Abstract
The paper of van der Ent et al. (Plant Soil 485:247–257, 2023), published in the previous issue, reports the hyperaccumulation of rare earth elements (REE) in plant species from the Proteaceae for the first time. Indeed, the high REE accumulation in Proteaceae is not completely unexpected, given that the plants release large amounts of carboxylates to acquire phosphorus and micronutrients. However, it is somewhat questionable that the efficiency of element mobilization alone sufficiently explains the large variability in REE accumulation among different taxa of Proteaceae or other P-efficient species that typically show low concentrations of REE. Given that REE3+ share chemical similarities to Ca2+ but form stable complexes with ligands similar to Al3+, it is reasonable that uptake and accumulation of REE depend not solely on element mobility but also on the dynamics of element speciation governed by the formation, stability, and fate of carboxylate-REE-complexes in the rhizosheaths. The rationale behind this contention is that for elements with low mobility in soil, changes in chemical speciation may increase the availability only if the complex stabilities that depend on rhizosphere pH allow a breakdown during uptake. In this commentary, we explore the idea that REE accumulation depends on rhizosphere processes related to nutrient acquisition and element exclusion that overlap in time, space, and function depending on the composition of metal-chelating ligands released by plant roots in concert with rhizosphere pH. Based on data from greenhouse and field experiments, we propose a model where plants with a P-mining strategy (hyper)accumulate REE when rhizosphere pH is below a critical value shifting the REE speciation to available forms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Phosphorus acquisition and rare earth elements availability
Plants that deploy a phosphorus (P)-acquisition strategy based on the release of carboxylates mobilize not only P and micronutrients but also a variety of non-essential elements, including rare earth elements (REEs) (Wenzel 2009; Wiche et. 2016a, b). This occurs because carboxylates alter the chemical and physical rhizosheath properties towards the dissolution of organic and inorganic host-phases of P and REE, that predominantly consist of the oxides of silicon (Si), aluminum (Al), iron (Fe), and manganese (Mn) (Shane and Lambers 2005; Lambers 2022). The ability to mobilize P and metals varies considerably among plant species, functional groups, and genotypes (Neumann et al. 2000; Lambers et al. 2013, 2015) and depends on the plant’s capacity for the release of protons, ligands (carboxylates, siderophores), phenolics, enzymes and formation of mutualistic relationships with bacteria and fungi (PGPR, mycorrhiza) (Neumann and Römheld 2001; Hinsinger et al. 2009). Non-mycorrhizal plant species adapted to P-impoverished or P-sorbing soils, of which Proteaceae and lupines have been most profoundly studied, respond to P-deficiency by the formation of proteoid roots (cluster roots) that release large amounts of carboxylates together with protons and phenolics during a narrow time window (Shane and Lambers 2005; Weisskopf et al. 2006; Lambers et al. 2013).
Enhanced element mobility in the rhizosphere is a prerequisite for metal hyperaccumulation (Merlot et al. 2018). Consequently, carboxylate release might also explain the hyperaccumulation of REE in Proteaceae species reported by van der Ent et al. (2023) and other rather unspecialized hyperaccumulators from the Phytolacaceae and Juglanaceae that accumulate multiple elements, including Al, REE, and Mn, even when they are growing on soils with relatively low element availability (Jansen et al. 2002; Delgado et al. 2019; Liu et al. 2021, 2022a, b; Pollard 2023). Nevertheless, it is questionable that the capacity for element mobilization alone sufficiently explains the large variability in REE accumulation among different Proteaceae species (van der Ent et al. 2023) or other P-efficient taxa that typically show low concentrations of non-essential elements, including REE (Römer et al. 2000; Wiche and Heilmeier 2016; Wiche et al. 2017b). Instead, it is reasonable that element accumulation in general and specifically REE-hyperaccumulation in Proteaceae depends not only on element mobility but also on the dynamics of element speciation, especially the formation, stability, and fate of carboxylate-REE-complexes in the rhizosheaths. The rationale behind this contention is that, with few exceptions, nutrient uptake systems involved in transporting non-essential elements shuttle elements exclusively in free ionic form and exclude organo-element-complexes during the uptake stage (Barber and Lee 1974; Marschner 1995; Comerford 1998; Shan et al. 2002). Hence, when element availability is limited by a low solubility, higher mobility in the rhizosphere through complex formation enhances uptake and accumulation only if the stability of complexes allows a breakdown of the complex at the sites of metal uptake.
Carboxylates and other root-derived metal chelators ultimately enhance the availability of elements that do not react with the ligands directly (phosphate) or when the complexes formed are relatively unstable such as the carboxylate complexes of Ca, Mn, and Zn with relatively low stability constants (log K < 5) (Martell et al. 2004). The complexes formed prevent the reabsorption of the elements to the soil matrix and enhance diffusion towards the rhizoplane, where the elements are liberated from the complex. This might also explain why hyperaccumulators for Ni, Zn, Cd, and REE that evolved in metalliferous environments characterized by high element availability rely on specialized uptake, transport, and detoxification systems and do not deploy carboxylates for element mobilization (Pollard 2023). In these plants, enhanced element mobility is most probably achieved by changes in rhizosphere pH and the release of weak ligands such as amino acids (Ni-Histidine log K < 2) that increase mobility but do not interfere with uptake (Puschenreiter et al. 2005; Dessureault-Rompré et al. 2010; Álvarez-López et al. 2021; Liu et al. 2022a, b). In contrast, forming stable complexes is especially advantageous for element mobilization in severely nutrient-impoverished environments, where the root-derived ligands must compete with the inorganic or organic bonding partners (Fe, Mn, Al) during ligand exchange in the soil. However, their contribution to the availability of essential metals with high charge density additionally depends on the plant’s capacity to liberate the element from the complex, which explains the development of the ferric iron reductases that liberate Fe3+ from highly stable Fe(III)-complexes (Fe(III)-citrate log K = 11.5) (Martell et al. 2004) in dicots (a strategy I species) to cover the nutritional demands for Fe (Marschner and Römheld 1994).
