Abstract
Aims
Root exudates contain polymers that form crosslinks and can create a jelly like substance known as mucilage, which adheres to soil and thus promotes the formation of rhizosheaths, i.e. soil that remains attached to the roots after gentle shaking. We hypothesized that rhizosheath formation is optimal at an intermediate chia seed mucilage concentration and water content, but that its formation is limited at both a high concentration of chia seed mucilage and under dry conditions as well as at a low concentration of chia seed mucilage and under wet conditions. We used an artificial root soil system in which soil moisture and mucilage concentrations could be varied independently from one another with respect to their effect on rhizosheath formation.
Methods
Jute cords were disposed in sandy loam soil and in quartz sand. In a subsequent study, they were also amended to different moisture contents with five different concentrations of mucilage (from 0 to 0.2 g dry mucilage g−1 water), before being isolated from chia and flaxseed mucilage after swelling of the respective seeds in distilled water for 15 min.
Results
We found that in dry soil, rhizosheath formation peaked at an intermediate chia seed mucilage concentration. This behavior was supported by our conceptual model of mucilage spreading and rhizosheath formation, which relies on a radial diffusion equation and assumes that at low mucilage concentration, molecule numbers are insufficient to support polymer-like networks that stick soil particles together. In a very concentrated gel, however, mucilage is too sticky to diffuse far into the soil. Increasing soil moisture promotes rhizosheath formation both in a low and a high mucilage concentration range, although only up to an intermediate volumetric water content of 0.15cm3 cm–3.
Conclusions
We conclude that both water and chia seed mucilage concentration are important drivers of rhizosheath formation. The effects are not additive but can combine to an optimum range, with a maximum formation of rhizosheaths observed in this study at 0.12 g mucilage g−1 rhizosphere water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil is compacted by root growth, which in turn reduces spatial rhizosphere extension. Exudates are produced in the rhizosphere by plant roots and microorganisms, thus affecting the soil structure along with alternating drying and wetting cycles (Czarnes et al. 2000). Some of these changes affect the subsequent uptake of water and nutrients as well as the associated root penetration resistance and microbial population dynamics (Hinsinger et al. 2009; Oleghe et al. 2017; York et al. 2016). Plant exudates that trigger these processes comprise molecules of various molecular weights (Cortez and Billes 1982; Mench et al. 1988). A dominant high molecular weight exudate from the root tips is mucilage (Morré et al. 1967).
Mucilage is a polymeric gel, mostly made up of polysaccharides from plants and microorganisms, which also contains a trace amount of lipids (Vermeer and McCully 1982; Read et al. 2003). McCully and Boyer (1997) reported that mucilage has a large water holding capacity. Mucilage that is fully hydrated can contain up to 1000 times its own dry weight. The authors discovered that mucilage drops a considerable portion of its water at water potentials lower than—0.01 MPa, concluding that the water content of mucilage does not play a major role in drought mitigation through water storage. However, it was suggested that mucilage can enhance the drought tolerance of plants by sustaining a better liquid connectivity via mucilage bridges between soil particles and thus a better hydraulic conductivity in the rhizosphere under rather dry soil conditions (Carminati et al. 2011, 2017; Ahmed et al. 2014).
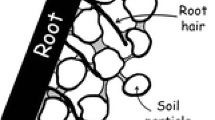
Mucilage consists of long polymers that build up crosslinks that form a network-like structure in soil, which can attach to soil surfaces (Fig. 1). The applicability of the typical diffusion equation that was originally developed for spreading low molecular solutes in soils is limited. When freshly exuded, mucilage – a mixture of polymers, water, and other components – has a certain concentration. At typical concentrations of exudation, mucilage is a fluid of high viscosity. When exuded into dry or wet soil, this exuded liquid can become more concentrated or diluted, transitioning to a more solid-like behavior at high mucilage concentrations or towards a more Newtonian liquid with a low viscosity at lower concentrations (Carminati et al. 2017; Schnepf et al. 2022). A proper simulation of this spreading would require both (a) the simulation of the hydrological process, i.e. the dynamics of the spreading of exuded water into the soil, and (b) the simulation of the distribution of mucilage components, i.e. diffusion, convection, adsorption, and the formation and rupture (due to friction) of crosslinks, and subsequently also the degradation of mucilage.
(a) During drying, crosslinks are formed between polymers and mucilage undergoes a phase transition from a Newtonian fluid at a high water content to a polymeric solution of increased viscosity, a hydrogel, and, finally, a solid. At low concentrations, diffusion is important for polymer dynamics, while at higher concentrations crosslinks are created and network dynamics become dominant. (b) Over time, polymer adhesion to the soil particle surface and the formation of crosslinks reduce polymer mobility and form a spider-web-like structure in the pore space
Hydrological processes and the spreading of mucilage are dynamics that affect each other: the mobility of water molecules is strongly reduced when mucilage polymers at high concentration increase the viscosity of soil solution, build up crosslinks, and become attached to the soil surface, thus forming a spider-web-like structure within the pore space (Brax et al. 2020; Kroener et al. 2018). However, diffusion, convection, adsorption, and the rupture and formation of crosslinks are strongly affected by water content and hydraulic dynamics (Bittelli et al. 2015).
