Abstract
Background and aims
Proteaceae are a prominent plant family in south-western Australia. Most Proteaceae are ‘calcifuge’, occurring exclusively on old phosphorus (P)-impoverished acidic soils, with a few ‘soil-indifferent’ species also found on young P-richer calcareous soils. Calcium (Ca)-enhanced P toxicity explains the calcifuge habit of Proteaceae. However, previous research has so far been focused exclusively on the roles of Ca and P in determining Proteaceae distribution, and consequently there is little knowledge on how other soil-based strategies influence this distribution. We aimed to study the effects of young calcareous soils on four soil-grown Proteaceae and assess differences between calcifuge and soil-indifferent Proteaceae to better understand their natural distribution.
Methods
Two calcifuge and two soil-indifferent Proteaceae from south-western Australia were grown in six contrasting soils, including young calcareous, and old acidic soils.
Results
When grown in calcareous soils all species showed root growth inhibition, micronutrient deficiency, Ca-enhanced P toxicity, and negative impacts on physiology. Calcifuge species were more sensitive to calcareous soils than soil-indifferent ones, although this varied between genera. Soil-indifferent species tended to produce more cluster roots, release more carboxylates per root mass, and allocate less Ca to their leaves, compared with calcifuges; they also had smaller seeds and were less sensitive to Ca-enhanced P toxicity.
Conclusion
We surmise that a combination of these traits allows soil-indifferent species to tolerate calcareous soils. This study provides insight into how Proteaceae respond to young calcareous soils and how this influences their distribution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calcareous and alkaline soils cover more than 30% of the earth’s surface, contain up to 95% free calcium carbonate and have a high pH (Chen and Barak 1982; Marschner 2012). This creates an extremely harsh environment that excludes most plants, and, consequently, calcareous soils have a strong influence on the distribution of species (Lee 1998). In fact, the role of calcareous soils in influencing species distribution is so apparent and important in ecology that it was one of the first systems to be studied in the field of experimental plant ecology; however, despite over a century of research it is still not fully understood (Tansley 1917; Burström 1968; Grubb et al. 1969; Lee 1998; Bothe 2015; Vélez-Bermúdez and Schmidt 2022).
Species that are unable to grow on calcareous soils are considered ‘calcifuge’, whilst those that preferentially grow on calcium-rich or calcareous soils are ‘calcicole’ (de Silva 1934). Species that occur on both acidic soils and calcareous soils are ‘soil-indifferent’ (Zohlen and Tyler 2000; Marschner 2012; Hayes et al. 2019b). Edaphic factors including low soil phosphorus (P) availability, low micronutrient (iron (Fe), zinc (Zn), and manganese (Mn)) availability at high pH, high bicarbonate concentrations, and high calcium (Ca) concentrations have all been shown to restrict calcifuge species from calcareous soils (Jefferies and Willis 1964; Lee and Woolhouse 1969; Grundon 1972; Tyler 1992; Tyler and Olsson 1993; Zohlen and Tyler 1997, 2000; Zohlen 2002; Hayes et al. 2019a, b; Kotula et al. 2020; Guilherme Pereira et al. 2021). Soil-indifferent and calcicole species show a range of strategies to overcome these edaphic factors (Tyler and Ström 1995; Ström 1997; Zohlen and Tyler 2000; Hayes et al. 2019a, b). However, most of our understanding of calcicole/soil-indifferent strategies has been developed in regions where older calcareous soils have a P availability that is lower than that of adjacent acidic soils (Tyler 1992). In these systems, the primary limitation for many calcifuges on calcareous soils is thought to be their inability to access enough P in P-poor calcareous soils (Tyler 1992; Tyler and Olsson 1993; Zohlen and Tyler 2000).
Recent studies have highlighted an exciting and novel system on the young calcareous soils of the Swan Coastal Plain, south-western Australia (Turner and Laliberté 2015; Hayes 2018; Hayes et al. 2019a, b; Kotula et al. 2020; Guilherme Pereira et al. 2021). Unlike other calcareous soils, these calcareous dunes (< 7,000 year old) exhibit relatively high total and plant-available soil P concentrations when compared with the immediately adjacent and much older acidic dunes (> 120,000 year) (McArthur and Bettenay 1974; McArthur 2004; Hayes et al. 2014; Turner and Laliberté 2015; Turner et al. 2018). These younger calcareous soils also exhibit very high carbonate concentrations, high Ca concentrations, and high soil pH (Turner and Laliberté 2015). This system is located within a biodiversity hotspot with an exceptional level of species diversity and species turnover, making it a unique system to study species distributions across calcareous and acidic soils (Zemunik et al. 2015, 2016). The most dramatic shift in species composition occurs between the young calcareous soils and the older acidic ones (Zemunik et al. 2016).
Proteaceae are an iconic and prominent plant family in south-western Australia, with > 650 species found in the region, representing almost 40% of all Proteaceae globally and contributing significantly to the region’s exceptional biodiversity (Cowling and Lamont 1998; George 1998; Myers et al. 2000; Hopper and Gioia 2004; Weston 2007; Brundrett 2021). Although Proteaceae are quite widespread in south-western Australia, very few species occur on young calcareous dunes, with most only found on much older P-impoverished acidic dunes (Zemunik et al. 2015, 2016). Therefore, most Proteaceae are ‘calcifuge’, with only a few ‘soil-indifferent’ species that naturally occur on both calcareous and acidic dunes, and none known to occur exclusively on calcareous soils (Hayes 2018).
Many Proteaceae are highly P sensitive and exhibit Ca-enhanced P toxicity, with calcifuge species being more P sensitive than soil-indifferent ones (Hayes et al. 2019b). It has, therefore, been proposed that it is the higher soil [P] of young calcareous soils, in combination with high soil [Ca] and low available micronutrients that excludes most Proteaceae from calcareous habitats, and that soil-indifferent species are able to overcome these limitations by tightly regulating leaf cellular [Ca] and [P], and by strategies that increase leaf micronutrient concentrations (Hayes et al. 2019a, b; Guilherme Pereira et al. 2021).
Proteaceae produce carboxylate- and proton-releasing cluster roots, allowing them to mobilise soil P and micronutrients, as well as acidifying the rhizosphere (Dinkelaker et al. 1995; Shane and Lambers 2005). These structures would, therefore, be expected to allow them to mobilise and take up micronutrients from calcareous soils. However, studies on this system have so far been restricted to hydroponic experiments, and it is, therefore, not yet known how roots of these species are impacted by calcareous soils. This leads to the question: are Proteaceae species able to produce functional cluster roots that are effective in calcareous soils and does this differ between calcifuge and soil-indifferent species?
We grew four Proteaceae species in six contrasting soils. These soils included three unamended field-collected soils: two calcareous and one acidic, as well as three amended acidic soils, where combinations of P and CaCO3 were added. Species included two calcifuge and two soil-indifferent Banksia and Hakea species. Our aims were to: (1) study the effects of calcareous soils on all species, (2) assess differences in the sensitivities of calcifuge and soil-indifferent species, and (3) compare traits of calcifuge and soil-indifferent species when grown in soil, to explain their different natural distributions.
First, we hypothesised that all species would show reduced growth and micronutrient deficiency symptoms under calcareous conditions, driven by high pH, high bicarbonate, and high Ca concentrations. Second, we hypothesised that calcifuge species would be more sensitive to calcareous soils than soil-indifferent ones, as demonstrated in previous hydroponic experiments (Hayes et al. 2019b). Finally, a comparison of calcifuge and soil-indifferent traits would allow us to discover how soil-indifferent species tolerate calcareous conditions, providing an ecologically relevant understanding of the calcifuge habit of Proteaceae.
Materials and methods
Species selection
We selected four Proteaceae from two genera, Hakea and Banksia, found along the Jurien Bay dune chronosequence on the Swan Coastal Plain, south-western Australia, ~ 200 km north of Perth (Hayes et al. 2014; Turner and Laliberté 2015; Zemunik et al. 2015). We selected two calcifuge species (Hakea incrassata R.Br. and Banksia menziesii R.Br.) and two soil-indifferent species (H. prostrata R.Br. and B. prionotes Lindl.). The distribution of these species and their sensitivities to Ca and P are known (Hayes et al. 2014, 2019a, b; Zemunik et al. 2015; Guilherme Pereira et al. 2021). All studied species can be considered to use Strategy I for Fe acquisition (Kobayashi and Nishizawa 2012); however, recent research has indicated that, unlike in other Strategy I species, the regulation of Fe uptake in Banksia may not be based on the regulation of root-mediated Fe reduction (Cawthray et al. 2021).
Soil collection, treatments, and analyses
Plants were grown in soils collected from three stages of the Jurien Bay chronosequence; the young calcareous Quindalup Medium (QM, stage 2) and Quindalup Old (QO, stage 3), and the oldest acidic Bassendean (B, stage 6) (Turner and Laliberté 2015; Zemunik et al. 2015). Bulk soil (0–20 cm) was collected from 10 spatially distributed permanent plots in each stage (Turner and Laliberté 2015). Soil from each stage was air-dried, mixed, and sieved (2 mm), before being triple pasteurised at 80˚C for 2 h per day over seven days.
This study included six soil conditions. Three unamended (but pasteurised) field-collected soils: Bassendean (B), Quindalup Medium (QM), and Quindalup Old (QO), and three amended B soils. The three amended B soils were B with added P (B + P), B with added CaCO3 (B + Ca), and B with added CaCO3 and P (B + Ca + P). Phosphorus was added as dissolved KH2PO4 (2.8 and 4.2 mg P kg–1 dry soil, B + P and B + Ca + P, respectively) to achieve a resin-P (a measure of readily-available soil P) close to the average of QO and QM soils (Table 1). Calcium carbonate was added as powdered CaCO3 (30 g kg–1 dry soil) to increase soil pH to the average of QO and QM soils (Table 1). Calcium carbonate amendments involved mixing CaCO3 into dry soil (end-over-end mixing, 300 revolutions). Pots (2.8 L) containing soil were watered and after seven days, P was added. All pots were then left to stabilise for a further seven days before planting.
Three extra pots per soil type were collected immediately prior to planting and used to determine soil chemical properties (Table 1 and S1). The content of each pot was homogenised, air dried, and analysed for pH, carbonate, total P, exchangeable P, total N, and exchangeable and total cations at the Smithsonian Tropical Research Institute (Panamá, Repúblic de Panamá). Soil pH was determined in deionised (DI) water, and in a 1:2 soil-to-solution ratio with 10 mM CaCl2 using a glass electrode. Carbonate content was determined by mass loss after the addition of 3 M HCl (Loeppert and Suarez 1996). Total P and total cations (Ca, Fe, Zn, Mg, Mn, Cu, K, and Al) were determined by nitric acid digestion followed by inductively coupled plasma optical-emission spectrometry (ICP-OES; Optima 7300DV; Perkin Elmer Inc, Wellesley, MA, USA). Readily-exchangeable phosphate (resin-P) was determined by extraction with anion-exchange membranes in DI water, followed by molybdate colorimetry using a Lachat Quickchem 8500 (Hach Ltd, Loveland, CO, USA) (Turner and Romero 2009; Turner and Laliberté 2015). Total N was measured by dry combustion and gas chromatography on a Thermo Flash 1112 analyser (CE Elantech, Lakewood, NJ, USA). Exchangeable cations (Ca, Fe, Zn, Mg, Mn, Cu, K, and Al) were determined by Mehlich-III extraction and ICP-OES (Optima 7300DV; Perkin Elmer Inc, Wellesley, MA, USA ) (Mehlich 1984).