Given that plants have not developed similar reductases for non-essential elements, the release of carboxylates and other chelators forms, at the same time, an essential strategy of plants to avoid metal toxicity in the rhizosphere, where elements are excluded at the sites of uptake based on charge density, and consequently complex stability. The concept of element exclusion through extracellular complexation has been studied in detail for Al in Al-resistant species (Zheng et al. 1998; Ma and Hiradate 2000; Ma et al. 2001; Kochian et al. 2004) and Cd in Lupinus albus (Römer et al. 2000). This strategy predominantly relies on the high complex stability of Al-complexes with carboxylates that are otherwise released during nutrient acquisition (Al-malate log K 6.0; Al-citrate log K 7.9) (Martell et al. 2004). We recently demonstrated that REE accumulation is lower in plants that release higher amounts of carboxylates, especially citrate (Wiche et al. 2023), and REE accumulation was inversely related to rhizosphere pH. We proposed a model where rhizosphere acidification counteracts element exclusion of REE by shifting the carboxylate /carboxylic acid ratio towards the acid form, thus rendering them ineffective and causing the elements to be present in available ionic REE forms (Wiche et al. 2023) as it has been previously demonstrated for Al and P (Pearse et al. 2006). Given that REE share chemical similarities to Ca (Brown et al. 1990; Tyler 2004) and Al (Ishikawa et al. 1996; Ma and Hiradate 2000; Kataoka et al. 2002; Purwadi et al. 2021; Fehlauer et al. 2022) it is reasonable that REE accumulation in plants results from the remarkable chemical features of REE in concert with chemical rhizosphere traits involved in nutrient acquisition and element exclusion that overlap in the rhizosphere in time, space, and function and determine uptake or exclusion based on REE-speciation.
Chemical features of rare earth elements
The REE comprise a group of 16 elements from the lanthanide series, including lanthanum, yttrium (Y), and scandium (Sc) that occur widespread in soils with concentrations comparable to those of some essential plant nutrients (Reimann et al. 2003; Wiche et al. 2017a). The concentrations of REE vary from 66 µg g− 1 (Ce), 30 µg g− 1 (La), and 28 µg g− 1 (Nd) to 0.5 µg g− 1 (Tm) and 0.3 µg g− 1 (Lu) (McLennan 2001; Davranche et al. 2017). Compared with other elements of the periodic system, no other group with a uniform oxidation state, e.g., the alkali or the earth alkali metals, displays such a remarkable similarity as the REE (Brown et al. 1990; Tyler 2004). As a unique feature in this group, all 16 REE exhibit ionic radii similar to Ca2+ (Ca2+ 100 pm, La 3+ 103.1 pm) (Shannon 1976); however, under most pedologically-relevant conditions, REE form trivalent cations (Ce and Eu may also occur as 4 + ions (Ce) or 2 + ions (Eu) (Wyttenbach et al. 1998; Pourret et al. 2022), which strongly interact with phosphate, negatively charged soil constituents and plant cell constituents (Diatloff et al. 1999; Cao et al. 2001; Kovarikova et al. 2019). Due to the element’s higher reactivity, the biogeochemical behavior of REE in the soil-plant system is not simply analogous to Ca2+ but may resemble that of other trivalent metals, particularly Al3+ (Ma and Hiradate 2000; Kataoka et al. 2002). In particular, they can form stable complexes with dissolved organic compounds (Pourret et al. 2007; Wiche et al. 2017b), and their stability depends on the nature of the ligand and the REE involved. As an exception of this group, the effective ionic radius of the trivalent ions decreases slightly but systematically from La3+ (103.1 pm) to Lu3+ (86.1 pm) (Wyttenbach et al. 1998), leading to differences in their sorption and complexation behavior in soil, plants and the rhizosphere (Tyler 2004; Monei et al. 2022; Wiche et al. 2023) and fractionation of light REE (LREE: REE with a lower mean atomic mass than 153 and a larger effective radius than 95 pm (La, Ce, Pr, Nd, Sm, and Eu) relative to heavy REE (HREE: REE with a higher mean atomic mass than 153 and a lower effective ion radius than 95 pm (Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, and Y) (Tyler 2004). REE fractionation has been most profoundly studied during petro- and pedogenesis (Aide and Aide 2012) and has great potential to study processes during nutrient acquisition; however, until nowadays, rhizosphere effects on REE accumulation and REE fractionation have been widely neglected in plant research.
Mobility and chemical speciation of rare earth elements in soil-plant systems
The mobility of REE in soils is a function of their ionic radii and speciation in solution, pH, Eh, water fluxes, and the nature of secondary intermediate minerals formed under different conditions (Tyler 2004). High contents of clay minerals (Wenming et al. 2001), presence of phosphates and Fe-Mn hydroxides (Cao et al. 2001; Ding et al. 2005), and particulate soil organic matter (SOM) (Pourret et al. 2007; Davranche et al. 2015) typically decrease the solubility and mobility of REEs in soils. In contrast, the mobility of REE increases with decreasing soil pH (Cao et al. 2001). Lowering of pH unspecifically mobilizes REE together with P, Fe, and Al in the soil (Fig. 1A) by desorption of surface-complexed elements and dissolution of mineral element forms, especially REE-bearing Fe-oxyhydroxides, Al-oxides, and REE-phosphate-precipitates (Tyler and Olsson 2001a). Depending on the plant species and the nutritional status, especially of P and Fe, rhizosphere pH in most forbs can be more than 1 unit lower due to a higher ATPase activity (Hinsinger et al. 2003). Higher element solubility following proton release results in the protolysis of geochemical soil phases that act as pH buffers. Consequently, changes in pH integrate the plant’s capacity for proton release and the physicochemical soil properties in the root environment that interact with each other.
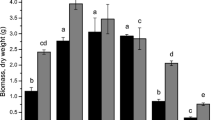
Effect of organic ligands (citrate (cit), malate, desferrioxamine B (DFOB) and pH on elements solubility and plant uptake. A mobilization of P, Fe, Al, Si, REE in soil treated with 0.1 mM DFOB (pH 7) or 1 mM citric acid (pH 4.5) compared to deionized water (H2O pH 7) or deionized water acidified with HNO3 (H2O pH 4.5); according to Wiche et al. (2017b). B accumulation of LREE and HREE in Phalaris arundinacea supplied with REE-DFOB, REE-citrate, or REE in ionic forms (according to Wiche et al. (2017b). C carboxylate release and REE accumulation in Brassica napus and Lupinus albus supplied with 0P (P-deficient reference plants) or 100 µM P in the watering solution (Wiche et al. 2023). D proposed model of the effects of rhizosphere pH on carboxylate activity and REE uptake by plants
The mobilized elements are initially in ionic form in the soil solution and subsequently interact with dissolved and colloidal compounds. They are reabsorbed on soil particles or diffuse into the root apoplast, where they can be absorbed by root cells. REE precipitation with phosphates and Fe-oxy hydroxides has been reported to control the solubility of REEs in natural surface waters as well as in the vicinity of the epidermis cells of roots (Johannesson et al. 1995; Saatz et al. 2016; Martinez et al. 2018). The presence of inorganic and organic ligands such as sulfate, carbonates, fluorides (Diatloff et al. 1999; Gu et al. 2000), carboxylates (Shan et al. 2002; Han et al. 2005), siderophores (Wiche et al. 2017b; Schwabe et al. 2021) and humic and fulvic acids (Pourret et al. 2007; Davranche et al. 2015) counteracts reabsorption/precipitation and strongly increase REE mobility in the soil-plant-system. The aqueous inorganic complexes of REE are relatively unstable compared to organo-metal-complexes, and thus their relevance is most likely restricted to aquatic environments or soils with low DOC.