Many of these processes are highly non-linear and we assume that these processes depend on (a) physical and chemical soil properties, and (b) on the chemical composition of the root exudates, i.e. plant species, root age, and growth conditions. For most of these processes, there is still a lack of not only parameters but also a model to describe these mucilage-specific individual physical processes. Although urgently needed, the development of such models and the systematic experimental determination of all required parameters goes far beyond a single research study. In this study, to provide a qualitative description of the effect of mucilage concentration under dry soil conditions, all previously mentioned coupled processes were considered in the model and the simplified diffusion equation was applied.
We assume that these liquid bridges between the soil particles and the mucilage have a strong influence on the formation of rhizosheaths, especially under dry soil conditions. The rhizosheath is formed by mucilage and root hairs that assist the soil clinging to the roots. Rhizosheath was observed in the 19th century as soil particles adhering to grass roots in the desert (Volkens 1887), although its name was established much later (Wullstein et al. 1979). Today, it is sometimes described as the weight of soil that adheres to roots when excavated from the pot or field (George et al. 2014; McCully 1999). The rhizosheath should also not be confused with the rhizosphere. Rhizosheath refers to soil that physically adheres to the root system, whereas rhizosphere refers to soil influenced by roots (Hassan and Mathesius 2015), which means that the rhizosphere spreads beyond the boundaries of the rhizosheath (York et al. 2016). Under dry conditions, rhizosheaths are very common, especially in cereals and wild grasses (Price 1911; Watt et al. 1994; Young 1995: Wullstein et al. 1979).
The rhizosheath is thus only a part of the rhizosphere, and is commonly discovered when the soils are dry (Watt et al. 1994), but is hardly found when they are wet. There was no rhizosheath present when plant species lacked root hairs (Lawrie K Brown et al. 2017), which suggests that mucilage exudation alone is insufficient for the formation of rhizosheaths (Margaret E McCully 1999). However, the presence of root hair is not an absolute requirement for rhizosheath formation, instead depending on numerous factors such as root type, root system, root length, or the amount of composition of root mucilage (Fan et al. 2001; Muszyński et al. 2015; Peña et al. 2012; Vančura and Hanzlíková 1972). However, in the root-hairless mutants, the adhesiveness of the exudate became more prominent. This was due to variations in the chemical composition of the root mucilage, which partially compensated for the lack of root hairs that would normally physically entangle soil particles (Burak et al. 2021). Overall, the rhizosheath includes soil particles, an intricate structure of root hairs, a local bacterial community, and mucilage, even in the case of maize (Gochnauer et al. 1989). The surface tension of mucilage increases with the dehydration of the mucilage in dry soil, meaning that the elevated viscosity additionally contributes to the stabilization of both aggregates and its surrounding rhizosheath (Read and Gregory 1997).
The main aim of this study was to disentangle the complex interactions between mucilage concentration and moisture content and their effect on rhizosheath formation. We hypothesized that the effects were not simply additive. We tested the following hypotheses in depth: (a) at low concentrations of mucilage, and thus low viscosity, mucilage can easily spread into distant parts of the soil, with the result that the concentration and binding properties of mucilage next to the root are not sufficient to establish a stable, large rhizosheath; (b) at a higher mucilage concentration, the substance can no longer diffuse far into the soil, meaning that the extension of the rhizosheath is small. As a consequence, c) the largest extension of the rhizosheath can be expected at an intermediate concentration of mucilage. Finally, we assume that d) the optimum value of rhizosheath formation shifts with variations in the soil moisture content and the associated mucilage concentrations. To qualitatively test our hypotheses, we used a simple model for spreading mucilage by radial diffusion.
Materials & methods
Extraction of mucilage from chia seeds
Physico-chemical conditions of root mucilage can vary significantly depending on plant species, plant age, and soil conditions. Sufficient quantities of real root mucilage are difficult to extract from roots in soil. Therefore, mucilage, both from chia seeds (Salvia hispanica L.) and from flax seeds (Linum usitatissimum L.), were used in this study as plant model mucilage. Both chia and flax seed mucilage have been used to resemble root mucilage in artificial root–soil systems (Hayat et al. 2021; Naveed et al. 2017, 2019; Oleghe et al. 2017; Paporisch et al. 2021).
The chemical composition of chia seed mucilage is similar to maize (Zea mays L.) root mucilage. They are both composed of xylose, glucose, and uronic acids, with the latter making up around 25% of the composition (Carminati and Vetterlein 2013; Lin et al. 1994). Moreover, chia seed mucilage has similar physical characteristics to maize and lupin (Lupinus albus L.) mucilage, as it forms a gel when hydrated and becomes hydrophobic after drying. Hydrophobicity is lower in flax seed mucilage compared to chia seed mucilage. Moreover, flax seed mucilage is less attached to seeds than chia seed mucilage.