Soil chemical properties are detailed in Table 1 and Supplementary Table S1. In brief, the control B soil and the amended B + P soil were acidic (pH 5.7–5.9), whilst field-collected calcareous (QO and QM) and CaCO3-amended soils (B + Ca + P and B + Ca) were alkaline (pH 8.2–8.6). Soil carbonate concentration was highest in field-collected calcareous soils (QM and QO) and lowest in the acidic soils (B and B + P), while amended calcareous soils (B + Ca + P and B + Ca) were intermediate. Soil total P concentration was highest in field-collected calcareous soils (QM and QO) and was similarly low in all other soils. Resin-P concentration was highest in field-collected calcareous soils (QM and QO) and in P-amended soils (B + P, and B + Ca + P).
Plant growth
The study was split into two consecutive experiments, with a common control of B soil (all species naturally occur on B). The first experiment (December 2014 to September 2015) included only unamended field-collected soils: B, QO, and QM. The second experiment (March to December 2015) included the control B and the amended soils: B + P, B + Ca + P, and B + Ca. As expected, results for plants grown in the control soil were consistent between experiments and were combined in the analyses.
Plants were grown from seeds collected along the Jurien Bay chronosequence. Seeds were collected from two or more populations on either acidic (calcifuge) or calcareous (soil-indifferent) soils (Hayes et al. 2019b). Seeds were collected from 10 to 30 individual mature healthy plants at each population in November 2013 and stored under cool, dry conditions. Seeds from the same collection were also used in several other studies (Hayes 2018; Hayes et al. 2019a, b; Guilherme Pereira et al. 2021). Seeds were surface sterilised in 1% (w/v) sodium hypochlorite (20 s) and 70% (v/v) ethanol (20 s), rinsed with DI water between and after each treatment. Sterilised seeds were germinated on moist filter paper in Petri dishes, until the primary root began to emerge (~ three weeks, 15˚C, 12 h light : 12 h dark); they were then transferred to 64-cell seedling trays containing sterilised low-nutrient acidic Spearwood sand (Turner and Laliberté 2015). Seedlings were grown for ~ 10 weeks in a glasshouse before seedlings of uniform size were selected and transferred after removing loose soil to 2.8 L sealed pots containing the final treatments. Each pot contained a single plant, with six pots per species*treatment combination (n = 6). Pots were watered with DI water to weight, three times per week, to 80% pot capacity. Plants were grown in a temperature-controlled glasshouse at the University of Western Australia; at a mean temperature/relative humidity of 18˚C / 57% (first experiment) and 19˚C / 54% (second experiment).
Harvest measurements
Plants were grown for six months, after which light-saturated net photosynthetic rates (Asat) and chlorophyll fluorescence were measured. Less than one week later, plants were harvested. Light-saturated net photosynthetic rates were measured using an infrared gas analysis system (LI-COR 6400, LI-COR Inc., Lincoln NE, USA). The two youngest fully-expanded leaves of each plant were measured separately (later averaged), using a 6 cm2 chamber (2 × 3 cm) under a saturating light level of 2,000 µmol photons m–2 s–1 (red-blue light source; 6400-02B) at 400 µmol mol–1 CO2. The flow rate was set at 200–500 µmol s–1, leaf temperature was controlled at 25˚C, and relative humidity maintained at 60–80%. Where the leaf area in the chamber was < 6 cm2, the measured area was marked and later removed during the harvest, traced onto graph paper, scanned, and the area determined using Fiji image analysis software (Schindelin et al. 2012).
Chlorophyll fluorescence was measured on the same two leaves as Asat, using a Plant Efficiency Analyser fluorometer (PEA, Hansatech Instruments Ltd., King’s Lynn, Norfolk, UK). The variable over maximal fluorescence (Fv/Fm) reflects the maximum potential quantum efficiency of photosystem II and was measured on dark-adjusted leaves (30 min using leaf clips with shutter).
During harvest, plants were separated into mature leaves (ML; fully expanded leaves), immature leaves (IL; soft expanding leaves and shoots), stems (ST), cluster roots (CR), and non-cluster roots (NCR). Aboveground parts were separated into ML, IL and ST. All ML were rinsed in 0.1 M HCl and DI water, before patting dry and sub-sampling the two youngest fully-mature leaves for chlorophyll concentration (described below). Root systems were then removed from the soil, and rhizosheath carboxylates collected (described below). Roots were thoroughly rinsed with DI water to remove all soil before separating them into CR and NCR. All plant parts were weighed fresh (FW) before being oven dried (70˚C, 72 h) and weighed dry (DW).
Plant nutrient analyses
Dried subsamples of mature leaves, stems, and non-cluster roots were ground in a vertical ball-mill grinder using plastic vials and yttrium-stabilised zirconium ceramic beads (Geno/Grinder, SPEX SamplePrep, Metuchen, NJ, USA). Samples were acid-digested using concentrated HNO3:HCl (1:1) and analysed for Ca, Cu, Fe, K, Mg, Mn, Na, P, S, and Zn by ICP-OES (National Measurements Institute, Perth, WA, Australia) using either an axially configured Varian Vista Pro (Varian Australia Pty Ltd, Mulgrave, Vic, Australia), or PlasmaQuant PQ9000 in radial view (Analytik Jena, Jena, Germany), depending on element concentrations.
Rhizosheath carboxylates
During the harvest, plants were removed from the soil and gently shaken to remove bulk soil. The soil left attached to the roots was defined as rhizosheath (Pang et al. 2017). The entire root system was then submerged in a beaker containing a known volume of 0.2 mM CaSO4 (30 to 250 mL depending on plant size), and the majority of the rhizosheath removed by washing it away from the root mass with a syringe, taking care not to damage roots. A subsample of the rhizosheath extract was collected and filtered through a 0.22 μm syringe filter into a 10 mL tube and immediately frozen at − 20˚C. This known volume of extract was freeze-dried and resuspended in a 95 : 5, 25 mM KH2PO4 : methanol solution adjusted to pH 2.5 with orthophosphoric acid. Resuspension volumes ranged from 400 µL to 800 µL, depending on concentration of analytes. A 200 µL subsample was transferred to a full-recovery high-performance liquid chromatography (HPLC) vial and analysed for carboxylates. Carboxylates, including oxalate, were determined using reverse phase high performance liquid chromatography, RP-HPLC (600E pump, 717plus auto-injector, 996 photodiode array detector (PAD); Waters, Milford, MA, USA) as described in detail in Cawthray (2003) and Uloth et al. (2015). Organic acid standards were obtained from ICN Biomedicals (Aurora, Ohio, USA), of analytical quality. The carboxylates determined and their limits of detection (µM) were: citrate (5), malate (7), iso-citrate (10), oxalate (8), trans-aconitate (0.1), cis-aconitate (0.1), fumarate (0.06), maleate (0.05), shikimate (0.1), succinate (15), acetate (24), lactate (13), and malonate (8) (Cawthray 2003; Uloth et al. 2015). Acetate, lactate, and malonate were always below detection and removed from the analysis. Total carboxylates was the sum of all detected carboxylates, mainly citrate, malate, iso-citrate, oxalate, and trans-aconitate. All carboxylate amounts were expressed per unit root FW.
Chlorophyll
During the harvest, we removed two 5-mm discs from each of the two youngest fully-mature leaves using a cork borer; they were weighed fresh, immediately frozen in liquid N, and stored in 2 mL cryovials at − 80˚C. Leaf discs were ground under cryo conditions using a vertical ball mill grinder and ceramic beads (Geno/Grinder, SPEX SamplePrep, Metuchen, NJ, USA), and later homogenised in 2 mL methanol. Samples were then incubated for one hour on a platform mixer (230 rpm), before centrifugation at 14,462 g for 5 min. The supernatant was then transferred to a fresh tube. Samples were diluted with methanol, if necessary, and the absorbance determined at 470, 653, and 666 nm. Samples were always kept in the dark and below 4˚C. Chlorophyll a and b concentrations were calculated using published equations (Wellburn 1994; Warren 2008).
Seedling emergence experiment
A separate experiment was conducted to assess the ability of the studied species to complete germination and emerge in calcareous versus acidic soils. Nine lots of 46 seeds were randomly selected for each species and surface sterilised as described above. Seeds were sown 10 mm deep into 64-cell seedling trays containing either B, QO, or QM soil (pasteurised). Two seeds were sown per cell, using a randomised block design. Trays were covered with a transparent plastic cover and lightly watered as necessary. All seeds and soil were the same as those used in the main experiment. Seedling emergence was recorded twice weekly over 98 days, with emergence recorded when seedlings emerged > 3 mm above the soil surface, or when the cotyledons were fully expanded, whichever came first. The experiment was conducted in a temperature-controlled room (15˚C, 12 h light : 12 h dark). This temperature was chosen because 15˚C is between the mean daily minimum (10˚C) and maximum (20˚C) temperatures in Jurien Bay in July (Australian Bureau of Meteorology, http://www.bom.gov.au/climate/data/), approximately when seeds of these species naturally germinate and this temperature has been used successfully in previous experiments (Hayes et al. 2019a, b; Guilherme Pereira et al. 2021). Seeds from this experiment were previously analysed to determine seed mass, nutrient concentration, and nutrient content, see Hayes et al. (2019b) for further details.
Calculations and statistics
The relative growth rate (RGR, mg g–1 week–1) was calculated as
where \({DW}_{total}\) and \({DW}_{seed}\) are the total plant and seed DW, respectively, and \({T}_{1}\) and \({T}_{2}\) are the times of sowing and harvesting (in weeks), respectively. We acknowledge that using seed DW instead of an initial plant DW is not ideal and if species have different seed:seed coat weight ratios it can impact estimates of RGR (Pérez-Harguindeguy et al. 2013).
The Emergence Value (EV), an index combining speed and completeness of emergence, was determined from the seedling emergence experiment (Czabator 1962).
where \(PV\) is the peak value, derived from the cumulative percent emergence on any day divided by the number of days since sowing, and MDE is the mean daily emergence, calculated as the percent emergence divided by the number of days to the end of the experiment (98 days). Leaf nutrient allocation was calculated as the percentage of total plant nutrient allocated to leaves, i.e. total mature leaf nutrient content divided by the sum of the total mature leaf, total stem, and total root nutrient content, reported as a percentage.
Differences in traits among treatments and species, including their interaction, were tested using generalised least squares models. Differences in leaf nutrient allocation among species were tested using general linear mixed-effects models with treatment set as the random effect (Pinheiro and Bates 2000). The residuals of each model were visually inspected for heteroskedasticity. In the presence of heteroskedasticity, appropriate variance structures were specified if they significantly improved the model based on Akaike’s Information Criterion corrected for finite sample sizes (AICc) (Pinheiro and Bates 2000). Differences among species and treatments were defined using Tukey HSD post-hoc tests (Hothorn et al. 2008).