Complex stability depends on the structure and amount of ligands released by the roots or microbes, determining the ligand/element ratio, presence of competing ions in solution, ionic radii, and charge density of the central atom. For any given metal, carboxylate-complexes’ stability increases with the number of bonds to the central atom by the ligand. It thus increases with the number of carboxylate groups in the order citrate > malate > fumerate > acetate (Fig. 1E), while for a specific carboxylate (e.g., citrate), the complex stabilities decrease in the order Fe3+ > Al3+ > HREE3+ > LREE3+ > Zn2+ >Ca2+ > Mn2+ > K+ (Byrne and Li 1995; Martell et al. 2004). The high complex stabilities of tricarboxylates (citrate) and dicarboxylates (malate, succinate, fumarate) compared to monocarboxylates, and effective desorption of soil cations with decreasing pH determines the success of the P-mining strategy in severely nutrient impoverished environments (Lambers 2022).
The release of ligands by plants is initially controlled by the cellular nutritional status as a consequence of metabolic shifts in carbohydrate allocation from shoot to roots in concert with increased biosynthesis of malate and citrate and decreased citrate turnover in the tricarboxylic acid cycle (Neumann and Römheld 2001). However, the compounds produced interact with a broad spectrum of cations in soil solution, change their dissolution equilibria and liberate REE from their organic and inorganic host phases in soil by ligand exchange (Wiche et al. 2016a, b, 2017b). The presence of 1 mM citrate substantially increased the dissolved concentrations of P, Fe, Al, Si, and REE at pH 4.5 compared to water acidified with an inorganic acid to the same pH or the presence of the microbial siderophore desferrioxamine B (DFOB) (Fig. 1A). In contrast to most catecholate and hydroxamate siderophores that mobilize REE and other elements without requiring pH action, deprotonation of the carboxylic acid headgroup could further exacerbate the complexation of REE and have thus a higher impact when pH increases (Fig. 1D). Due to differences in charge density, the stability of HREEs with most inorganic and organic ligands (La-citrate log K 7.63, Lu-citrate log K 8.12) typically increases with increasing atomic number and consequently decreasing atomic radius (Byrne and Li 1995; Martell et al. 2004). Also, shifts in the redox-sensitive representatives of REEs (Ce) may mirror the environmental conditions during mobilization (Pepi et al. 2016). Therefore, the REE pattern in soils corresponds to the REE pattern of minerals as modified by the sorption/complexation REE constants with ligands, colloids, and particles (Davranche et al. 2015) as well as by biological activities in the rhizosphere of plants (Stille et al. 2006; Schwabe et al. 2021).
Uptake and accumulation of rare earth elements in plants
Plant uptake of elements requires the elements to be dissolved in the soil solution in a chemical form that can diffuse through the apoplast and is accessible for uptake systems (Barber and Lee 1974; Marschner 1995; Comerford 1998). Generally, the availability and uptake of REEs increase with increasing concentrations in the soil solution, for instance, through decreasing soil pH (Tyler and Olsson 2001a, b; Thomas et al. 2014; Cheng et al. 2015). However, only very few studies observed correlations of soil parameters with shoot concentrations of soil-grown plants, possibly due to processes in the rhizosphere affecting their chemical speciation (Wiche et al. 2017b, 2023). It is generally assumed that plants take up REEs from soil solution in their ionic form as REE3+ (Wang et al. 2004; Han et al. 2005; Brioschi et al. 2013; Wiche et al. 2017b), which is performed mainly by Ca2+, Na+, K+ channels, as Han et al. (2005) demonstrated for La3+ in roots of Hordeum vulgare. From hydroponic studies, there is evidence that the presence of inorganic ligands such as phosphate (Diatloff et al. 1999; Ding et al. 2005), as well as the formation of iron plaque at the root surface, lowers the availability of REEs to plants through the formation of poorly soluble precipitates (Ding et al. 2005; Saatz et al. 2016; Wiche and Heilmeier 2016; Martinez et al. 2018). However, these processes are most likely less relevant in severely phosphorus-impoverished environments and in the rhizosphere of plants with a P-mining strategy that strongly acidify the rhizosphere and mobilize P and Han et al. (2005) demonstrated that organic acids promote the uptake of La by barley, but the effect of the acid decreased in the order acetic acid > malic acid > citric acid, which can be explained mainly by decreased sorption of La onto the apoplast and prevention of REE-phosphate precipitates in the presence of the acid anion but a reduced uptake with increasing complex stability (Han et al. 2005).
There are considerable differences between plants from the functional groups of grasses and forbs to utilize different element forms for uptake (Marschner and Römheld 1994). The addition of REE-DFOB complexes to Phalaris arundinacea (reed canary grass), a strategy II plant in Fe nutrition, did not alter REE accumulation, most probably through the uptake of intact REE-siderophore complexes similar to Fe(III)-DMA complexes (Wiche et al. 2017b). In contrast, the presence of REE-citrate complexes substantially reduced REE uptake, especially of HREE, and caused a fractionation of HREE relative to LREE due to the higher complex stabilities of HREE (Fig. 1B). Similarly, split-root experiments showed that REE accumulation in Brassica napus and Lupinus albus was inversely related to carboxylate release (Fig. 1C). A higher carboxylate release in P-deficient plants led to higher LREE/HREE ratios than in P-supplied plants (Wiche et al. 2023). However, this effect disappeared in plants that strongly acidified the rhizosheaths (Wiche et al. 2023). Some studies demonstrated that the artificial addition of chelators like EDTA increases the accumulation of REE in plants (Lihong et al. 1999; Liu et al. 2022a, b). We emphasize that the artificial supply of chelators in single doses or repeated pulses in hydroponics differs from the natural situation in the rhizosphere, where plants continuously release the compounds in the apoplastic fluids. When applied in single doses, the chelators interact with the soil (Fig. 1A), transfer REE from stable to more labile element pools, and become more available when the ligands are microbially decomposed. In mixed culture experiments of Lupinus albus with Avena sativa and Hordeum vulgare, L. albus strongly mobilized REE but did not accumulate the elements. Instead, the elements were taken up by the neighboring cereals, most likely through the decomposition of complex while moving from the rhizosphere of the lupins to the roots of the grasses that do not release carboxylates (Wiche et al. 2016a, b; Monei et al. 2022).