Mucilage was extracted by the method described by Ahmed et al. (2014). In this method, 5 g of seeds were mixed in 50 g of water. The mixture was stirred with a magnetic stirrer for 2 min and kept for 2 h at room temperature. We pushed this mixture through a sieve using a syringe that was cut at the end, thus separating the seeds from their mucilage. It is worth mentioning that the stickiest and gel-like part of the mucilage remained attached to the seeds. The extracted wet mucilage was freeze-dried to obtain a powder of dry mucilage, which can be easily mixed with a certain amount of water to obtain mucilage at a desired concentration.
Preparation of soil
We used two different soil textures for this study: a sandy loam and quartz sand. The soils were air-dried and sieved to pass through the particle size of 2 mm. The particle size distributions of both soils are presented in Table 1. The soil was homogeneously packed in PVC cylinders with a height of 1.4 cm and an internal diameter of 4.5 cm, corresponding to a soil volume of 22 cm3. For the soil packing, soil was poured into the cylinder lying horizontally in order to minimize soil layering. This process resulted in a bulk density of 1.45 g cm–3 and 1.7 g cm–3 for the sandy loam and quartz sand soils, respectively. First, soil was poured into the PVC cylinders. We then inserted the artificial root at the top of the soil layer. The artificial root should resemble a plant root with root hairs and was made up of jute material with a diameter of 3 mm. A wet mucilage solution was prepared at five concentrations: 0.0 g (control), 0.02 g, 0.04 g, 0.12 g, and 0.2 g dry mucilage g−1 water. To prepare the desired mucilage concentrations, freeze-dried mucilage was diluted with deionized water and kept in a sealed container for 15 min to swell. The wet mucilage (2 g) was then uniformly injected by syringe onto the artificial root model to resemble the exudation of mucilage into soil. As a control, we simply used 2 g of water instead of mucilage. Finally, additional soil was uniformly poured over the sample and soil samples were kept for 48 h at 25 °C ± 1 °C room temperature. For the application of wet mucilage, we calculated its total area [cm2] assuming that the root had a cylindrical shape with a radius of 3 mm and a root length of 70 mm. Finally, additional soil was uniformly poured over the sample and soil samples were kept for 48 h at 25 °C ± 1 °C room temperature.
At the end of this experiment, the artificial root was removed from the soil and weighed. This experiment consisted of four replicates for each treatment. We performed two studies using the same method to investigate rhizosheath formation in two soils – sandy loam and quartz – under the influence of chia seed mucilage concentration. In the other study, we simply monitored the effect of chia and flax seed mucilage in only quartz sand. The purpose of this experiment was to compare the influence of the gravimetric concentration of chia and flax seed mucilage on rhizosheath formation. We did not observe a visible swelling of the jute material. We repeated these two experiments in four replicates.
In another separate study to investigate the effect of volumetric moisture contents on rhizosheath formation, 40 g of oven-dried soil samples were gently packed into a sample holder using a similar method as mentioned above. The soil water content was maintained at five different volumetric moisture contents: control (without moisture), 0.5cm3 cm–3, 0.15cm3 cm–3, 0.30cm3 cm–3, and 0.35 cm3 cm–3 by adding an appropriate amount of distilled water to an oven-dried soil. The soil was prepared by putting some soil into the PVC cylinders. The artificial root was subsequently placed on the soil surface and then (2 g) mucilage was spread on the root in similar concentrations (0.0 g, 0.02 g, 0.4 g, and 0.12 g dry mucilage g−1 water), as outlined above. Soil water content was maintained by weighing soil samples with the addition of an appropriate amount of distilled water. Once the required moisture content was ensured, the samples were kept for 48 h at 25 °C ± 1 °C room temperature. The samples were covered with a plastic sheet to avoid any evaporation. After 48 h, the artificial roots were removed from the soil. They were then gently shaken to remove extra soil from the roots. The roots were then weighed on an electric balance to obtain the mass of the fresh rhizosheaths. The rhizosheaths were subsequently oven-dried at 405 °C for 48 h to obtain the dried mass of the rhizosheaths. We repeated this experiment with three replicates.