Principal Component Analysis (PCA) was used to visually illustrate separation of species based on key traits (Clarke 1993). These traits were leaf Zn allocation, leaf Ca allocation, total rhizosheath carboxylates per unit root FW, percent iso-citrate, root : shoot DW ratio, percent DW allocated to cluster roots, and RGR. PCAs were performed on Banksia or Hakea plants grown in field-collected calcareous soils (QO and QM combined). There was a small number of missing values for Hakea (8 of 312) due to random factors. To create complete data sets, missing values were imputed using an iterative PCA approach (Josse and Husson 2016). PCA was then performed on full data sets using the R function ‘prcomp’; results were extracted using ‘get_pca’ and plotted using ‘ggbiplot’ (Vu 2011; Kassambara and Mundt 2020).
All statistical analyses were performed using the R software platform (R Core Team 2021) with the packages: ‘emmeans’ (Lenth 2022), ‘factoextra’ (Kassambara and Mundt 2020), ‘missMDA’ (Josse and Husson 2016), ‘multcomp’ (Hothorn et al. 2008), ‘nlme’ (Pinheiro et al. 2021), ‘stats’ (R Core Team 2021), and ‘tidyverse’ (Wickham et al. 2019).
Results
Visual symptoms
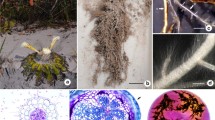
Visual symptoms were most severe for plants grown in field-collected calcareous soils (QO and QM), with similar, but less severe symptoms in the amended calcareous soils (B + Ca + P and B + Ca) (Fig. 1). There were no visual symptoms in acidic soils (B and B + P). In field-collected calcareous soils, all species showed signs of chlorosis and necrosis, indicating severe nutrient imbalance but the degree of response was different among species and particularly between genera. In all species except H. prostrata, the B + Ca + P treatment tended to show slightly more severe visual symptoms than B + Ca, while there were no visual symptoms in H. prostrata in either soil. In summary, all species grew best in acidic soils and worst in calcareous soils, and H. prostrata appeared least sensitive.
Visual symptoms of four Proteaceae from two genera (Hakea and Banksia) grown in six contrasting soils for six months. Soils included one unamended acidic soil (Bassendean, B), two unamended calcareous soils (Quindalup Old, QO; Quindalup Medium, QM), and three amended Bassendean soils with added phosphorus (P) and/or calcium carbonate (Ca). Bassendean was considered the control. Calcifuge (CF) species do not naturally occur on calcareous soils, whilst soil-indifferent (SI) species occur on both calcareous and acidic soils. Inset images illustrate typical leaf symptoms in calcareous soils (insets not to scale). Black lines were added to some leaves during the experiment to determine areas for photosynthetic measurements. See Table 1 for soil chemical properties. Bars, 10 cm
When comparing within each genus calcifuges showed more severe visual symptoms than the soil-indifferent species of the same genus (Fig. 1). In the calcifuge H. incrassata, the main visual symptoms were severe chlorosis and mild leaf-tip necrosis, whilst soil-indifferent H. prostrata showed only minor interveinal chlorosis. The calcifuge B. menziesii showed the most severe symptoms of all species: almost complete chlorosis, severe necrosis leading to leaf loss, stunted leaf development and rosetting of terminal leaves in some plants. Soil-indifferent B. prionotes, however, showed less severe chlorosis and necrosis, and began developing slightly greener leaves without rosetting (Fig. 1).
Biomass response
The RGR was slower in calcareous soils by an average of 39% in Banksia plants and 10% in Hakea plants than in the control (P < 0.05, Fig. 2a). In general, soil-indifferent species had a faster RGR than calcifuge species (Fig. 2a). Soil-indifferent B. prionotes had, on average, a 22% faster RGR than calcifuge B. menziesii (P < 0.05, Fig. 2a). Similarly, soil-indifferent H. prostrata had a 12% faster RGR on QM soil than calcifuge H. incrassata (P < 0.001).
a Relative growth rate (RGR), b percentage of total plant biomass allocated to cluster roots, and c leaf, stem, cluster root, and non-cluster root dry weights of calcifuge (CF) and soil-indifferent (SI) Hakea and Banksia species, grown in six contrasting soils for six months. Soils included one unamended acidic soil (Bassendean, B), two unamended calcareous soils (Quindalup Old, QO; Quindalup Medium, QM), and three amended Bassendean soils with added phosphorus (P) and/or calcium carbonate (Ca). Bassendean was considered the control. Soils B + Ca + P, B + Ca, QO, and QM are calcareous and B, and B + P are acidic. Bar heights show means and error bars show standard errors (n = 5–12). Different letters indicate significant differences within (a, b) each species and (c) for above- and belowground total dry weights (post-hoc Tukey test, P < 0.05). P-values (*, < 0.05; **, < 0.01; ***, < 0.001) represent significant differences between species of contrasting distribution types (CF and SI) (post-hoc Tukey test)
Except for H. prostrata, plants grown in field-collected calcareous soils showed a lower percentage biomass allocated to cluster roots compared with the control, and lower total root biomass, (P < 0.05, Fig. 2b–c). For example, in calcifuge H. incrassata the investment in cluster roots was halved (Fig. 2b), whilst in soil-indifferent H. prostrata biomass investment in cluster roots was maintained across all soils (P > 0.05). In soil-indifferent B. prionotes cluster-root biomass was lower in calcareous soils, but some investment was maintained; 2% cluster-root biomass in QO/QM compared to 18% in B (Figs. 2b–c, S1a). By contrast, calcifuge B. menziesii produced almost no cluster roots in calcareous soils compared with an investment in cluster-root biomass of 11% in acidic soils down to 0% in most calcareous soils (Figs. 2b–c, S1a).
All species had lower total biomass in calcareous soils than in acidic ones (Figs. 2c, S1b). The acidic B + P treatment produced the largest biomass in almost all species, followed by the control. There were no consistent differences in total biomass between distribution types (Fig. S1b). Soil-indifferent H. prostrata had a greater root : shoot ratio than H. incrassata in all calcareous soils (P < 0.01, Fig. S1c). This was largely accounted for by a greater allocation of biomass to cluster roots in H. prostrata (Figs. 2b–c, S1a). Banksia distribution types had similar root : shoot ratios (P > 0.05). In summary, calcareous soils reduced RGR, cluster-root investment and total root biomass, while soil-indifferent species showed a greater cluster-root investment and faster RGR than calcifuges.
Rhizosheath carboxylates
Rhizosheath carboxylate amounts per unit root FW differed significantly among soils within Hakea plants but were consistently low among Banksia plants (Fig. 3a). Importantly, in H. prostrata, total rhizosheath carboxylate amounts were greater in calcareous soils than in acidic soils (P < 0.05, Fig. 3a). By contrast, Banksia plants showed no clear difference between calcareous and acidic soils. Soil-indifferent H. prostrata showed by far the highest amount of carboxylates, 34 µmol g–1 root FW when grown in QM. This amount was 45-fold greater than in the control and 6.5-fold greater than for H. incrassata in the same soil. In Banksia plants, both distribution types generally had similar amounts of rhizosheath carboxylates; however, soil-indifferent B. prionotes showed greater amounts than B. menziesii in the most carbonate-rich soil (QM, P < 0.05).
a Total amount of carboxylates per unit root fresh weight (FW), and b composition of rhizosheath carboxylates of calcifuge (CF) and soil-indifferent (SI) Hakea and Banksia species, grown in six contrasting soils for six months. Soils included one unamended acidic soil (Bassendean, B), two unamended calcareous soils (Quindalup Old, QO; Quindalup Medium, QM), and three amended Bassendean soils with added phosphorus (P) and/or calcium carbonate (Ca). Bassendean was considered the control. Soils B + Ca + P, B + Ca, QO, and QM are calcareous and B, and B + P are acidic. Carboxylates listed as ‘others’ included cis-aconitate, fumarate, maleate, shikimate, and succinate. Bar heights show means and error bars show standard errors (n = 5–12). Different letters indicate significant differences within each species (post-hoc Tukey test, P < 0.05). P-values (*, < 0.05; **, < 0.01; ***, < 0.001) represent significant differences between species of contrasting distribution types (CF and SI) (post-hoc Tukey test)
The composition of rhizosheath carboxylates differed to a major extent between calcareous and acidic soils. All species released iso-citrate almost exclusively in calcareous soils, with amounts of about 3% of all carboxylates in acidic soils, and 42% in calcareous soils (Figs. 3b, S2). By contrast, oxalate was released in acidic soils, but barely detected in calcareous ones (Fig. 3b). Furthermore, soil-indifferent species tended to show a slightly higher percentage of iso-citrate than calcifuges (Figs. 3b, S2). For example, iso-citrate accounted for, on average, 13% more of the total carboxylates in B. prionotes than in B. menziesii, and, on average 8%, more in H. prostrata than in H. incrassata in calcareous soils.
In summary, Hakea plants had a higher rhizosheath carboxylate amount than Banksia plants. Both Hakea species released more carboxylates in calcareous soils than in acidic ones, with soil-indifferent H. prostrata releasing substantially more than calcifuge H. incrassata. All species in calcareous soils had higher iso-citrate and lower oxalate amounts than in acidic soils, with soil-indifferent species tending to release more iso-citrate than calcifuges.
Nutrient concentrations and allocations
Soil-indifferent species allocated less Ca to their leaves than calcifuges did (P < 0.05, Figs. 4a, S3b). For example, differences in Ca allocation were 46% (H. prostrata) versus 67% (H. incrassata), and 49% (B. prionotes) versus 56% (B. menziesii) (Fig. S3b). Leaf Ca allocation tended to be lower in calcareous soils than in the acidic control soil (B), except for H. incrassata (Fig. 4a). The difference in leaf Ca allocation between soil types was greatest in soil-indifferent species, with H. prostrata and B. prionotes showing 19% and 37% lower values in calcareous QM than in the control. Leaf and root [Ca] were generally higher in calcareous soils than in acidic ones, with the highest [Ca] in QO and QM (Fig. 4b–c). The [Ca] among soil types increased more in roots than in leaves. Leaf [Ca] showed no clear difference between distribution types in calcareous soils, whereas the root [Ca] was generally higher in soil-indifferent species. For example, in QM, the root [Ca] was two-fold (H. prostrata) and 2.3-fold (B. prionotes) higher than in calcifuges.
a Calcium (Ca) allocation to leaves, b leaf Ca concentrations ([Ca]), c root [Ca], and d leaf phosphorus concentrations ([P]) of calcifuge (CF) and soil-indifferent (SI) Hakea and Banksia species grown in six contrasting soils for six months. Leaf Ca allocation was calculated as the percentage of total plant Ca allocated to leaves. Soils included one unamended acidic soil (Bassendean, B), two unamended calcareous soils (Quindalup Old, QO; Quindalup Medium, QM), and three amended Bassendean soils with added phosphorus (P) and/or calcium carbonate (Ca). Bassendean was considered the control. Soils B + Ca + P, B + Ca, QO, and QM are calcareous and B, and B + P are acidic. Bar heights show means and error bars show standard errors (n = 5–12). Different letters indicate significant differences within each species (post-hoc Tukey test, P < 0.05). P-values (*, < 0.05; **, < 0.01; ***, < 0.001) represent significant differences between species of contrasting distribution types (CF and SI) (post-hoc Tukey test)
All species showed a similarly low leaf [P] in the control, 244–317 µg g–1 (Fig. 4d). As expected, the highest leaf [P] was in B + P, ranging from 598 to 1035 µg g–1, while B + Ca + P gave similar results to the other calcareous soils. There was no consistent difference in leaf [P] between distribution types; however, H. prostrata did tend to have a higher leaf [P] than H. incrassata in calcareous soils. Leaf Ca : P ratios mainly reflected differences in leaf [Ca] and less so leaf [P] (Fig. S4).