Rare earth element accumulation in plants with different nutrition strategies
Twenty-five years ago, Wyttenbach et al. (1998) highlighted the unexplainable high variation of REE accumulation observed among plant species and even within different individuals of a single species growing on the same soil. Given that REE availability depends on both element mobility and REE speciation based on pH and the presence of ligands in the rhizosphere, it is reasonable that on a given soil, REE accumulation in plants varies among species with different nutrient acquisition strategies, P-acquisition efficiency, and depends on the P and Fe nutrition status of plants. In addition to the plant-associated factors, chemical and physical rhizosheath properties arise from soil-associated factors, of which initially soil pH, buffer capacity, nutrient availability, and concentrations of REE in labile, potentially plant-available element pools are the most profoundly studied. Do we find evidence of relationships between below-ground functional traits related to nutrient acquisition and shoot REE accumulation in the field? In a field experiment, nine crop species differing in their efficiency to access P and micronutrients through the release of siderophores, carboxylates, and acidification of the rhizosphere were cultivated on a slightly alkaline Luvisol (soil pH H2O 7.8) characterized by low P, Fe, and Mn availability (Wiche 2016; Wiche and Heilmeier 2016). A principal component analysis (PCA) based on shoot Mn, Fe REE, Ge, and Si concentrations (Ge and Si were used to differentiate between grasses forbs) revealed that plant species that deploy a carboxylate-based nutrient-acquisition strategy (Lupinus albus, Lupinus angustifolius) were characterized by highest concentrations of Mn, which is an indicator of carboxylate release (Lambers et al. 2015) but lowest concentrations of REEs, most likely due to the exclusion of REE-carboxylate complexes during uptake (Fig. 2A, B). The highest concentrations of REEs were found in Brassica napus, Fagopyrum esculentum, and Avena sativa (Fig. 2A) distributed along PC 1, which was fully loaded with REE and Fe (Fig. 2B).
A Results of a Principal Component Analysis based on the leaf concentrations of Ge, La, Nd, Gd, Er, Si, Fe, and Mn of nine crop species growing on homogeneous, slightly alkaline soil (pH 7.8) and B loading values of the respective geochemical groups of elements (Wiche 2016). C, D leaf Mn and REE concentrations in mycorrhizal (AM) and non-mycorrhizal (NM) plant species (Acanthocarpus preissii, Senecio pinnatifolius var. latilobus, Lepidosperma calcicola, Acacia lasiocarpa, Conostylis candicans subsp. Calcicola, Melaleuca systena, Mesomelaena pseudostygia, Acacia pulchella, Banksia menziesii, Stirlingia latifoia) collected on young Quindalup dunes and old Bassendean dunes of the Jurien Bay Soil Chronosequence, Western Australia (Hayes et al. 2014)
Brassica napus and Fagopyrum esculentum are phosphophile plant species that release less carboxylates, especially dicarboxylates like malate and fumarate, under conditions of P-deficiency but strongly acidify the rhizosphere (Pearse et al. 2006; Wiche et al. 2023). Avena sativa is a strategy II species concerning Fe nutrition and releases siderophores when the Fe supply is low (Marschner and Römheld 1994). At this point, it has to be noticed that P and Fe deficiency may often appear together in natural ecosystems (Chapin et al. 2002), and siderophores increase mobility and plant availability of a variety of different trace metals (Römheld and Marschner 1986; Reichman and Parker 2005; Oburger et al. 2014; Kraemer et al. 2017) including REE (Fig. 1A). Leaf samples collected from plant species along the Jurien Bay Soil Chronosequence (Hayes et al. 2014; Turner et al. 2018) showed higher REE concentrations in plants collected from the acidic (pH H2O 4.6) and severely P-impoverished Bassendean soils than on the younger calcareous (pH H2O 7.8) Quindalup dunes (Fig. 2C, D). On the alkaline Quindalup soils, leaf REE concentrations were highest in mycorhizal plants than in non-mycorrhizal plants, and REE concentrations decreased significantly with increasing Mn concentrations, indicating that carboxylate release decreased REE uptake under the alkaline conditions.
In contrast, on the acidic Bassendean soils, mycorrhizal species had the lowest REE concentration, and REE and Mn showed a positive relationship, most probably due to the efficient element mobilization through carboxylate release in the non-mycorrhizal species coupled with inhibited complex formation under acidic conditions (Fig. 2D, Fig. 1D). It is reasonable that these processes also explain the anomalous REE concentrations in Helicia species collected from habitats with low REE mineralization (van der Ent et al., 2023). Plants of the genus Helicia belong to the family Proteaceae which develop proteoid roots in severely P-impoverished soils and release large amounts of carboxylates, most likely citrate, and malate together with protons in an ‘exudative burst’ for P mobilization after reaching the maturity state (Shane and Lambers 2005). Given that plants that had REE anomaly occurred in habitats with acidic soils with low Ca-base saturation and low pH-buffer capacity (Caritat et al. 2011), the resulting pH of the rhizosheaths may favor the decomposition of REE-carboxylate-complexes and by rendering the carboxylate /carboxylic acid ratio towards the ineffective form (Fig. 1D). In addition, there is evidence that the metabolism and P acquisition mechanism of Proteaceae is altered with soil pH (Veneklaas et al. 2003; Griebenow et al. 2022). Veneklaas et al. (2003) demonstrated that the citrate/malate ratio in acidic soils is lower than in soils with higher pH, which favors labile complexes and uptake of REE in acidic habitats. This contrasts with situations in habitats where Proteaceae or other species that rely on carboxylates grow on near-neutral soil or soil with higher pH buffer capacity and higher P-availability. Here, the resulting pH changes in the rhizosphere should be comparatively low, leading to a low solubility of REE and high activity of carboxylates that exclude REE during uptake.
