3):336–342. https://doi.org/10.1007/s004420100663
Dungait JAJ, Docherty G, Straker V, Evershed RP (2010) Seasonal variations in bulk tissue, fatty acid and monosaccharide δ13C values of leaves from mesotrophic grassland plant communities under different grazing managements. Phytochemistry 71(4):415–428. https://doi.org/10.1016/j.phytochem.2009.10.010
Köhler IH, Poulton PR, Auerswald K, Schnyder H (2010) Intrinsic water-use efficiency of temperate seminatural grassland has increased since 1857: an analysis of carbon isotope discrimination of herbage from the Park Grass Experiment. Glob Chang Biol 16(5):1531–1541. https://doi.org/10.1111/j.1365-2486.2009.02067.x
Drewer J, Dufosse K, Skiba UM, Gabrielle B (2016) Changes in isotopic signatures of soil carbon and CO2 respiration immediately and one year after Miscanthus removal. Glob Chang Biol Bioenergy 8(1):59–65. https://doi.org/10.1111/gcbb.12230
Díaz-Pinés E, Molina-Herrera S, Dannenmann M, Braun J, Haas E, Willibald G, Arias-Navarro C, Grote R, Wolf B, Saiz G, Aust C, Schnitzler J-P, Butterbach-Bahl K (2017) Nitrate leaching and soil nitrous oxide emissions diminish with time in a hybrid poplar short-rotation coppice in southern Germany. Glob Chang Biol Bioenergy 9:613–626. https://doi.org/10.1111/gcbb.12367
Fitton N, Datta A, Cloy JM, Rees RM, Topp CFE, Bell MJ, Cardenas LM, Williams J, Smith K, Thorman R, Watson CJ, McGeough KL, Kuhnert M, Hastings A, Anthony S, Chadwick D, Smith P (2017) Modelling spatial and inter-annual variations of nitrous oxide emissions from UK cropland and grasslands using DailyDayCent. Agric Ecosyst Environ 250:1–11. https://doi.org/10.1016/j.agee.2017.08.032
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Glob Chang Biol 18(6):1781–1796. https://doi.org/10.1111/j.1365-2486.2012.02665.x
Poeplau C, Don A, Vesterdal L, Leifeld J, van Wesemael B, Schumacher J, Gensior A (2011) Temporal dynamics of soil organic carbon after land-use change in the temperate zone—carbon response functions as a model approach. Glob Chang Biol 17(7):2415–2427. https://doi.org/10.1111/j.1365-2486.2011.02408.x
Gregory AS, Dungait JAJ, Watts CW, Bol R, Dixon ER, White RP, Whitmore AP (2016) Long-term management changes topsoil and subsoil organic carbon and nitrogen dynamics in a temperate agricultural system. Eur J Soil Sci 67(4):421–430. https://doi.org/10.1111/ejss.12359
Acknowledgements
We thank the following colleagues at Rothamsted Research for their help with this work: A Karp, PJ Murray (planning and discussion); TJP Barraclough, M Castle, I Durenkamp, P Fruen (sampling); M Masna, DJW Steele (sample processing and root analysis); M Brookman, A Duffy, M Redmile-Gordon, R Skilton and X Zhou (soil analysis); farm staff (maintaining the field experiments). We also thank two anonymous reviewers and the editors for their constructive comments in improving the paper.
Funding
This work formed part of the ‘Maximising carbon retention in soils’ project (BBS/E/C/00005214) funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC). The field experiment was established under the Bioenergy from Miscanthus And Salix Species (BioMASS) project (BB/G016216/1) of the BBSRC Sustainable Bioenergy Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gregory, A.S., Dungait, J.A.J., Shield, I.F. et al. Species and Genotype Effects of Bioenergy Crops on Root Production, Carbon and Nitrogen in Temperate Agricultural Soil. Bioenerg. Res. 11, 382–397 (2018). https://doi.org/10.1007/s12155-018-9903-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-018-9903-6