In summary, soil-indifferent species allocated less Ca to their leaves than calcifuges. This was likely driven by a higher root [Ca] in soil-indifferent species in calcareous soils, compared with calcifuge species. Leaf [P] was highest in B + P, and there was no consistent difference in leaf [P] between distribution types.
Banksia prionotes allocated a greater proportion of plant total Zn to leaves than B. menziesii did, 36% versus 27% (P < 0.05) and had a higher leaf Zn allocation in calcareous soils, ranging from 23% in the acidic control (B) to 63% in the most carbonate-rich soil (QM) (Figs. 5a, S3a). By contrast, the two Hakea species had similar leaf Zn-allocation patterns (P > 0.05). Leaf Mn and Fe allocation was also similar between distribution types (Fig. S5). In comparison to what is considered adequate for crop growth (Bloom and Epstein 2005), leaf [Zn] was very low in calcareous soils, ranging from 1 to 21 µg g–1 (Fig. 5b). The leaf [Zn] of soil-indifferent species were similar to or greater than those in calcifuge species in calcareous soils. Leaf [Fe] was extremely low in calcareous soils (Bloom and Epstein 2005), ranging from 5 to 36 µg g–1 (Fig. 5c). Soil-indifferent species generally had a higher leaf [Fe] than calcifuge species in calcareous soils. For example, H. prostrata had a 2.3-fold higher leaf [Fe] than H. incrassata in the most carbonate-rich soil, QM, and it was similarly high in most calcareous soils. Leaf [Mn] was lower in calcareous soils by about 74% in Hakea species and 93% in Banksia species, compared with the control (P < 0.05, Fig. 5d). Soil-indifferent species generally had a higher leaf [Mn] than calcifuge species in calcareous soils. For example, H. prostrata had a 2.6-fold higher leaf [Mn] than H. incrassata in QM and its leaf [Mn] was higher than that of H. incrassata in most calcareous soils.
a Zinc (Zn) allocation to leaves, b leaf Zn concentrations ([Zn]), c leaf iron concentrations ([Fe]), and d leaf manganese concentrations ([Mn]) of calcifuge (CF) and soil-indifferent (SI) Hakea and Banksia species grown in six contrasting soils for six months. Leaf Zn allocation was calculated as the percentage of total plant Zn allocated to leaves. Soils included one unamended acidic soil (Bassendean, B), two unamended calcareous soils (Quindalup Old, QO; Quindalup Medium, QM), and three amended Bassendean soils with added phosphorus (P) and/or calcium carbonate (Ca). Bassendean was considered the control. Soils B + Ca + P, B + Ca, QO, and QM are calcareous and B, and B + P are acidic. Bar heights show means and error bars show standard errors (n = 5–12). Different letters indicate significant differences within each species (post-hoc Tukey test, P < 0.05). P-values (*, < 0.05; **, < 0.01; ***, < 0.001) represent significant differences between species of contrasting distribution types (CF and SI) (post-hoc Tukey test). The grey dashed lines represent the nutrient concentrations considered adequate for crop growth (Epstein and Bloom 2005)
In summary, the soil-indifferent B. prionotes had a greater allocation of Zn to leaves than the calcifuge B. menziesii, increasing in more calcareous soils. Concentrations of key leaf micronutrients (Zn, Fe, and Mn) were all very low when grown in calcareous soils, and soil-indifferent species tended to have equal or higher concentrations than calcifuges. Hakea prostrata had the most consistent and largest differences in leaf micronutrients (Zn, Fe, and Mn) compared with the calcifuge H. incrassata.
Soil-indifferent species had a seed P content that was less than half that of calcifuges (Table 2). This difference was mainly due to differences in seed mass, because seed [P] did not consistently differ between distribution types.
Chlorophyll and photosynthesis
Leaf chlorophyll (a + b) concentrations were lower in calcareous soils for all species, except H. prostrata (Fig. 6a). This reflected visual symptoms, as all species except H. prostrata were severely chlorotic under calcareous conditions (Fig. 1). Soil-indifferent H. prostrata showed a 2.4-fold higher chlorophyll concentration than calcifuge H. incrassata in calcareous soils (P < 0.01; Fig. 6a). Both Banksia species showed similar chlorophyll concentrations, with a much lower concentration in calcareous soils compared with acidic ones, averaging ~ 10% (B. menziesii) and 12% (B. prionotes) of the those grown in the control.
a Chlorophyll concentration (a + b), b light-saturated photosynthetic rate (Asat), and c chlorophyll fluorescence (Fv/Fm) of calcifuge (CF) and soil-indifferent (SI) Hakea and Banksia species grown in six contrasting soils for six months. Soils included one unamended acidic soil (Bassendean, B), two unamended calcareous soils (Quindalup Old, QO; Quindalup Medium, QM), and three amended Bassendean soils with added phosphorus (P) and/or calcium carbonate (Ca). Bassendean was considered the control. Soils B + Ca + P, B + Ca, QO, and QM are calcareous and B, and B + P are acidic. Bar heights show means and error bars show standard errors (n = 5–12). Different letters indicate significant differences within each species (post-hoc Tukey test, P < 0.05). P-values (*, < 0.05; **, < 0.01; ***, < 0.001) represent significant differences between species of contrasting distribution types (CF and SI) (post-hoc Tukey test)
Like chlorophyll concentrations, light-saturated net photosynthetic rates (Asat) were also slower in calcareous soils for all species, except H. prostrata (Fig. 6b). Soil-indifferent H. prostrata showed equal to or higher values than calcifuge H. incrassata when grown in calcareous conditions, with H. prostrata showing a faster Asat rate in the most carbonate-rich soil (QM, P < 0.01). Both Banksia species had similar Asat rates, which were much slower in calcareous soils compared with acidic ones.
Chlorophyll fluorescence (Fv/Fm) was lower in H. incrassata across most calcareous soils than in the control, while H. prostrata did not show a difference across treatments (Fig. 6c). Soil-indifferent H. prostrata maintained a fairly high average Fv/Fm of 0.77 across all soils. By contrast, Fv/Fm of calcifuge H. incrassata was lower in most calcareous soils than in the control; average 0.73 (calcareous) versus 0.83 (control). The Fv/Fm for H. prostrata was equal to or higher than that of H. incrassata when grown in calcareous soils. Both Banksia species showed similar Fv/Fm, with a large difference ranging from 0.81 in the control to 0.45 in calcareous treatments.
In summary, all species showed lower chlorophyll concentrations, Asat rates, and Fv/Fm when grown in calcareous soils, except for soil-indifferent H. prostrata. Between distribution types, soil-indifferent H. prostrata showed higher chlorophyll concentrations and similar or higher Asat and Fv/Fm than calcifuge H. incrassata in all calcareous soils. There were no differences between Banksia distribution types, with both species showing substantially lower values in calcareous soils.
Seedling emergence
Seedling emergence was similar or greater in calcareous soils compared with acidic soils, except for H. incrassata, which had lower seedling emergence in QO (Figs. 7a, S6). In calcareous soils, soil-indifferent species tended to have the highest emergence. The Emergence Value (EV) is an index combining both the speed and completeness of emergence. Soil-indifferent B. prionotes had a significantly higher EV than B. menziesii across all soils (P < 0.05), while that of H. prostrata tended to be higher than H. incrassata, but this was not significant (P > 0.05).
a Percent emergence over time, and b Emergence Value (EV) of calcifuge (CF) and soil-indifferent (SI) Hakea and Banksia species grown in three contrasting field-collected soils for 98 days. The EV is an index combining speed and completeness of emergence (Eq. 2). Soils included one unamended acidic soil (Bassendean, B), and two unamended calcareous soils (Quindalup Old, QO; Quindalup Medium, QM). Bassendean was considered the control. Soils QO and QM are calcareous, and B is acidic. Bar heights show means and error bars show standard errors (n = 3). Different letters indicate significant differences (a) within each soil type or (b) within each species (post-hoc Tukey test, P < 0.05). P-values (*, < 0.05; **, < 0.01; ***, < 0.001) represent significant differences between species of contrasting distribution types (CF and SI) (post-hoc Tukey test)
In summary, calcareous soils did not dramatically reduce seedling emergence. All species in QM were able to complete germination and emerge at rates similar to, or greater than on the control soil; emergence for all species was above 75%, and with no differences among species. Soil-indifferent species appeared to have a slight advantage in calcareous soils, tending to have a higher percent emergence and higher EVs.
Principal component analysis
Principal component analysis of key traits in plants grown in field-collected calcareous soils (QM and QO) revealed a clear separation of the two distribution types (Fig. 8). Soil-indifferent species were separated from calcifuges along the horizontal axes (PC 1), primarily due to differences in the percentage biomass allocated to cluster roots, rhizosheath carboxylate amount per unit root FW, and leaf Ca allocation (Figs. 8, S7). Soil-indifferent species were associated with a greater allocation of biomass to cluster roots, a higher rhizosheath carboxylate amount, and a greater allocation of Ca to leaves, while calcifuges showed the opposite. These three traits were common to the top four traits most contributing to PC 1 across both genera (Fig. S7).
Principal component analysis (PCA) biplot of key traits, comparing calcifuge (CF) and soil-indifferent (SI) a Hakea and b Banksia species grown in field-collected calcareous soils (QM and QO). These traits include leaf calcium allocation (%leafCa), leaf Zn allocation (%leafZn), total amount of rhizosheath carboxylates per unit root FW (Carboxylates), percent iso-citrate in the total rhizosheath carboxylate amount (%iso-citrate), percentage of biomass allocated to cluster roots (%CR), relative growth rate (RGR), and root : shoot ratio (R:S). Only 12.4% (Hakea) and 12.9% (Banksia) of variance was explained by PC 3
Discussion
All species showed reduced growth and micronutrient deficiency symptoms when grown in calcareous soils. The calcifuge Hakea species was far more sensitive to calcareous soils than the soil-indifferent one, while Banksia species showed only minor differences in visual symptoms. Soil-indifferent species produced more cluster roots, released more carboxylates, and allocated less Ca to their leaves, compared with calcifuge species; they also tended to have smaller seeds and have been shown to be less sensitive to Ca-enhanced P toxicity (Hayes et al. 2019b). We surmise that these traits allow soil-indifferent Proteaceae species in south-western Australia to exhibit greater tolerance to calcareous soils. We first focus our discussion on the responses to and consequences of growing in calcareous soils, and then present and discuss the strategies enabling soil-indifferent Proteaceae to tolerate calcareous soils.