Root–shoot transport of REEs depends on the efficiency of transport systems and REE mobility within the plant (Kovarikova et al. 2019), most likely governed by cell-wall absorption, phosphate deposition (Saatz et al. 2016; Liu et al. 2022a, b), and intracellular complexation with carboxylates (Ma and Hiradate 2000). It can be reasonably assumed that in REE-accumulating Helicia species, root uptake and root–shoot transfer of REE is achieved via unspecific Al, Mn and Ca pathways as a consequence of their functional adaptation to nutrient-impoverished environments (Lambers et al. 2015). Notably, Proteaceae function at very low cellular P concentrations and produce large amounts of carboxylates in root cells (Shane et al. 2004; Shane and Lambers 2005), which most likely supports root-shoot transport of REE through the formation of REE-carboxylate complexes (Ma and Hiradate 2000; Fehlauer et al. 2022), reduced REE-sorption onto cell walls and low REE-phosphate precipitation fostering the accumulation of REE in older leaves of the perennial plants similar to Ca (van der Ent et al. 2023). Most species from the Proteaceae tend to allocate Ca and P to different cell types (Hayes et al. 2019), which enhances P-use efficiency by avoiding the deleterious precipitation of Ca-phosphate (Hayes et al. 2018) and might play a key role in the accumulation of REE (Saatz et al. 2016; Liu et al. 2022a, b). The presence of specialized plasma membrane-localized REE-selective transporters, as identified in the specialized hyperaccumulator Dicranopteris linearis (Zheng et al. 2023), cannot be completely ruled out and is yet to be tested experimentally on Proteaceae. Still, future will need to elucidate the processes by bridging methods of plant ecophysiology with analytical chemistry in elemental speciation. Nonetheless, the proposed model provides a mechanistic understanding of differences in REE-accumulation in plants based on element speciation in the rhizosphere. Conversely, leaf REE signatures (total REE, LREE/HREE ratios) could be used together with Mn to screen for changes in rhizosphere chemistry related to plant nutrition following an ionomic approach.
Data availability
All data obtained during the experiment are contained in the manuscript.
References
Aide MT, Aide C (2012) Rare earth elements: their importance in understanding soil genesis. ISRN Soil Sci 2012:783876. https://doi.org/10.5402/2012/783876
Álvarez-López V, Puschenreiter M, Santner J, Lehto N, Prieto-Fernández Á, Wenzel WW, Monterroso C, Kidd PS (2021) Evidence for nickel mobilisation in rhizosphere soils of Ni hyperaccumulator Odontarrhena serpyllifolia. Plant Soil 464:89–107. https://doi.org/10.1007/s11104-021-04944-7
Barber DA, Lee RB (1974) The effect of micro-organisms on the absorption of manganese by plants. New Phytol 73:97–106. https://doi.org/10.1111/j.1469-8137.1974.tb04610.x
Brioschi L, Steinmann M, Lucot E, Pierret MC, Stille P, Prunier J, Badot PM (2013) Transfer of rare earth elements (REE) from natural soil to plant systems: implications for the environmental availability of anthropogenic REE. Plant Soil 366:143–163. https://doi.org/10.1007/s11104-012-1407-0
Brown PH, Rathjen AH, Graham RD, Tribe DE (1990) Rare earth elements in biological systems. In: Gschneider KA, Eyring L (eds) Handbook on the physics and chemistry of rare earths, vol 13. Elsevier, North Holland, Amsterdam, pp 423–450
Byrne RH, Li B (1995) Comparative complexation behavior of the rare earths. Geochim Cosmochim Acta 59:4575–4589. https://doi.org/10.1016/0016-7037(95)00303-7
Cao X, Chen Y, Wang X, Deng X (2001) Effects of redox potential and pH value on the release of rare earth elements from soil. Chemosphere 44:655–661. https://doi.org/10.1016/S0045-6535(00)00492-6
Chapin FS, Matson PA, Vitousek P (2002) Principles of terrestrial ecosystem ecology. Springer, New York
Cheng J, Ding C, Li X, Zhang T, Wang X (2015) Rare earth element transfer from soil to navel orange pulp (Citrus sinensis Osbeck cv. Newhall) and the effects on internal fruit quality. PLoS One 10:1–15. https://doi.org/10.1371/journal.pone.0120618
Comerford NB (1998) Soil P bioavailability. In: Lynch JP, Deikman J (eds) Phosphorus in plant biology: regulatory roles in molecular, cellular, organismic, and ecosystem processes. American Society Plant Physiologists, Rockville, pp 136–147
Davranche M, Gruau G, Dia A, Bouhnik-Le Coz M, Marsac R, Pédrot M, Pourret O (2017) Rare earth elements in wetlands, trace elements in waterlogged soils and sediments. Taylor & Francis Group/CRC Press, p 135–162
Davranche M, Gruau G, Dia A, Marsac R, Pédrot M, Pourret O (2015) Biogeochemical factors affecting rare earth element distribution in shallow wetland groundwater. Aquat Geochem 21:197–215. https://doi.org/10.1007/s10498-014-9247-6
de Caritat P, Cooper M, Wilford J (2011) The pH of australian soils: field results from a national survey. Soil Res 49:173–182. https://doi.org/10.1071/SR10121
Delgado M, Valle S, Barra PJ, Reyes-Díaz M, Zúñiga-Feest A (2019) New aluminum hyperaccumulator species of the Proteaceae family from southern South America. Plant Soil 444:475–487. https://doi.org/10.1007/s11104-019-04289-2
Dessureault-Rompré J, Luster J, Schulin R, Tercier-Weiber M-L, Nowack B (2010) Decrease of labile Zn and Cd in the rhizosphere of hyperaccumulating Thlaspi caerulescens with time. Environ Pollut 158:1955–1962. https://doi.org/10.1016/j.envpol.2009.10.032
Diatloff E, Asher CJ, Smith FW (1999) The effects of rare earth elements on the growth and nutrition of plants. Rare Earths ’98. Mater Sci Forum 315:354–360. https://doi.org/10.4028/www.scientific.net/MSF.315-317.354