Model of mucilage spreading and rhizosheath formation using the radial diffusion equation
Root systems are often described by complex three-dimensional root architecture models that require large computational resources. Since the artificial root–soil system used in our experiment exhibits translational symmetry along the root, it is sufficient to consider the 2D cross section. For this model, we also assume rotational symmetry. This is a simplification of the experimental geometry, which is not exactly rotational symmetric due to gravity and due to the application of mucilage from the top on the horizontally lying root. The radial rotational symmetric diffusion–adsorption equation for solutes in soil (Bittelli et al. 2015; Landl et al. 2021) is:
where \({\uprho }_{b}\) is the soil bulk density (g cm−1), \(\mathrm{C}\) (g g−1 dry soil) is the mucilage concentration per dry soil, \(\uptheta\) (cm3 cm−3) is the volumetric water content, \(\mathrm{t}\) (s) is time, \({\uprho }_{l}\) is the water density (g cm−1), c (g g−1 water) is the mucilage concentration in soil solution, \(\mathrm{r}\) (m) is the radial coordinate, \(\mathrm{D}\) (m2 s) is the diffusion coefficient, and \(\mathrm{k}\) (d−1) is the adsorption coefficient. Assuming the simplification of a constant soil water content and using \({\uprho }_{b}\mathrm{C}=\uptheta {\uprho }_{l}c\), this becomes:
Numerous diffusion studies have shown that the diffusion equation can provide reasonable results for solutes of low molecular weights. As discussed in the previous paragraph for the diffusion of mucilage polymers, this is a simplified model. Various mobilities of the polymers of mucilage exuded at high, medium, and low concentrations are represented in the model by choosing a low, medium, and high diffusion coefficient in liquid (Table 2). The relation between mucilage concentration, polymer mobility, and the diffusion coefficient is highly non-linear. The diffusion coefficient in soil is obtained from the liquid diffusion coefficient by considering soil tortuosity using the equation \(D(\theta )={D}_{0}\cdot \left(\frac{{\theta }^{10/3}}{{\varphi }^{2}}\right)\) (Landl et al. 2021; Millington and Quirk 1961).
After a certain time \({t}_{end}\), we assume that – due to the formation of crosslinks between polymers and due to the attachment of polymers to soil particle surfaces – the spreading of mucilage comes to an end and mucilage polymers can then be considered to be immobile. This assumption of a \({t}_{end}\) when mucilage becomes immobile is in agreement with experimental observations by neutron radiography (Moradi et al. 2012) showing the region of soil around the roots where the presence of mucilage alters the soil hydraulic dynamics. Indeed, the region affected by mucilage has a similar extension around one-day-old roots as around two-week-old roots, which suggests that mucilage diffusion occurs to a large extent during the first few days before coming to an end. In this simplified model, the transition of mucilage from a mobile phase to an immobile mucilage phase is represented by choosing certain values of \({t}_{end}\) where the diffusion process stops. Technically, the mucilage turning immobile is not an adsorption process where solutes are fully adsorbed to soil particle surfaces. Instead, it is largely a formation of polymer crosslinks, which strengthens the polymeric network, with only partial binding to surfaces, but which may still occupy the entire pore space. We therefore set the adsorption parameter \(k=0\) and the solution of Eq. (3) is:
\({C}_{0}\) accounts for the initial mucilage distribution, representing the radial exudation from the point source: \(\mathrm{C}\left(r,t=0\right)={C}_{0}\delta \left(0\right)\), where \(\delta \left(0\right)\) is the delta function in space.
From a simulated mucilage distribution \(\mathrm{C}\left(r,{t}_{end}\right)\), the rhizosheath extension can roughly be obtained by assuming that it may occur when the mucilage concentration is high enough to glue soil particles to each other, i.e. in the regions where \(\mathrm{C}\left(r,{t}_{end}\right)>{\mathrm{C}}_{rhiz}\) with \({\mathrm{C}}_{rhiz}\) as a threshold value that may depend on the physico-chemical properties of mucilage and soil.
Results
Rhizosheath formation in a sandy loam and quartz sand soil
The key results of this experimental study are presented in Fig. 2. The rhizosheaths measured [g cm–1] in a sandy loam soil were studied under various concentrations of applied chia seed mucilage [g dry mucilage per g water]. At intermediate mucilage concentrations (0.12 g dry mucilage g−1 water), the average dry mass of consolidated and coherent rhizosheaths per dry mass of root peaked at 3.63 g cm–1. In contrast, the formation and development of rhizosheaths at low (0.02 g dry mucilage g−1 water) and high (0.2 g dry mucilage g−1 water) mucilage concentrations resulted in an average mass of only 0.13 g cm–1 and 0.36 g cm–1 respectively. Likewise, there was no significant difference in rhizosheath formation in a quartz sandy soil (Fig. 2) compared to sandy loam soil under the same concentrations of chia seed mucilage. Overall, it followed the same trend in terms of stable rhizosheath formation, i.e. the peak in rhizosheath formation was replicated, which was recorded at 4 g cm–1 and again at intermediate mucilage concentrations (0.12 g dry mucilage g−1 water). In addition, similar to the sandy loam soil, rhizosheath formation was smaller at higher (0.2 g dry mucilage g−1 water) and lower mucilage concentrations (0.02 g dry mucilage g−1 water) of applied chia seed mucilage.
Comparison of chiaseed and flaxseed mucilage with respect to rhizosheath formation
Figure 3 shows rhizosheath formation under numerous concentrations of flax seed mucilage. The average mass of rhizosheaths was negligibly small in relation to that of chia seed mucilage. This is in agreement with our own observations during mucilage extraction from seeds, i.e. chia seed mucilage was much stickier than flax seed mucilage.