Calcareous soils reduced growth in all species
All species were negatively impacted by calcareous soils. Symptoms included reduced biomass and RGR, as well as severe chlorosis and necrosis (Fig. 9a). Such responses are typical for sensitive species and are due primarily to high soil pH, and high bicarbonate and Ca concentrations (Tyler and Ström 1995; Lee 1998; Zohlen and Tyler 2000; Zohlen 2002; Giel and Bojarczuk 2011). These soil properties are typical of calcareous soils and directly and indirectly impact plant functioning.
Schematic illustrating a key responses of calcifuge Proteaceae to calcareous soils and b five traits common to soil-indifferent versus calcifuge species that allow soil-indifferent species to survive in calcareous habitats. Illustrated species are (a) Hakea incrassata (calcifuge) grown in acidic versus calcareous soils, and (b) H. incrassata (calcifuge) and H. prostrata (soil-indifferent) grown in calcareous soils. These responses and strategies are common among the four studied species. CO3 = soil carbonate content, Ex. = soil exchangeable cations, Asat = light-saturated photosynthetic rate
Direct inhibition of roots
Cluster-root and total root biomass were severely reduced under calcareous conditions, reflecting an inhibition of root development and growth. High pH and high bicarbonate concentrations are typical of calcareous soils and directly inhibit root growth (Tang et al. 1993; Zohlen and Tyler 2000; Peiter et al. 2001; Cross et al. 2019; Liao et al. 2020; Wala et al. 2023). The impacts of high pH and high bicarbonate concentrations on root growth have been demonstrated in a range of species including calcifuge Lupinus species (e.g., L. angustifolius, L. cosentinii, L. albus) (Tang et al. 1993, 1996; Ding et al. 2019, 2020), calcifuge grasses (e.g., Deschampsia flexuosa) (Lee and Woolhouse 1969), Zn-inefficient rice genotypes (Oryza sativa) (Yang et al. 2003), as well as barley (Hordeum vulgare), sorghum (Sorghum bicolor), and maize (Zea mays) (Alhendawi et al. 1997). Both high pH and high bicarbonate concentrations reduce root cell elongation (Lee and Woolhouse 1969; Tang et al. 1993, 1996; Peiter et al. 2001; Ding et al. 2019, 2020). High bicarbonate concentrations directly impact root elongation through interference of root cell metabolism, adverse effects on root plasma membrane integrity, and through pH-buffering in the root apoplast preventing apoplastic acidification in the root elongation zone (Alhendawi et al. 1997; Peiter et al. 2001). Similarly, high external pH interferes with the establishment and maintenance of the transmembrane pH gradient and the acidification of the apoplast, thus inhibiting root cell elongation (Tang et al. 1996; Marschner 2012). Hence, both high pH and high bicarbonate concentrations interfere with root cell elongation and consequently root growth, in accordance with the acid growth theory (Rayle and Cleland 1992).
Soil pH and bicarbonate concentrations are tightly interrelated in calcareous soils; it is, therefore, difficult to determine which is the primary cause of root inhibition (van Breemen 1991). Because soil bicarbonate concentrations increase at high pH and are a strong pH buffer, it is likely that at very high pH, such as that in young calcareous soils (pH > 8.2 in this study) both bicarbonate and pH will act together to inhibit root growth. This is supported by a recent study on Lupinus species where high pH inhibited root growth, while a high bicarbonate concentration was important in buffering pH and determining recovery and survival in calcareous soils (Ding et al. 2019). All calcareous soils in our study had a similarly high pH (pH 8.2–8.6) but differed substantially in carbonate content (3–85%) and thus bicarbonate concentration and buffering capacity. Interestingly, soils with the highest bicarbonate concentration (QO and QM) also showed the strongest inhibition of root growth, emphasising that not only soil pH, but also bicarbonate concentration and buffering capacity, are important in inhibiting root growth in calcareous soils.
Under calcareous conditions, both Banksia species and the calcifuge H. incrassata showed a much lower investment in cluster roots, with all species showing lower cluster-root biomass. The size of individual cluster roots appeared reduced under calcareous conditions, compared with those grown in acidic soils, producing shorter stunted rootlets along the main cluster root axis (Hayes pers. obs.). This is likely associated with the combination of high soil pH and high bicarbonate concentration interfering with root elongation in the cluster roots and buffering against acidification of the rhizosphere.
Micronutrient deficiency
Plants grown in calcareous soils showed symptoms consistent with severe micronutrient deficiency (e.g., Fe, Zn, Mn). These symptoms included chlorosis, necrosis, lower chlorophyll concentrations, lower biomass, and lower micronutrient concentrations (Fe, Zn, Mn). Total concentrations of Mn and Zn decreased more than that of Fe when grown in calcareous soils. However, the concentrations of all micronutrients were exceptionally low under calcareous conditions, compared with both plants grown in the control soil and concentrations considered adequate for crop growth (Fig. 5b–d; (Bloom and Epstein 2005). Without additional information on the physiological availability of these micronutrients, we refrain from further speculation of their relative importance in limiting growth (Cakmak and Marschner 1987; Zohlen and Tyler 1997, 2000; Lambers et al. 2002). Micronutrient deficiency symptoms are common in sensitive species and may be caused indirectly as a result of lower root biomass/elongation, which would lower the capacity to acquire micronutrients, as well as directly, due to soil chemistry (Tyler and Ström 1995; Lee 1998; Zohlen and Tyler 2000; Zohlen 2002).
Lower root growth and cluster-root investment would have resulted in a reduced capacity to acquire micronutrients. Reduced root elongation, caused by high pH and high bicarbonate concentrations would reduce the ability of plants to explore the soil matrix and thus reduce their capacity to acquire micronutrients. We surmise that this would have reduced the plants’ ability to mobilise sparingly-available micronutrients, limiting micronutrient uptake under calcareous conditions.
High soil pH would also have directly reduced the availability of micronutrients in calcareous soils. Reduced soil Fe availability at high pH often explains the exclusion of calcifuge species from calcareous soils (Steele 1955; Zohlen 2002; Ding et al. 2019). The strong buffering capacity of high soil bicarbonate concentrations is also important in explaining why some species normally able to acidify the rhizosphere still fail to function in calcareous habitats. For example, soil-indifferent H. prostrata only showed chlorosis under the most extreme calcareous soil, QM, which had a much higher carbonate content than other calcareous soils (85% vs. 3–42%). Amazingly, H. prostrata showed no chlorosis in other calcareous soils, despite those soils all having the same pH (pH 8.2–8.6) and producing active cluster roots in all soils (Figs. 2b and 3a). We surmise that the higher bicarbonate concentration in QM resulted in a much higher buffering capacity in the rhizosheath. This would have reduced the capacity of H. prostrata to acidify the rhizosphere, despite producing active cluster roots, and thus would have reduced the mobilisation and uptake of micronutrients, explaining why chlorosis only occurred in the most carbonate-rich soil.
Calcium-enhanced P sensitivity
A high soil Ca concentration is characteristic of calcareous soils and negatively impacts certain species (Jefferies and Willis 1964; Grundon 1972; Nichols and Beardsell 1981; Ding et al. 2018; Hayes et al. 2019b). For example, Ca enhances P sensitivity in species that are P-sensitive, such as Proteaceae (Grundon 1972; Hayes et al. 2019a, b). Species are susceptible to Ca-enhanced P toxicity if they: (1) lack the ability to strongly down-regulate P-uptake capacity (Shane et al. 2004; de Campos et al. 2013), (2) preferentially allocate P to mesophyll cells as a strategy that improves P-use efficiency (Hayes et al. 2018), and (3) poorly regulate leaf cellular [Ca] and [P] (Hayes et al. 2019a). Alternatively, in other non-P-sensitive species Ca can stimulate P uptake (Robson et al. 1970; McClure 1972; Nichols and Beardsell 1981; Hayes et al. 2019b), but this is not the case here.
High leaf [Ca] can enhance the relative distribution of leaf P to palisade mesophyll cells, resulting in a greater [P] within these cells, despite no change in whole leaf [P] (Hayes et al. 2019a). This increase in cellular [P] can be substantial; for example, Hayes et al. (2019a) showed a more than 10-fold increase (from 9 to 100 µmol g–1) in the mesophyll [P] of H. incrassata between low- and high-Ca treatments, resulting in severe chlorosis and necrosis, despite no change in total leaf [P]. An increase in mesophyll [P] is thought to reduce the availability of essential micronutrients within photosynthetic cells, most likely through precipitation of sparingly soluble micronutrients with excess phosphate (Cakmak and Marschner 1987; Takagi et al. 2020). This P-induced reduction in the cellular availability of micronutrients results in micronutrient-deficiency symptoms that are often observed as a result of Ca-enhanced P toxicity (Cakmak and Marschner 1987; Lambers et al. 2002; Broadley et al. 2012; Hayes et al. 2019a, b; Guilherme Pereira et al. 2021). All studied species experience Ca-enhanced P toxicity, with variation among species of contrasting distribution types (Hayes et al. 2019a).
High leaf [P] alone does not necessarily cause or indicate P-induced micronutrient deficiency. For example, Hayes et al. (2019b) found that for a range of Proteaceae species grown in hydroponics, there was no difference in whole leaf [P] across all Ca treatments, yet those under high Ca availability showed severe symptoms, and those under low Ca showed no symptoms. Similarly, in the current study the leaf [P] was consistently highest in B + P treatment, yet plants grown under this treatment showed no symptoms. Thus, in such situations it is important to also determine differences in cellular nutrient concentrations, as these may vary substantially from whole leaf nutrient concentrations (Hayes et al. 2019b).
Impacts on physiology
We have shown that root inhibition, micronutrient deficiency, and Ca-enhanced P sensitivity were common responses to calcareous soils. These calcareous-soil-induced responses had a significant impact on plant physiology, and consequently growth, including lower chlorophyll concentrations, Asat rates and Fv/Fm. Reduced photosynthetic rates and Fv/Fm may be linked to reduced cellular P or micronutrient availabilities, associated with Ca-enhanced P toxicity, as noted in Guilherme Pereira et al. (2021). This is the first study to demonstrate how calcareous soils impact Proteaceae species, thus helping to explain why most Proteaceae are excluded from calcareous habitats. Although all species in this study were exposed to the same conditions, and all exhibited a negative response to calcareous soils, the soil-indifferent Hakea species was less sensitive than the calcifuge one.
Strategies common to soil-indifferent Proteaceae
When comparing within each genus, the soil-indifferent species showed less severe visual symptoms and faster growth rates than calcifuges of the same genus, supporting previous findings (Hayes 2018; Hayes et al. 2019a, b; Guilherme Pereira et al. 2021). Differences between soil-indifferent and calcifuge species was most pronounced in Hakeas. Here, we identified three traits common to soil-indifferent Proteaceae: (1) high allocation of biomass to cluster roots, (2) high rhizosheath carboxylate amounts, and (3) low allocation of Ca to leaves. Principal Component Analysis showed a clear separation of the two distribution types based mainly on these traits (Figs. 8, S7). We will also discuss two additional traits: seed size and cellular regulation of Ca and P, drawing on results from this study and previous work (Hayes et al. 2019b; Kotula et al. 2020). We surmise that five traits, common to soil-indifferent species, are important in counteracting the negative impacts of calcareous soils and thus explain how soil-indifferent species tolerate calcareous habitats (Fig. 9b).