Ding SM, Liang T, Zhang CS, Yan JC, Zhang ZL (2005) Accumulation and fractionation of rare earth elements (REEs) in wheat: controlled by phosphate precipitation, cell wall absorption and solution complexation. J Exp Bot 56:2765–2775. https://doi.org/10.1093/jxb/eri270
Fehlauer T, Colline B, Angletti B, Negahi MM, Dentant C, Chaurand P, Lallemand C, Levard C, Rose J (2022) Multiscale imaging on Saxifragapaniculata provides new insights into yttrium uptake by plants. Sci Rep: 18268. https://doi.org/10.1038/s41598-022-23107-x
Griebenow S, Makunga NP, Privett S, Strauss P, Veste M, Kleinert A, Valentine AJ (2022) Soil pH influences the organic acid metabolism and exudation in cluster roots of Protea species from the Mediterranean-type fynbos ecosystem, Western Cape, South Africa. Rhizosphere 21:100486. https://doi.org/10.1016/j.rhisph.2022.100486
Gu Z, Wang X, Cheng J, Wang L, Dai L (2000) Effects of sulfate on speciation and bioavailability of rare earth elements in nutrient solution. Chem Speciat Bioavailab 12:52–58. https://doi.org/10.3184/095422900782775544
Han F, Shan X-Q, Zhang J, Xie Y-N, Pei Z-G, Zhang S-Z, Zhu Y-G, Wen B (2005) Organic acids promote the uptake of lanthanum by barley roots. New Phytol 165:481–492. https://doi.org/10.1111/j.1469-8137.2004.01256.x
Hayes PE, Clode PL, Oliveira RS, Lambers H (2018) Proteaceae from phosphorus-impoverished habitats preferentially allocate phosphorus to photosynthetic cells: an adaptation improving phosphorus-use efficiency. Plant Cell Environ 41:605–619. https://doi.org/10.1111/pce.13124
Hayes PE, Clode PL, Pereira CG, Lambers H (2019) Calcium modulates leaf cell-specific phosphorus allocation in Proteaceae from south-western Australia. J Exp Bot 70:3995–4009. https://doi.org/10.1093/jxb/erz156
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410. https://doi.org/10.1111/1365-2745.12196
Hinsinger P, Bengough AG, Vetterlein D, Young IM (2009) Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321:117–152. https://doi.org/10.1007/s11104-008-9885-9
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-induced pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59. https://doi.org/10.1023/A:1022371130939
Ishikawa S, Wagatsuma T, Ikarashi T (1996) Comparative toxicity of A13+, Yb3+ and La 3+ to root-tip cells differing in tolerance to high A13+ in terms of ionic potentials of dehydrated trivalent cations. Soil Sci Plant Nutr 42:613–625. https://doi.org/10.1080/00380768.1996.10416330
Jansen S, Broadley MR, Robbrecht E, Smets E (2002) Aluminum hyperaccumulation in angiosperms: a review of its phylogenetic significance. Bot Rev 68:235–269. https://doi.org/10.1663/0006-8101(2002)068[0235:AHIAAR]2.0.CO;2
Johannesson KH, Lyons WB, Stetzenbach KJ, Byrne RH (1995) The solubility control of rare earth elements in natural terrestrial waters and the significance of PO43– and CO32– in limiting dissolved rare earth concentrations: a review of recent information. Aquat Geochem 1:157–173
Kataoka T, Stekelenburg A, Nakanishi TM, Delhaize E, Ryan PR (2002) Several lanthanides activate malate efflux from roots of aluminium-tolerant wheat. Plant Cell Environ 25:453–460. https://doi.org/10.1046/j.0016-8025.2001.00821.x
Kochian LV, Hoekenga OA, Piñeros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493. https://doi.org/10.1146/annurev.arplant.55.031903.141655
Kovarikova M, Tomášková I, Soudek P (2019) Rare earth elements in plants. Biol Plant 63:20–32. https://doi.org/10.32615/bp.2019.003
Kraemer D, Tepe N, Pourret O, Bau M (2017) Negative cerium anomalies in manganese (hydr)oxide precipitates due to cerium oxidation in the presence of dissolved siderophores. Geochim Cosmochim Acta 196:197–208. https://doi.org/10.1016/j.gca.2016.09.018
Lambers H (2022) Phosphorus acquisition and utilization in plants. Annu Rev Plant Biol 73:1.1-1.26. https://doi.org/10.1146/annurev-arplant-102720-125738
Lambers H, Clements JC, Nelson MN (2013) How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am J Bot 100:263–288. https://doi.org/10.3732/ajb.1200474
Lambers H, Hayes PE, Laliberté E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90. https://doi.org/10.1016/j.tplants.2014.10.007
Lihong Y, Xiaorong W, Hao S, Haishi Z (1999) The effect of EDTA on rare earth elements bioavailability in soil ecosystem. Chemosphere 38:2825–2833. https://doi.org/10.1016/S0045-6535(98)00496-2
Liu C, Ding T-X, Liu W-S, Tang Y-T, Qiu R-L (2022a) Phosphorus mediated rhizosphere mobilization and apoplast precipitation regulate rare earth element accumulation in Phytolacca americana. Plant Soil 483:697–709. https://doi.org/10.1007/s11104-022-05743-4
Liu C, Sun D, Zheng H-X, Wang G-B, Liu W-S, Cao Y, Tang Y-T, Qiu R-L (2022b) The limited exclusion and efficient translocation mediated by organic acids contribute to rare earth element hyperaccumulation in Phytolacca americana. Sci Total Environ 805:150335. https://doi.org/10.1016/j.scitotenv.2021.150335
Liu C, Liu W-S, van der Ent A, Morel JL, Zheng H-X, Wang G-B, Tang Y-T, Qiu RL (2021) Simultaneous hyperaccumulation of rare earth elements, manganese and aluminum in Phytolaccaamericana in response to soil properties. Chemosphere 282:131096. https://doi.org/10.1016/j.chemosphere.2021.131096
Ma JF, Hiradate F (2000) Form of aluminium for uptake and translocation in buckwheat (Fagopyrum esculentum Moench). Planta 211:355–360. https://doi.org/10.1007/s004250000292
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6:273–278. https://doi.org/10.1016/S1360-1385(01)01961-6
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, London
Marschner H, Römheld V (1994) Strategies of plants for acquisition of iron. Plant Soil 165:261–274. https://doi.org/10.1007/BF00008069
Martell AE, Smith RM, Motekaitis RJ (2004) NIST critically selected Stability Constants of Metal 452 complexes, database 46, Version 8. NIST, Gaithersburg