Rhizosheath formation as a function of water content
In the quartz sandy soil, rhizosheath formation was also studied as a function of volumetric content Vol. WC (cm3 cm–3), which is depicted in Fig. 4. In general, the soil water content (dry: soil not very sticky; medium: most sticky; wet: hardly sticky) also had a major influence on the volume of the rhizosheath. At the highest water content (0.35 cm3 cm–3), the concentration of mucilage did not have a strong impact on rhizosheath formation, and the former peak disappeared (Fig. 4). Similarly, at a low water content, the formation of rhizosheaths hardly required the presence of mucilage. However, this was different under drought conditions, which are typically critical for plants to take up water and mineral nutrients. Under such conditions, intense root–soil contact is crucial for plant nutrition. In line with these ecological requirements, at 0 (no water) Vol. WC (cm3 cm–3), the mucilage concentration had a significant influence on rhizosheath extension, with the amount of rhizosheaths peaking at an intermediate content, as indicated above. Mucilage is therefore crucial in facilitating nutrient acquisition by increasing the root surface area, for example by enhancing nutrient availability through chelating agents and by enhancing plant resilience to various abiotic stresses, such as drought, salinity, and heavy metal toxicity.
Formation of rhizosheaths as a function of Vol. WC [cm3 cm–3] in a quartz sandy soil under various chia seed mucilage concentrations. Control means no water was added in an oven-dried quartz sandy soil by a mean value of ± SD, n = 3, control Vol. WC [cm3 cm–3], while the rest of the soils were irrigated by 0.05, 0.15, 0.30, and 0.35[cm3 cm–3] Vol. WC, and analyzed for rhizosheath development by a mean value of ± SD, n = 3
Model of mucilage spreading and rhizosheath formation
The simulated spreading of mucilage in soil (Fig. 5) shows that when mucilage was applied at high (0.2 g dry mucilage g−1 water) concentrations, it could only spread a short distance with a high concentration near the initial mucilage pulse. In contrast, when mucilage was applied at intermediate concentrations (0.12 g dry mucilage g−1 water), it could spread a few millimeters, occupying a considerable soil volume with a relevant amount of mucilage. At a low concentration of applied mucilage (0.02–0.04 g dry mucilage g−1 water), the polymers were likely to have easily diffused into more distant areas, making it impossible to form a polymeric network between soil particles that could glue them together to form a rhizosheath. As a result, the formation of rhizosheaths was largest at an intermediate concentration of applied mucilage. At lower and higher concentrations, rhizosheath extension was much shorter, i.e. the conceptual model was able to mimic the observations of the laboratory experiments.
(a) Simulated diffusion of mucilage in soil based on the parameters of Table 2. (b) The extension of rhizosheaths that is expected to form where mucilage concentration in soil is larger than a certain threshold value
Discussions
Rhizosheath formation measurements under varied mucilage concentrations as well as the conceptual model of mucilage spreading and rhizosheath extension in dry soil (Fig. 1 and 2) were able to confirm our hypothesis. We hypothesized that mucilage has a strong influence on rhizosheath formation under dry soil conditions. In dry soils, the intermediate mucilage concentrations of the polymeric network is sufficient to glue and stick soil particles around the root for amplified rhizosheath formation in both soil types. This can be better explained in terms of mucilage diffusion, which allowed a greater volume of rhizosheath extension around the roots. We expected to observe a negligent and weak formation of rhizosheaths at lower concentrations of applied mucilage due to the high diffusion of polymers and, therefore, the wide spreading of mucilage distribution and thus a low concentration in soil. Similarly, higher mucilage concentrations exhibit a lower mobility of polymers. This limits the distribution of mucilage in soil, which in turn resulted in a reduced region of soil agglutination for the development of rhizosheath volume. At higher concentrations, the viscosity of mucilage is expected to be 1000 times higher than pure water, as shown for chia mucilage by Ahmed et al. (2016). Our experiments show that, similar to viscosity, the mobility of mucilage polymers in soil solution also varies strongly with mucilage concentration. We found the same behavior of rhizosheath development in both soils: sandy loam and quartz sand. Moreover, at a low mucilage concentration, the formation of rhizosheaths in sandy loam is higher than in quartz sand. We assume this could be attributed to the presence of large amount of fine particles in sandy loam as compared to quartz sand.
We also compared the effect of chia seed and flax seed mucilage under the same mucilage concentrations on the development of rhizosheaths (Fig. 3). Our findings indicate no significant effect on rhizosheath formation by flax seed mucilage. We assume that this effect is due to the difference in the viscosity of chia seed and flax seed mucilage. This is in agreement with the results reported by Brax et al. (2020) and Mazza and Biliaderis (1989), in which they compared the viscosity of chia and flax seed mucilage. The findings of both studies confirmed the higher viscosity of chia seed mucilage compared to flax seed mucilage at all concentrations studied. Bemiller et al. (1993) also recorded a high viscosity of chia seed mucilage compared to flax seed mucilage. Similarly, Naveed et al. (2019) and Brütsch et al. (2019) reported a higher viscosity of chia seed mucilage than barley.