Greater investment in cluster roots
Soil-indifferent species allocated more biomass to cluster roots than calcifuges did. This was evident in plants grown under field-collected calcareous soils and was strongly associated with soil-indifferent species (Figs. 2 and 8, S7). For example, the soil-indifferent H. prostrata showed a consistent investment in cluster roots across all soils, while investment in H. incrassata was halved under the most carbonate-rich soils compared with the control. Similarly, soil-indifferent B. prionotes showed some cluster-root investment across all soils, whereas the calcifuge B. menziesii produced no cluster roots under the most carbonate-rich soils. Given the ability of cluster roots to enhance nutrient acquisition, we surmise that a greater investment in cluster roots under calcareous soils allows soil-indifferent species to acquire more micronutrients and therefore exhibit greater tolerance to calcareous habitats, compared with calcifuges.
We have demonstrated that soil-indifferent species allocated more biomass to cluster roots under calcareous conditions than calcifuge species which we surmise confers an advantage in calcareous soils. Understanding how cluster roots function in calcareous habitats and the role(s) they play in pedogenic decarbonation and ecosystem development has significant implications for the selection and management of species in rehabilitation of highly alkaline mine-site tailings and limestone quarries, and for the restoration/stabilisation of young calcareous dune systems worldwide (Diem et al. 2000; Hanslin and Kollmann 2016; Cross and Lambers 2017, 2021; Cross et al. 2018).
High rhizosheath carboxylate amount
A higher rhizosheath carboxylate amount was strongly associated with soil-indifferent Proteaceae (Figs. 3a and 8, S7). Soil-indifferent H. prostrata had by far the highest rhizosheath carboxylate amount per unit root FW and also appeared the most vigorous under calcareous treatments (Fig. 1). This species also showed an increase in carboxylate amount with increasingly calcareous soil types. Banksia species showed lower carboxylate amounts than Hakea species, but the rhizosheath carboxylate amount of soil-indifferent B. prionotes was still higher than that of calcifuge B. menziesii in the most carbonate-rich soil. Although, this difference between Banksia species may also be partly influenced by small differences in the low amount of root biomass used to calculate rhizosheath carboxylate concentrations per unit root FW. The higher carboxylate amount associated with soil-indifferent species was presumably due to either faster rates of release per unit root, or longer cluster-root longevity, releasing more carboxylates over time. The ability of soil-indifferent species to allocate more biomass to cluster roots might also have influenced their total rhizosheath carboxylate amount as could have differences in the amount of rhizosheath recovered per unit root FW, but based on our data and additional personal observations, this appears unlikely (Figs. 3a, S1a).
Higher carboxylate amounts are associated with a greater ability to survive in calcareous habitats (Ström et al. 1994; Ström 1997; Wang et al. 2006; Shi et al. 2020). For example, soil-indifferent B. sessilis produces more carboxylates per unit cluster root than B. attenuata and this is associated with its ability to acquire more P from limestone substrates, contributing to its soil-indifferent distribution (Shi et al. 2020). We surmise that increased rhizosheath carboxylate amounts, coupled with the ability to increase this in calcareous soils, as exemplified by H. prostrata, would allow soil-indifferent species to access more of the poorly-available nutrients, including Fe and Zn, allowing them to grow in calcareous habitats.
The composition of carboxylates also varied among treatments and between distribution types. All four species released iso-citrate almost exclusively in calcareous soils, and oxalate in acidic soils. Of eight common low-molecular-weight organic acids, Ström et al. (1994) showed that citric acid is by far the most effective at solubilising Fe (at 10 mM). Furthermore, Shi et al. (2020) showed that iso-citrate is even more effective than citrate at solubilising P from limestone, and therefore presumably also highly efficient at mobilising other micronutrients, such as Fe, Zn, and Mn (Shi et al. 2020). Interestingly, these soil-indifferent species tended to release proportionally more iso-citrate than calcifuge species in calcareous soils, suggesting that this strategy may be advantageous in calcareous soils.
Our results suggest that the release of iso-citrate is an adaptive response to calcareous soils. Furthermore, Ström et al. (1994) showed that plants grown in calcareous soil continue to release iso-citrate up to 48 h after having been removed from this substrate, suggesting that the process is due to changes within the roots and not to the external substrate alone. However, without determining the absorption kinetics and solubility of iso-citrate in the different soil types we are not able to exclude the possible influence of the external substrate. Further research is needed to determine the mechanism(s) responsible for the adaptive release of iso-citrate in calcareous soils, if it is directly beneficial, and if it is a widespread response among plants.
Low allocation of Ca to leaves
Soil-indifferent Proteaceae allocated less Ca to their leaves than calcifuge species. Calcifuge H. incrassata had a 1.5-fold greater leaf Ca allocation than H. prostrata, while the two Banksia species only had a 1.1-fold difference. Low leaf Ca allocation was mainly driven by higher root [Ca], at least in the most carbonate-rich soils. This supports earlier work by Kotula et al. (2020) showing that H. prostrata intercepts Ca by accumulating it in root cortical cells, thereby reducing transport of Ca to shoots and generally achieving lower leaf [Ca] than in H. incrassata (Lux et al. 2021). This Ca accumulation in root cortical cells is associated with the development of a strongly suberised endodermis in H. prostrata, a trait absent in H. incrassata (Kotula et al. 2020). Both Banksia species also lack a distinct suberised endodermis and consequently show no accumulation of Ca within the root cortex and generally similar leaf [Ca] when grown in hydroponics (Kotula et al. 2020). Differences in Banksia root [Ca] observed here may instead be due to the soil growth medium, as opposed to hydroponics in earlier studies (Hayes et al. 2019b; Kotula et al. 2020). It is also possible that plants grown in (bi)carbonate-rich soils may accumulate Ca in the form of Ca-carbonate in or on the root surface (Kinzel 1989; Jaillard et al. 1991; Alhendawi et al. 1997).
In summary, soil-indifferent H. prostrata allocated less Ca to leaves by accumulating Ca in the root cortex and thereby had generally lower leaf [Ca] than calcifuge H. incrassata. Although Banksia species also showed a slight difference in leaf Ca allocation, this difference did not appear to reduce leaf [Ca] of soil-indifferent B. prionotes, and therefore may simply be due to the precipitation of Ca-carbonate in or on the root surface.
Low seed P content and early development of cluster roots
Soil-indifferent species had lower seed P content than calcifuges, primarily related to their much smaller seed mass. This supports previous work that included four additional Proteaceae species (Hayes et al. 2019b). High seed P content is advantageous in P-impoverished acidic soils, as it reduces seedling reliance on external P and allows establishment in extremely low-P soils in seasonally-dry environments (Mustart and Cowling 1992; Kołodziejek 2017; Hayes et al. 2021). In P-richer calcareous soils, however, a larger seed P content may lead to higher leaf [P] in young seedlings that can systemically suppress the formation of cluster roots and consequently the uptake of poorly-available micronutrients (Shane and Lambers 2006). This was evident in calcifuge seedlings, which formed fewer cluster roots and later than soil-indifferent species did (Hayes pers. obs.) and often had lower micronutrient concentrations. The correlation between seed P content and calcifuge distribution likely only occurs within species that have evolved in severely P-impoverished environments, as nutrient-acquisition in these species is more likely to be determined by soil P availability (Bartelheimer and Poschlod 2016).
Tight regulation of cellular Ca and P
Higher leaf [Ca] can result in Ca-enhanced P toxicity in P-sensitive Proteaceae, resulting in an increase in mesophyll [P] with no increase in total leaf [P] (Hayes et al. 2019a, b). Soil-indifferent H. prostrata and B. prionotes can tightly regulate the allocation of Ca and P among leaf cell types, allowing them to maintain a constant mesophyll [P], despite increasing Ca supplies. By contrast, the calcifuge H. incrassata and B. menziesii have a low capacity to regulate allocation of these elements and, consequently, their mesophyll [P] increases under high Ca supply (Hayes et al. 2019a). This means that soil-indifferent species maintain the same mesophyll [P] regardless of leaf [Ca], whilst calcifuge species can exhibit increases of up to 10-fold under high Ca supplies, resulting in severe Ca-enhanced P toxicity.
We surmise that although total leaf [P] remains similar between calcareous soils and the control, the increased leaf [Ca] would induce Ca-enhanced P toxicity under calcareous conditions, and this would most severely impact the calcifuge species.
Why are most Proteaceae excluded from calcareous habitats?
All four Proteaceae responded negatively when grown in calcareous soil. This included an inhibition of root growth, symptoms of micronutrient deficiency, Ca-enhanced P toxicity and subsequent negative impacts on physiology. The soil-indifferent Hakea species was less impacted by calcareous soils than the calcifuge one and although both Banksia species were severely impacted, the soil-indifferent Banksia species also exhibited less severe symptoms than the calcifuge one. Soil-indifferent species exhibited five common traits allowing them to counteract and, therefore, better tolerate calcareous soils. We surmise that low soil micronutrient availability, in combination with high soil Ca and P concentrations, prevents most Proteaceae from establishing in calcareous habitats.
Low soil micronutrient availability and Ca-enhanced P toxicity both result in a reduced concentration/availability of essential micronutrients within leaves (i.e. Fe, Zn, Mn), resulting in micronutrient deficiency and reduced growth. High soil pH directly reduces the availability of micronutrients, while high soil bicarbonate/pH reduces root growth, and along with a strong soil buffering capacity indirectly reduces the capacity of plants to acquire sparingly available micronutrients.
High soil [Ca] can also cause micronutrient deficiency symptoms, via Ca-enhanced P toxicity. Ca-enhanced P toxicity increases mesophyll [P], reducing the physiological availability of micronutrients within the mesophyll, resulting in micronutrient deficiency. Previous work conducted in hydroponics highlighted the importance of Zn in the symptoms of Ca-enhanced P toxicity. By contrast, the current study reveals Fe to be important; this difference is likely due to the difference in growth media. In hydroponics, all nutrients are readily available for uptake, including Fe, in a chelated form. Conversely, in soil, Fe and other micronutrients are far less available, especially under high-pH conditions. Further research is needed to determine the relative importance of micronutrients, Fe, Zn, and Mn, including concentrations of their metabolically active forms within leaves (Cakmak and Marschner 1987; Zohlen 2000).
Soil-indifferent Proteaceae can overcome the limitations of calcareous soils by producing more cluster roots, releasing more carboxylates and protons, and allocating less Ca to their leaves. These same species also form cluster roots earlier, presumably related to their smaller seed P content and faster RGR, requiring external P uptake at an earlier stage. Previous research demonstrated that soil-indifferent species are also able to regulate leaf cellular Ca and P allocation more tightly, reducing their Ca-enhanced P sensitivity.