Martinez RE, Pourret O, Faucon M-P, Dian C (2018) Effect of rare earth elements on rice plant growth. Chem Geol 489:28–37. https://doi.org/10.1016/j.chemgeo.2018.05.012
McLennan SM (2001) Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem Geophys 2. https://doi.org/10.1029/2000GC000109
Merlot S, Sanchez V, de la Torre G (2018) Physiology and molecular biology of traceelement hyperaccumulation. In: van der Ent A, Baker AJM, Echevarria G, Simonnot M-O, Morel JL (eds) Agromining: farming for metals, mineral resource reviews series. Springer International Publishing, p 93–116
Monei N, Hitch M, Heim J, Pourret O, Heilmeier H, Wiche O (2022) Effect of substrate properties and P-supply on facilitating the uptake of rare earth elements (REE) in mixed-culture cropping systems of Hordeum vulgare, Lupinus albus and Lupinus angustifolius. Environ Sci Poll Res 29:57172–57189. https://doi.org/10.1007/s11356-022-19775-x
Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E (2000) Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann Bot 85:909–919. https://doi.org/10.1006/anbo.2000.1135
Neumann G, Römheld V (2001) The release of root exudates as affected by the plant’s physiological status. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere – biochemistry and organic substances at the soil–plant interface. Marcel Dekker AG, Basel
Oburger E, Gruber B, Schindlegger Y, Schenkeveld WDC, Hann S, Kraemer SM, Wenzel WW, Puschenreiter M (2014) Root exudation of phytosiderophores from soil-grown wheat. New Phytol 203:1161–1174. https://doi.org/10.1111/nph.12868
Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H (2006) Triticum aestivum shows a greater biomass response to a supply of aluminium phosphate than Lupinus albus, despite releasing less carboxylates into the rhizosphere. New Phytol 169:515–524. https://doi.org/10.1111/j.1469-8137.2005.01614.x
Pepi S, Sansone L, Chicca M, Marrocchino E, Vaccaro C (2016) Distribution of rare earth elements in soil and grape berries of Vitis vinifera cv.“Glera”. Environ Monit Assess 188:477. https://doi.org/10.1007/s10661-016-5490-1
Pollard AJ (2023) Inadvertent uptake of trace elements and its role in the physiology and evolution of hyperaccumulators. Plant Soil 483:711–719. https://doi.org/10.1007/s11104-022-05856-w
Pourret O, Davranche M, Gruau G, Dia A (2007) Rare earth elements complexation with humic acid. Chem Geol 243:128–141. https://doi.org/10.1016/j.chemgeo.2007.05.018
Pourret O, van der Ent A, Hursthouse A, Irawan DE, Liu H, Wiche O (2022) The ‘europium anomaly’ in plants: facts and fiction. Plant Soil 476:721–728. https://doi.org/10.1007/s11104-021-05210-6
Purwadi I, Nkrumah PN, Paul AL, van der Ent A (2021) Uptake of yttrium, lanthanum and neodymium in Melastoma malabathricum and Dicranopteris linearis from Malaysia. Chemoecology 31:335–342. https://doi.org/10.1007/s00049-021-00348-2
Puschenreiter M, Schnepf A, Millán IM, Fitz WJ, Horak O, Klepp J, Schrefl T, Lombi E, Wenzel WW (2005) Changes of Ni biogeochemistry in the rhizosphere of the hyperaccumulator Thlaspi goesingense. Plant Soil 271:205–218. https://doi.org/10.1007/s11104-004-2387-5
Reichman SM, Parker DR (2005) Metal complexation by phytosiderophores in the rhizosphere. In: Huang PM, Gobran GR (eds) Biogeochemistry of trace elements in the rhizophere. Elsevier, p 129–155
Reimann C, Siewers U, Tarvainen T, Bityukova L, Eriksson J, Giucis A, Gregorauskiene V, Lukashev VK, Matinian NN, Pasieczna A (2003) Agricultural soils in northern Europe. Schweizerbart, Stuttgart
Römer W, Kang D-K, Egle K, Gerke J, Keller H (2000) The acquisition of cadmium by Lupinusalbus L., Lupinusangustifolius L., and Loliummultiflorum Lam. J Plant Nutr Soil Sci 163:623–628. https://doi.org/10.1002/1522-2624(200012)163:6<623::AID-JPLN623>3.0.CO;2-C
Römheld V, Marschner H (1986) Mobilization of iron in the rhizosphere of different plant species. Adv Plant Nutr, Vol. II, Praeger, New York, p 155–204
Saatz J, Stryhanyuk H, Vetterlein D, Musat N, Otto M, Reemtsma T, Richnow HH, Daus B (2016) Location and speciation of gadolinium and yttrium in roots of Zea mays by LA-ICP-MS and ToF-SIMS. Environ Pollut 216:245–252. https://doi.org/10.1016/j.envpol.2016.05.069
Schwabe R, Dittrich C, Kadner J, Senges CHR, Bandow JE, Tischler D, Schlömann M, Levicán G, Wiche O (2021) Secondary metabolites released by the rhizosphere bacteria Arthrobacter oxydans and Kocuria rosea enhance plant availability and soil–plant transfer of germanium (Ge) and rare earth elements (REEs). Chemosphere 285:131466. https://doi.org/10.1016/j.chemosphere.2021.131466
Shan X-Q, Lian J, Wen B (2002) Effect of organic acids on adsorption and desorption of rare earth elements. Chemosphere 47:701–710. https://doi.org/10.1016/S0045-6535(02)00032-2
Shane MW, Cramer MD, Funayama-Noguchi S, Cawthray GR, Millar AH, Day DA, Lambers H (2004) Developmental physiology of cluster-root carboxylate synthesis and exudation in harsh hakea. Expression of phosphoenolpyruvate carboxylase and the alternative oxidase. Plant Physiol 135(1):549–560. https://doi.org/10.1104/pp.103.035659
Shane MW, Lambers H (2005) Cluster roots: a curiosity in context. Plant Soil 274:101–125. https://doi.org/10.1007/s11104-004-2725-7
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr A 32:751–767. https://doi.org/10.1107/S0567739476001551
Stille P, Steinmann M, Pierret M-C, Gauthier-Lafaye F, Chabaux F, Viville D, Pourcelot L, Matera V, Aouad G, Aubert D (2006) The impact of vegetation on REE fractionation in stream waters of a small forested catchment (the Strengbach case). Geochim Cosmochim Acta 70:3217–3230. https://doi.org/10.1016/j.gca.2006.04.028