In a parallel study, rhizosheath formation as a function of soil Vol. WC (cm3 cm–3) showed a smaller formation of rhizosheaths at low soil Vol. WC (cm3 cm–3) due to the reduced stickiness and cohesiveness of dry soil. In these conditions, there is insufficient moisture available in the soil to facilitate the binding of soil particles to the root surface, resulting in a smaller and less developed rhizosheath of the soil. Conversely, with high soil WC (cm3 cm–3), the formation of rhizosheaths was again reduced as well. Excessive soil moisture can lead to waterlogged or saturated conditions, where water fills the pore spaces between soil particles, creating a physical barrier between the root surface and soil particles. This barrier limits the adhesion and interaction between roots and soil particles, hindering the formation of a well-developed rhizosheath. These observations underscore the critical role of maintaining an optimal balance of soil moisture for effective root-soil interactions and nutrient uptake by plants, as variations in soil moisture content can significantly influence rhizosheath formation. This phenomenon aligns with previous studies that investigate the relationship between soil stickiness and moisture content, providing valuable insights into root-soil dynamics (Hallett & Young 1999; Watt et al. 1994). These observations are also in line with a previous study of Liu et al. (2019), who measured rhizosheath development in response to moisture content. They reported the highest formation of rhizosheaths at 10–14% (W/W) compared to other soil moisture levels. Similarly, we can also assume that at higher moisture contents, the actual influence of mucilage is not significant, particularly because mucilage can dissolve under wet conditions, thus diffusing along with water into the surrounding soil (Watt et al. 1994), but not concentrating near the roots to form a network of polymers.
Here, we used artificial roots. In real soil, the rhizosphere may dry out again following the transpiration of the plant. Unlike conditions in the model system, there is therefore the chance for mucilage to dry in a system with living plants and to form a polymeric network again, unless it is decomposed by soil microorganisms.
Rhizosheath formation under dry conditions was closely related to mucilage concentration. The maximum rhizosheath formation was found at intermediate concentrations and was about 10 times higher than rhizosheath formation at other concentrations. These findings were in line with the outcomes of Watt et al. (1993, 1994), who reported that under dry conditions, larger and coherent rhizosheaths were bound to the roots than under wetter conditions. Moreover, in dry soils, the rhizosheaths of certain grasses were approximately three times larger than in wet soils. At low mucilage concentration, facilitated diffusion seemingly resulted in a spreading of the compounds to a degree that soil mucilage concentration was likely not particularly significant with respect to gluing soil particles together, i.e. it only formed a thin, transient rhizosheath layer, whereas rhizosheath formation declined again towards even higher mucilage concentrations (Figs. 4, 5).
When the water content increased for the given optimum “intermediate” mucilage concentration in the spike solution, the peak of rhizosheath formation shifted towards higher concentrations of the added water (Fig. 4, 5), which likely reflects the respective dilution of the polymers to optimum concentrations again. In any case, mucilage and water content did not have a purely additively effect, and neither one nor the other appeared to be solely responsible for the degree and amount of rhizosheaths formed. Instead, it was the concentration of the compounds in the gel, i.e. in the available soil water volume.
Conclusions
Overall, the current study showed that rhizosheaths are formed at various mucilage concentrations. This was demonstrated experimentally and based on a radial model using a diffusion equation. The degree of rhizosheath formation, however, is dependent on concentration and moisture, thus requiring a calculation of an effective mucilage concentration in the pore water of the rhizosphere. In the experiment and using the modelling approach, we have seen that under dry soil conditions, i.e. In the oven-dried quartz sandy soil, when the soil's volumetric moisture content deviated from zero, it was observed that an intermediate mucilage concentration of 0.12 g dry mucilage g−1 water led to the most substantial volume of rhizosheaths, surpassing both low (0.02 g dry mucilage g−1 water) and high (0.2 g dry mucilage g−1 water) mucilage concentrations. This physical behavior of mucilage in rhizosheaths might be crucial to supporting plant nutrient uptake and water availability under drought and stress conditions. The presence of mucilage in rhizosheath formation provides promising conditions for plants to become more tolerant to abiotic stresses and to improve agricultural yield in drought-prone areas.
Data availability
“The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.”