This study furthers our understanding of the factors influencing the distribution of this critically-important plant family, allowing for improved management of Proteaceae in restoration projects and in the horticultural industry (Bunn and Dixon 1992; Enright and Lamont 1992; Fuss et al. 1992; Stephenson 2005; Cross and Lambers 2017, 2021; Cross et al. 2018). More fundamentally, this research proposes novel strategies to explain the calcifuge/soil-indifferent distribution of species, helping to understand the unique strategies that certain species have evolved to tolerate calcareous habitats. This has significant implications for mine-site rehabilitation, coastal dune restoration/stabilisation, and understanding pioneer species establishment in young calcareous habitats.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alhendawi RA, Römheld V, Kirkby EA, Marschner H (1997) Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum, and maize. J Plant Nutr 20:1731–1753. https://doi.org/10.1080/01904169709365371
Bartelheimer M, Poschlod P (2016) Functional characterizations of Ellenberg indicator values – a review on ecophysiological determinants. Funct Ecol 30:506–516. https://doi.org/10.1111/1365-2435.12531
Bloom AJ, Epstein E (2005) Mineral nutrition of plants: principles and perspectives, 2nd edn. Sinauer Associates, Sunderland, MA, USA
Bothe H (2015) The lime–silicate question. Soil Biol Biochem 89:172–183. https://doi.org/10.1016/j.soilbio.2015.07.004
Broadley M, Brown P, Cakmak I et al (2012) Function of nutrients: micronutrients. In: Marschner P et al (eds) Marschner’s mineral nutrition of higher plants, 3rd edn. Elsevier/Academic Press, Waltham, MA, USA, pp 191–248
Brundrett MC (2021) One biodiversity hotspot to rule them all: southwestern Australia—an extraordinary evolutionary centre for plant functional and taxonomic diversity. J R Soc West Aust 104:91–122
Bunn E, Dixon KW (1992) Micropropagation of Stirlingia latifolia (Proteaceae), an important cut flower from western Australia. HortScience 27:368–368
Burström HG (1968) Calcium and plant growth. Biol Rev 43:287–316. https://doi.org/10.1111/j.1469-185X.1968.tb00962.x
Cakmak I, Marschner H (1987) Mechanism of phosphorus-induced zinc deficiency in cotton. III. Changes in physiological availability of zinc in plants. Physiol Plant 70:13–20. https://doi.org/10.1111/j.1399-3054.1987.tb08690.x
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr A 1011:233–240
Cawthray GR, Denton MD, Grusak MA et al (2021) No evidence of regulation in root-mediated iron reduction in two Strategy I cluster-rooted Banksia species (Proteaceae). Plant Soil. https://doi.org/10.1007/s11104-021-04849-5
Chen Y, Barak P (1982) Iron nutrition of plants in calcareous soils. In: Brady NC (ed) Advances in Agronomy. Academic Press, pp 217–240
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Cowling RM, Lamont BB (1998) On the nature of Gondwanan species flocks: diversity of proteaceae in mediterranean south-western Australia and South Africa. Aust J Bot 46:335–355. https://doi.org/10.1071/bt97040
Cross AT, Ivanov D, Stevens JC, Cross AT, Ivanov D, Stevens JC, Sadler R, Zhong H, Lambers H, Dixon KW (2019) Nitrogen limitation and calcifuge plant strategies constrain the establishment of native vegetation on magnetite mine tailings. Plant Soil 461:181–201. https://doi.org/10.1007/s11104-019-04021-0
Cross AT, Lambers H (2021) Calcicole–calcifuge plant strategies limit restoration potential in a regional semi-arid flora. Ecol Evol n/a 6941–6961. https://doi.org/10.1002/ece3.7544
Cross AT, Lambers H (2017) Young calcareous soil chronosequences as a model for ecological restoration on alkaline mine tailings. Sci Total Environ 607–608:168–175. https://doi.org/10.1016/j.scitotenv.2017.07.005
Cross AT, Stevens JC, Sadler R et al. (2018) Compromised root development constrains the establishment potential of native plants in unamended alkaline post-mining substrates. Plant Soil 163–179. https://doi.org/10.1007/s11104-018-3876-2
Czabator FJ (1962) Germination value: an index combining speed and completeness of pine seed germination. For Sci 8:386–396. https://doi.org/10.1093/forestscience/8.4.386
de Campos MCR, Pearse SJ, Oliveira RS, Lambers H (2013) Downregulation of net phosphorus-uptake capacity is inversely related to leaf phosphorus-resorption proficiency in four species from a phosphorus-impoverished environment. Ann Bot 111:445–454. https://doi.org/10.1093/aob/mcs299
de Silva BLT (1934) The distribution of calcicole and calcifuge species in relation to the content of the soil in calcium carbonate and exchangeable calcium, and to soil reaction. J Ecol 22:532. https://doi.org/10.2307/2256188
Diem HG, Duhoux E, Zaid H, Arahou M (2000) Cluster roots in casuarinaceae: role and relationship to soil nutrient factors. Ann Bot 85:929–936. https://doi.org/10.1006/anbo.2000.1127
Ding W, Clode PL, Clements JC, Lambers H (2018) Effects of calcium and its interaction with phosphorus on the nutrient status and growth of three Lupinus species. Physiol Plant 163:386–398. https://doi.org/10.1111/ppl.12732
Ding W, Clode PL, Lambers H (2019) Is pH the key reason why some Lupinus species are sensitive to calcareous soil? Plant Soil 434:185–201. https://doi.org/10.1007/s11104-018-3763-x
Ding W, Clode PL, Lambers H (2020) Effects of pH and bicarbonate on the nutrient status and growth of three Lupinus species. Plant Soil 447:9–28. https://doi.org/10.1007/s11104-019-03980-8
Dinkelaker B, Hengeler C, Marschner H (1995) Distribution and function of proteoid roots and other root clusters. Bot Acta 108:183–200. https://doi.org/10.1111/j.1438-8677.1995.tb00850.x
Enright NJ, Lamont BB (1992) Survival, growth and water relations of Banksia seedlings on a sand mine rehabilitation site and adjacent scrub-heath sites. J Appl Ecol 29:663–671. https://doi.org/10.2307/2404474
Fuss AM, Pattison SJ, Aspinall D, Sedgley M (1992) Shoot growth in relation to cut flower production of Banksia coccinea and Banksia menziesii (Proteaceae). Sci Hortic 49:323–334
George AS (1998) Proteus in Australia. An overview of the current state of taxonomy of the Australian Proteaceae. Aust Syst Bot 11:257–266. https://doi.org/10.1071/SB98024
Giel P, Bojarczuk K (2011) Effects of high concentrations of calcium salts in the substrate and its pH on the growth of selected rhododendron cultivars. Acta Soc Bot Pol 80:105–114. https://doi.org/10.5586/asbp.2011.021
Grubb PJ, Green HE, Merrifield RCJ (1969) The ecology of chalk Heath: its relevance to the calcicole–calcifuge and soil acidification problems. J Ecol 57:175–212. https://doi.org/10.2307/2258215
Grundon NJ (1972) Mineral nutrition of some Queensland heath plants. J Ecol 60:171–181. https://doi.org/10.2307/2258049
Guilherme Pereira C, Hayes PE, Clode PL, Lambers H (2021) Phosphorus toxicity, not deficiency, explains the calcifuge habit of phosphorus-efficient Proteaceae. Physiol Plant 172:1724–1738. https://doi.org/10.1111/ppl.13384
Hanslin HM, Kollmann J (2016) Positive responses of coastal dune plants to soil conditioning by the invasive Lupinus nootkatensis. Acta Oecol 77:1–9. https://doi.org/10.1016/j.actao.2016.08.007
Hayes PE (2018) Does calcium-enhanced phosphorus toxicity explain the absence of most Proteaceae species from calcareous habitats? PhD Thesis, The University of Western Australia
Hayes PE, Clode PL, Guilherme Pereira C, Lambers H (2019a) Calcium modulates leaf cell-specific phosphorus allocation in Proteaceae from south-western Australia. J Exp Bot 70:3995–4009. https://doi.org/10.1093/jxb/erz156
Hayes PE, Pereira CG, Clode PL, Lambers H (2019b) Calcium-enhanced phosphorus toxicity in calcifuge and soil-indifferent Proteaceae along the Jurien Bay chronosequence. New Phytol 221:764–777. https://doi.org/10.1111/nph.15447
Hayes PE, Clode PL, Oliveira RS, Lambers H (2018) Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: an adaptation improving phosphorus-use efficiency. Plant Cell Environ 41:605–619. https://doi.org/10.1111/pce.13124
Hayes PE, Nge FJ, Cramer MD, Hayes PE, Nge FJ, Cramer MD, Finnegan PM, Fu P, Hopper SD, Oliveira RS, Turner BL, Zemunik G, Zhong H, Lambers H (2021) Traits related to efficient acquisition and use of phosphorus promote diversification in Proteaceae in phosphorus-impoverished landscapes. Plant Soil 462:67–88. https://doi.org/10.1007/s11104-021-04886-0
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410. https://doi.org/10.1111/1365-2745.12196
Hopper SD, Gioia P (2004) The south west Australian floristic region: evolution and conservation of a global hot spot of biodiversity. Annu Rev Ecol Evol Syst 35:623–650. https://doi.org/10.1146/annurev.ecolsys.35.112202.130201
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Jaillard B, Guyon A, Maurin AF (1991) Structure and composition of calcified roots, and their identification in calcareous soils. Geoderma 50:197–210. https://doi.org/10.1016/0016-7061(91)90034-Q
Jefferies RL, Willis AJ (1964) Studies on the calcicole-calcifuge habit: II. The influence of calcium on the growth and establishment of four species in soil and sand cultures. J Ecol 52:691–707
Josse J, Husson F (2016) missMDA: a package for handling missing values in multivariate data analysis. J Stat Softw 70. https://doi.org/10.18637/jss.v070.i01
Kassambara A, Mundt F (2020) factoextra: extract and visualize the results of multivariate data analyses. R Package Version 1:7
Kinzel H (1989) Calcium in the vacuoles and cell walls of plant tissue. Flora 182:99–125. https://doi.org/10.1016/S0367-2530(17)30398-5
Kobayashi T, Nishizawa NK (2012) Iron Uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152. https://doi.org/10.1146/annurev-arplant-042811-105522
Kołodziejek J (2017) Effect of seed position and soil nutrients on seed mass, germination and seedling growth in Peucedanum oreoselinum (Apiaceae). Sci Rep 7:1959. https://doi.org/10.1038/s41598-017-02035-1
Kotula L, Clode PL, Ranathunge K, Lambers H (2020) Role of roots in adaptation of soil-indifferent Proteaceae to calcareous soils in south-western Australia. J Exp Bot 72:1490–1505. https://doi.org/10.1093/jxb/eraa515
Lambers H, Juniper D, Cawthray GR, Lambers H, Juniper D, Cawthray GR, Veneklaas EJ, Martínez-Ferri E (2002) The pattern of carboxylate exudation in Banksia grandis (Proteaceae) is affected by the form of phosphate added to the soil. Plant Soil 238:111–122
Lee JA (1998) The calcicole—calcifuge problem revisited. Adv Bot Res 29:1–30