Thomas PJ, Carpenter D, Boutin C, Allison JE (2014) Rare earth elements (REEs): effects on germination and growth of selected crop and native plant species. Chemosphere 96:57–66. https://doi.org/10.1016/j.chemosphere.2013.07.020
Turner BL, Hayes PE, Laliberté E (2018) A climosequence of chronosequences in southwestern Australia. Eur J Soil Sci 69:69–85. https://doi.org/10.1111/ejss.12507
Tyler G (2004) Rare earth elements in soil and plant systems – a review. Plant Soil 267:191–206. https://doi.org/10.1007/s11104-005-4888-2
Tyler G, Olsson T (2001a) Concentrations of 60 elements in the soil solution as related to the soil acidity. Eur J Soil Sci 52:151–165. https://doi.org/10.1046/j.1365-2389.2001.t01-1-00360.x
Tyler G, Olsson T (2001b) Plant uptake of major and minor mineral elements as influenced by soil acidity and liming. Plant Soil 230:307–321. https://doi.org/10.1023/A:1010314400976
van der Ent A, Nkrumah PN, Purwadi I, Erskine PD (2023) Rare earth element (hyper)accumulation in some Proteaceae from Queensland, Australia. Plant Soil 485:247–257. https://doi.org/10.1007/s11104-022-05805-7
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197. https://doi.org/10.1023/A:1022367312851
Wang Z, Zhang S, Shan X-Q (2004) Effects of low-molecular-weight organic acids on uptake of lanthanum by wheat roots. Plant Soil 261:163–170. https://doi.org/10.1023/B:PLSO.0000035563.71887.15
Weisskopf L, Abou-Mansour E, Fromin N, Thomasi N, Santelia D, Edelkott I, Neumann G, Aragno M, Tabacchi R, Martinoia E (2006) White lupin has developed a complex strategy to limit microbial degradation of secreted citrate required for phosphate acquisition. Plant Cell Environ 28:919–927. https://doi.org/10.1111/j.1365-3040.2005.01473.x
Wenming D, Xiangke W, Xiaoyan B, Aixia W, Jingzhou D, Zuyi T (2001) Comparative study on sorption/desorption of radioeuropium on alumina, bentonite and red earth: effects of pH, ionic strength, fulvic acid, and iron oxides in red earth. Appl Radiat Isot 54:603–610. https://doi.org/10.1016/S0969-8043(00)00311-0
Wenzel WW (2009) Rhizosphere processes and management in plant-assisted bioremediation (phytoremediation) of soils. Plant Soil 321:385–408. https://doi.org/10.1007/s11104-008-9686-1
Wiche O (2016) Plant availability of germanium and rare earth elements in soil. Dissertation at TU Bergakademie Freiberg
Wiche O, Dittrich C, Pourret O, Monei N, Heim J, Lambers H (2023) Relationships between carboxylate-based nutrient-acquisition strategies, phosphorus-nutritional status and rare earth element accumulation in plants. Plant Soil. https://doi.org/10.1007/s11104-023-06049-9
Wiche O, Heilmeier H (2016) Germanium (Ge) and rare earth element (REE) accumulation in selected energy crops cultivated on two different soils. Miner Eng 92:208–215. https://doi.org/10.1016/j.mineng.2016.03.023
Wiche O, Kummer N-A, Heilmeier H (2016a) Interspecific root interactions between white lupin and barley enhance the uptake of rare earth elements (REEs) and nutrients in shoots of barley. Plant Soil 402:235–245. https://doi.org/10.1007/s11104-016-2797-1
Wiche O, Székely B, Kummer N-A, Moschner C, Heilmeier H (2016b) Effects of intercropping of oat (Avena sativa L.) with white lupin (Lupinus albus L.) on the mobility of target elements for phytoremediation and phytomining in soil solution. Int J Phytoremediation 18:900–907. https://doi.org/10.1080/15226514.2016.1156635
Wiche O, Tischler D, Fauser C, Lodemann J, Heilmeier H (2017a) Effects of citric acid and the siderophore desferrioxamine B (DFO-B) on the mobility of germanium and rare earth elements in soil and uptake in Phalaris arundinacea. Int J Phytoremediation 19:746–754. https://doi.org/10.1080/15226514.2017.1284752
Wiche O, Zertani V, Hentschel W, Achtziger R, Midula P (2017b) Germanium and rare earth elements in topsoil and soil-grown plants on different land use types in the mining area of Freiberg (Germany). J Geochem Explor 175:120–129. https://doi.org/10.1016/j.gexplo.2017.01.008
Wyttenbach A, Furrer V, Schleppi P, Tobler L (1998) Rare earth elements in soil and in soil-grown plants. Plant Soil 199:267–273. https://doi.org/10.1023/A:1004331826160
Zheng H-X, Liu W-S, Sun D, Zhu S-C, Li Y, Yang Y-L, Liu R-R, Feng H-Y, Cai X, Cao Y, Xu G-H, Morel JL, van der Ent A, Ma LQ, Liu Y-G, Rylott EL, Qiu R-L, Tang Y-T (2023) Plasma-membrane-localized transporter NREET1 is responsible for rare earth element uptake in hyperaccumulator Dicranopteris linearis. Environ Sci Technol 57:6922–6933. https://doi.org/10.1021/acs.est.2c09320
Zheng SJ, Ma JF, Matsumoto H (1998) High aluminum resistance in buckwheat: I. Al-induced specific secretion of oxalic acid from root tips. Plant Phys 117:745–751. https://doi.org/10.1104/pp.117.3.745
Acknowledgements
We are grateful to Hans Lambers and Patrick Hayes for collecting plant and soil samples along the Jurien Bay Chronosequence. We thank Michel-Pierre Faucon for his internal review on an earlier version of our manuscript. OW was funded by a research fellowship granted by the OECD in the Co-operative Research Programme: Sustainable Agricultural and Food Systems (CRP).
Funding
Open Access funding enabled and organized by Projekt DEAL. OW was supported by a research fellowship granted by the OECD (Contract Number PO500120275).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Oliver Wiche. The first draft of the manuscript was written by Oliver Wiche and Olivier Pourret commented on versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Hans Lambers.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wiche, O., Pourret, O. The role of root carboxylate release on rare earth element (hyper)accumulation in plants – a biogeochemical perspective on rhizosphere chemistry. Plant Soil 492, 79–90 (2023). https://doi.org/10.1007/s11104-023-06177-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06177-2