References
Ahmed MA, Kroener E, Holz M, Zarebanadkouki M, Carminati A (2014) Mucilage exudation facilitates root water uptake in dry soils. Funct Plant Biol 41(11):1129–1137
Ahmed MA, Kroener E, Benard P, Zarebanadkouki M, Kaestner A, Carminati A (2016) Drying of mucilage causes water repellency in the rhizosphere of maize: measurements and modelling. Plant Soil 407(1–2):161–171. https://doi.org/10.1007/s11104-015-2749-1
Bemiller JN, Whistler ROYL, Barkalow DG, Chen C-C (1993) Aloe, chia, flaxseed, okra, psyllium seed, quince seed, and tamarind gums. In Industrial gums (pp 227–256). Elsevier
Bittelli M, Campbell GS, Tomei F (2015) Soil physics with Python: transport in the soil-plant-atmosphere system. OUP Oxford
Brax M, Buchmann C, Kenngott K, Schaumann GE, Diehl D (2020) Influence of the physico-chemical properties of root mucilage and model substances on the microstructural stability of sand. Biogeochemistry 147(1):35–52
Brown LK, George TS, Neugebauer K, White PJ (2017) The rhizosheath–a potential trait for future agricultural sustainability occurs in orders throughout the angiosperms. Plant Soil 418(1–2):115–128
Brütsch L, Stringer FJ, Kuster S, Windhab EJ, Fischer P (2019) Chia seed mucilage–a vegan thickener: Isolation, tailoring viscoelasticity and rehydration. Food Funct 10(8):4854–4860
Burak E, Quinton JN, Dodd IC (2021) Root hairs are the most important root trait for rhizosheath formation of barley (Hordeum vulgare), maize (Zea mays) and Lotus japonicus (Gifu). Ann Bot 128(1):45–57
Carminati A, Vetterlein D (2013) Plasticity of rhizosphere hydraulic properties as a key for efficient utilization of scarce resources. Ann Bot 112(2):277–290
Carminati A, Schneider CL, Moradi AB, Zarebanadkouki M, Vetterlein D, Vogel HJ, Hildebrandt A, Weller U, Schüler L, Oswald SE (2011) How the rhizosphere may favor water availability to roots. Vadose Zone Journal 10(3):988–998
Carminati A, Benard P, Ahmed MA, Zarebanadkouki M (2017) Liquid bridges at the root-soil interface. Plant Soil 417(1):1–15
Cortez J, & Billes G (1982) Role of calcium ions in the formation of Zea mays mucigel
Czarnes S, Hallett PD, Bengough AG, Young IM (2000) Root-and microbial-derived mucilages affect soil structure and water transport. Eur J Soil Sci 51(3):435–443
Fan TW-M, Lane AN, Shenker M, Bartley JP, Crowley D, Higashi RM (2001) Comprehensive chemical profiling of gramineous plant root exudates using high-resolution NMR and MS. Phytochemistry 57(2):209–221
George TS, Brown LK, Ramsay L, White PJ, Newton AC, Bengough AG, Russell J, Thomas WTB (2014) Understanding the genetic control and physiological traits associated with rhizosheath production by barley (Hordeum vulgare). New Phytol 203(1):195–205
Gochnauer MB, McCully ME, Labbe H (1989) Different populations of bacteria associated with sheathed and bare regions of roots of field-grown maize. Plant Soil 114(1):107–120
Hallett PD, Young IM (1999) Changes to water repellence of soil aggregates caused by substrate-induced microbial activity. Eur J Soil Sci 50(1):35–40
Hassan S, & Mathesius U (2015) Flavonoids Play Multiple Roles in Symbiotic Root–Rhizosphere Interactions. Biological Nitrogen Fixation 499–510
Hayat F, Abdalla M, Munir MU (2021) Effect of Chia Seed Mucilage on the Rhizosphere Hydraulic Characteristics. Sustainability 13(6):3303
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321(1–2):117–152
Kroener E, Holz M, Zarebanadkouki M, Ahmed M, Carminati A (2018) Effects of mucilage on rhizosphere hydraulic functions depend on soil particle size. Vadose Zone Journal 17(1):1–11
Landl M, Phalempin M, Schlüter S, Vetterlein D, Vanderborght J, Kroener E, & Schnepf A (2021) Modeling the nfluence of rhizosphere bulk density and mucilage gradients on root water uptake. Front Agron 3(6)
Lin K-Y, Daniel JR, Whistler RL (1994) Structure of chia seed polysaccharide exudate. Carbohyd Polym 23(1):13–18
Liu T, Ye N, Song T, Cao Y, Gao B, Zhang D, Zhu F, Chen M, Zhang Y, Xu W (2019) Rhizosheathformation and involvement in foxtail millet (Setaria italica) root growth under drought stress. J Integr Plant Biol 61(4):449–462
Mazza G, Biliaderis CG (1989) Functional properties of flax seed mucilage. J Food Sci 54(5):1302–1305
McCully ME (1999) Roots in soil: unearthing the complexities of roots and their rhizospheres. Annu Rev Plant Biol 50(1):695–718
McCully ME, Boyer JS (1997) The expansion of maize root-cap mucilage during hydration. 3. Changes in water potential and water content. Physiologia Plantarum 99(1):169–177
Mench M, Morel JL, Guckert A, Guillet B (1988) Metal binding with root exudates of low molecular weight. J Soil Sci 39(4):521–527
Millington R, Quirk J (1961) Permeability of porous solids. Trans Faraday Soc 57:1200–1207