Lee JA, Woolhouse HW (1969) A comparative study of bicarbonate inhibition of root growth in calcicole and calcifuge grasses. New Phytol 68:1–11
Lenth R (2022) Emmeans: estimated marginal means, aka least-squares means. R Package Version 1(7):2
Liao D, Zhang C, Li H, Liao D, Zhang C, Li H, Lambers H, Zhang F (2020) Changes in soil phosphorus fractions following sole cropped and intercropped maize and faba bean grown on calcareous soil. Plant Soil 448:587–601. https://doi.org/10.1007/s11104-020-04460-0
Loeppert RH, Suarez DL (1996) Carbonate and gypsum. Methods of soil analysis. Part 3. John Wiley & Sons, Ltd, Madison, WI, USA, pp 437–474
Lux A, Kohanová J, White PJ (2021) The secrets of calcicole species revealed. J Exp Bot 72:968–970. https://doi.org/10.1093/jxb/eraa555
Marschner P (ed) (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Elsevier/Academic Press, Waltham, MA, USA
McArthur WM (2004) Reference soils of south-western Australia, 2nd edn. Department of Agriculture (WA), Perth, WA, Australia
McArthur WM, Bettenay E (1974) The development and distribution of the soils of the Swan Coastal Plain, Western Australia. CSIRO, Melbourne, Vic, Australia
McClure GW (1972) Nutrient distribution in root zones. III. Further studies of the effects of phosphorus distribution on corn and wheat. Can J Bot 50:2275–2282
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15:1409–1416. https://doi.org/10.1080/00103628409367568
Mustart PJ, Cowling RM (1992) Seed size: phylogeny and adaptation in two closely related Proteaceae species-pairs. Oecologia 91:292–295
Myers N, Mittermeier RA, Mittermeier CG, Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Nichols DG, Beardsell DV (1981) Interactions of calcium, nitrogen and potassium with phosphorus on the symptoms of toxicity in Grevillea cv. ‘Poorinda Firebird ’ Plant Soil 61:437–445. https://doi.org/10.1007/BF02182024
Pang J, Ryan MH, Siddique KHM, Simpson RJ (2017) Unwrapping the rhizosheath. Plant Soil 418:129–139. https://doi.org/10.1007/s11104-017-3358-y
Peiter E, Yan F, Schubert S (2001) Lime-induced growth depression in Lupinus species: are soil pH and bicarbonate involved? J Plant Nutr Soil Sci 164:165–172. https://doi.org/10.1002/1522-2624(200104)164:2<165::AID-JPLN165>3.0.CO;2-B
Pérez-Harguindeguy N, Díaz S, Garnier E, Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167. https://doi.org/10.1071/BT12225
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York, USA
Pinheiro J, Bates D, DebRoy S et al (2021) nlme: linear and nonlinear mixed effects models. R Package Version 3:1–153
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rayle DL, Cleland RE (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol 99:1271–1274
Robson AD, Edwards DG, Loneragan JF (1970) Calcium stimulation of phosphate absorption by annual legumes. Aust J Agric Res 21:601–612
Schindelin J, Arganda-Carreras I, Frise E et al. (2012) Fiji - an Open source platform for biological image analysis. Nat Methods 9. https://doi.org/10.1038/nmeth.2019
Shane M, Lambers H (2005) Cluster roots: a curiosity in context. Plant Soil 274:101–125
Shane MW, Lambers H (2006) Systemic suppression of cluster-root formation and net P-uptake rates in Grevillea crithmifolia at elevated P supply: a proteacean with resistance for developing symptoms of ‘P toxicity’. J Exp Bot 57:413–423. https://doi.org/10.1093/jxb/erj004
Shane MW, Szota C, Lambers H (2004) A root trait accounting for the extreme phosphorus sensitivity of Hakea prostrata (Proteaceae). Plant Cell Environ 27:991–1004. https://doi.org/10.1111/j.1365-3040.2004.01204.x
Shi J, Strack D, Albornoz FE, Shi J, Strack D, Albornoz FE, Han Z, Lambers H (2020) Differences in investment and functioning of cluster roots account for different distributions of Banksia attenuata and B. sessilis, with contrasting life history. Plant Soil 447:85–98. https://doi.org/10.1007/s11104-019-03982-6
Steele B (1955) Soil pH and base status as factors in the distribution of calcicoles. J Ecol 43:120–132. https://doi.org/10.2307/2257125
Stephenson R (2005) Macadamia: domestication and commercialization. Chron Hortic 45:11–15
Ström L, Olsson T, Tyler G (1994) Differences between calcifuge and acidifuge plants in root exudation of low-molecular organic acids. Plant Soil 167:239–245. https://doi.org/10.1007/BF00007950
Ström L, Ström L, Strom L (1997) Root exudation of organic acids: importance to nutrient availability and the calcifuge and calcicole behaviour of plants. Oikos 80:459–466. https://doi.org/10.2307/3546618
Takagi D, Miyagi A, Tazoe Y, Takagi D, Miyagi A, Tazoe Y, Suganami M, Kawai‐Yamada M, Ueda A, Suzuki Y, Noguchi Ko, Hirotsu N, Makino A (2020) Phosphorus toxicity disrupts Rubisco activation and reactive oxygen species defence systems by phytic acid accumulation in leaves. Plant Cell Environ 43:2033–2053. https://doi.org/10.1111/pce.13772
Tang C, Longnecker NE, Greenway H, Robson AD (1996) Reduced root elongation of Lupinus angustifolius L. by high pH is not due to decreased membrane integrity of cortical cells or low proton production by the roots. Ann Bot 78:409–414
Tang C, Robson AD, Longnecker NE, Greenway H (1993) Physiological responses of lupin roots to high pH. Plant Soil 155/156:509–512
Tansley AG (1917) On competition between Galium saxatile L. (G. hercynicum Weig.) and Galium sylvestre Poll. (G. asperum Schreb.) on different types of soil. J Ecol 5:173–179. https://doi.org/10.2307/2255655
Turner BL, Hayes PE, Laliberté E (2018) A climosequence of chronosequences in southwestern Australia. Eur J Soil Sci 69:69–85. https://doi.org/10.1111/ejss.12507
Turner BL, Laliberté E (2015) Soil development and nutrient availability along a 2 million-year coastal dune chronosequence under species-rich mediterranean shrubland in southwestern Australia. Ecosystems 18:287–309. https://doi.org/10.1007/s10021-014-9830-0
Turner BL, Romero TE (2009) Short-term changes in extractable inorganic nutrients during storage of tropical rain forest soils. Soil Sci Soc Am J 73:1972–1979. https://doi.org/10.2136/sssaj2008.0407
Tyler G (1992) Inability to solubilize phosphate in limestone soils—key factor controlling calcifuge habit of plants. Plant Soil 145:65–70. https://doi.org/10.1007/BF00009542
Tyler G, Olsson PA (1993) The calcifuge behaviour of Viscaria vulgaris. J Veg Sci 4:29–36. https://doi.org/10.2307/3235731
Tyler G, Ström L (1995) Differing organic acid exudation pattern explains calcifuge and acidifuge behaviour of plants. Ann Bot 75:75–78. https://doi.org/10.1016/S0305-7364(05)80011-3
Uloth MB, You MP, Cawthray G, Barbetti MJ (2015) Temperature adaptation in isolates of Sclerotinia sclerotiorum affects their ability to infect Brassica carinata. Plant Pathol 64:1140–1148. https://doi.org/10.1111/ppa.12338
van Breemen N (1991) Soil acidification and alkalinization. In: Ulrich B, Sumner ME (eds) Soil acidity. Springer, Berlin, Heidelberg, pp 1–7
Vélez-Bermúdez IC, Schmidt W (2022) Plant strategies to mine iron from alkaline substrates. Plant Soil 483:1–25. https://doi.org/10.1007/s11104-022-05746-1
Vu VQ (2011) Ggbiplot: a ggplot2 based biplot. R Package Version 0.55
Wala M, Kołodziejek J, Mazur J (2023) The diversity of iron acquisition strategies of calcifuge plant species from dry acidic grasslands. J Plant Physiol 280:153898. https://doi.org/10.1016/j.jplph.2022.153898
Wang Z, Shen J, Zhang F (2006) Cluster-root formation, carboxylate exudation and proton release of Lupinus pilosus Murr. As affected by medium pH and P deficiency. Plant Soil 287:247–256. https://doi.org/10.1007/s11104-006-9071-x
Warren CR (2008) Rapid measurement of chlorophylls with a microplate reader. J Plant Nutr 31:1321–1332. https://doi.org/10.1080/01904160802135092
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Weston PH (2007) Proteaceae. Flowering plants. Eudicots. Springer-Verlag, Berlin, pp 364–404
Wickham H, Averick M, Bryan J et al (2019) Welcome to the Tidyverse. J Open Source Softw 4:1686. https://doi.org/10.21105/joss.01686
Yang X, Hajiboland R, Römheld V (2003) Bicarbonate had greater effects than high pH on inhibiting root growth of zinc-inefficient rice genotype. J Plant Nutr 26:399–415. https://doi.org/10.1081/PLN-120017143
Zemunik G, Turner BL, Lambers H, Laliberté E (2015) Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat Plants 1:15050. https://doi.org/10.1038/nplants.2015.50
Zemunik G, Turner BL, Lambers H, Laliberté E (2016) Increasing plant species diversity and extreme species turnover accompany declining soil fertility along a long-term chronosequence in a biodiversity hotspot. J Ecol 104:792–805. https://doi.org/10.1111/1365-2745.12546
Zohlen A (2000) Use of 1,10-phenanthroline in estimating metabolically active iron in plants. Commun Soil Sci Plant Anal 31:481–500. https://doi.org/10.1080/00103620009370451
Zohlen A (2002) Chlorosis in wild plants: is it a sign of iron deficiency? J Plant Nutr 25:2205–2228. https://doi.org/10.1081/PLN-120014071
Zohlen A, Tyler G (1997) Differences in iron nutrition strategies of two calcifuges, Carex pilulifera L. and Veronica officinalis L. Ann Bot 80:553–559. https://doi.org/10.1006/anbo.1997.0493
Zohlen A, Tyler G (2000) Immobilization of tissue iron on calcareous soil: differences between calcicole and calcifuge plants. Oikos 89:95–106
Acknowledgements
PEH was supported by an Australian Government Research Training Program Scholarship supplemented by a top-up scholarship from the Australian Research Council (ARC; DP130100005). We thank Daniel Beeck, Georgie Buck, Calum Irvine, Shahab Nikabadi, Carmen Schoenjahn, and Jorik Smits for their assistance in the glasshouse experiment. We thank Dr Patrick Finnegan for his thoughtful comments on this manuscript. This research was supported by an ARC Discovery Project grant (DP130100005) awarded to HL and PLC.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
PEH, and HL planned and designed the experiment; PEH performed the glasshouse experiment; PEH, and HL analysed and interpreted the data; and PEH wrote the paper with contributions from PLC, and HL.
Corresponding author
Additional information
Responsible Editor: Stefano Cesco.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 4.18 MB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hayes, P.E., Clode, P.L. & Lambers, H. Calcifuge and soil-indifferent Proteaceae from south-western Australia: novel strategies in a calcareous habitat. Plant Soil 496, 95–122 (2024). https://doi.org/10.1007/s11104-023-06297-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06297-9