Moradi AB, Carminati A, Lamparter A, Woche SK, Bachmann J, Vetterlein D, ... Oswald SE (2012) Is the rhizosphere temporarily water repellent? Vadose Zone J 11(3)
Morré DJ, Jones DD, Mollenhauer HH (1967) Golgi apparatus mediated polysaccharide secretion by outer root cap cells of Zea mays. Planta 74(3):286–301
Muszyński A, O’Neill MA, Ramasamy E, Pattathil S, Avci U, Peña MJ, Libault M, Hossain MS, Brechenmacher L, York WS (2015) Xyloglucan, galactomannan, glucuronoxylan, and rhamnogalacturonan I do not have identical structures in soybean root and root hair cell walls. Planta 242:1123–1138
Naveed M, Brown LK, Raffan AC, George TS, Bengough AG, Roose T, Sinclair I, Koebernick N, Cooper L, Hackett CA (2017) Plant exudates may stabilize or weaken soil depending on species, origin and time. Eur J Soil Sci 68(6):806–816
Naveed M, Ahmed MA, Benard P, Brown LK, George TS, Bengough AG, Roose T, Koebernick N, Hallett PD (2019) Surface tension, rheology and hydrophobicity of rhizodeposits and seed mucilage influence soil water retention and hysteresis. Plant Soil 437:65–81
Oleghe E, Naveed M, Baggs EM, Hallett PD (2017) Plant exudates improve the mechanical conditions for root penetration through compacted soils. Plant Soil 421(1–2):19–30
Paporisch A, Bavli H, Strickman RJ, Neumann RB, Schwartz N (2021) Root exudates alters nutrient transport in soil. Water Resour Res 57(10):e2021WR029976
Peña MJ, Kong Y, York WS, O’Neill MA (2012) A galacturonic acid–containing xyloglucan is involved in Arabidopsis root hair tip growth. Plant Cell 24(11):4511–4524
Price SR (1911) The roots of some North African desert-grasses. New Phytol 10(9):328–340
Read DB, Gregory PJ (1997) Surface tension and viscosity of axenic maize and lupin root mucilages. New Phytol 137(4):623–628
Read DB, Bengough AG, Gregory PJ, Crawford JW, Robinson D, Scrimgeour CM, Young IM, Zhang K, Zhang X (2003) Plant roots release phospholipid surfactants that modify the physical and chemical properties of soil. New Phytol 157(2):315–326
Schnepf A, Carminati A, Ahmed MA, Ani M, Benard P, Bentz J, Bonkowski M, Knott M, Diehl D, Duddek P (2022) Linking rhizosphere processes across scales: Opinion. Plant and Soil 478(1–2):5–42
Vančura V, Hanzlíková A (1972) Root exudates of plants: IV. Differences in chemical composition of seed and seedlings exudates. Plant Soil 36:271–282
Vermeer J, McCully ME (1982) The rhizosphere in Zea: new insight into its structure and development. Planta 156(1):45–61
Volkens G (1887) Die Flora der aegyptisch-arabischen Wüste: auf Grundlage anatomisch-physiologischer Forschungen. Gerbrüder Borntraeger (Ed. Eggers)
Watt M, McCully ME, Jeffree CE (1993) Plant and bacterial mucilages of the maize rhizosphere: comparison of their soil binding properties and histochemistry in a model system. Plant Soil 151(2):151–165
Watt M, McCully ME, Canny MJ (1994) Formation and stabilization of rhizosheaths of Zea mays L. (Effect of soil water content). Plant Physiol 106(1):179–186
Wullstein LH, Bruening ML, Bollen WB (1979) Nitrogen fixation associated with sand grain root sheaths (rhizosheaths) of certain xeric grasses. Physiologia Plantarum 46(1):1–4
York LM, Carminati A, Mooney SJ, Ritz K, Bennett MJ (2016) The holistic rhizosphere: integrating zones, processes, and semantics in the soil influenced by roots. J Exp Bot 67(12):3629–3643
Young IM (1995) Variation in moisture contents between bulk soil and the rhizosheath of wheat (Triticum aestivum L. cv. Wembley). New Phytol 130(1):135–139
Acknowledgements
The authors would like to thank the Higher Education Commission of Pakistan (HEC) for the scholarship funds, which were jointly provided by the German Academic Exchange Service (DAAD) and the German Research Foundation (DFG) under Germany’s Excellence Strategy-EXC 2070-390732324 (PhenoRob).
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Higher Education Commission of Pakistan (HEC) in collaboration with the German Academic Exchange Service (DAAD). The author (Riffat Rahim) obtained funding from the German Research Foundation (DFG) as part of Germany’s Excellence Strategy-EXC 2070–390732324 (PhenoRob).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Conceptualization, methodology, software, validation, writing – original draft; writing – review & editing was performed by Riffat Rahim and Eva Kroener. The first draft of the manuscript was written by Riffat Rahim and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors confirm and declare that there is no conflict of interest.
Additional information
Responsible Editor: Jiayin Pang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahim, R., Jahromi, O.E., Amelung, W. et al. Rhizosheath formation depends on mucilage concentration and water content. Plant Soil 495, 649–661 (2024). https://doi.org/10.1007/s11104-023-06353-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06353-4