Abstract
Background and Aims
Phosphorus (P) is a restricting nutrient for crop productivity worldwide. P deficiency can lead to stunted growth and development, eventually affecting crop yield. But less is documented about the impact of P fertility on industrial hemp (Cannabis sativa L.) production in the low-P soils of south-western Australia. We aimed to investigate the effect of P rates on growth, physiology, rhizosphere carboxylate exudation, nutrient uptake and P-use efficiency in hemp.
Methods
The study was conducted in a randomised complete block design with four P rates (0, 40, 80 and 120 mg P kg–1 dry soil) and three hemp varieties (Morpeth, Han FNQ and Fedora 17). Plants were grown and raised in a controlled-environment phytotron until harvested 35 days after sowing at vegetative growth stage (3rd to nth leaf pair).
Results
Our results revealed a strong influence of treatment (P rate) on hemp growth, physiology, biomass, nutrient uptake and P-use efficiency compared to variety and the variety × treatment interaction. Hemp roots predominantly released citrate in P-deficient conditions and gradually shifted to malate exudation with increasing P supply. The N:P ratio, leaf chlorophyll, and gas exchange data coupled with shoot and root length data suggest that Morpeth and Fedora 17 differ in morpho-physiological adaptations for optimum photosynthesis and growth, with high leaf chlorophyll and coarse root length achieved by Morpeth and high intercellular CO2 concentration and shoot length by Fedora 17.
Conclusions
Morpeth and Fedora 17 had high shoot biomass, root length, root surface area and agronomic P-use and P-utilisation efficiencies in response to increasing soil P, while Han FNQ had moderate shoot yield, root growth, high citrate exudation, tissue P concentration and P-uptake efficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a major constituent in all living beings as it is present in the nucleic acids (DNA and RNA), cell membranes, enzymes and energy-containing molecules (Verlinden et al. 2022). In plants, P regulates diverse physiological, biochemical and metabolic processes such as photosynthesis, signal transduction, aerobic respiration and glycolysis. It also takes part in many key biosynthetic pathways including the synthesis of nucleic acids, fatty acids and energy carrier ATP (Bechtaoui et al. 2021; Chen et al. 2021; Shiponi and Bernstein 2021b; Verlinden et al. 2022). In addition, P is the critical constituent in producing ribosomes, a complex molecular machinery that manufactures N-rich proteins from amino acids that constitute carbon (C) and energy-harvesting organs (Agren 2008). Phosphorus deficiency can cause stunted shoot growth, premature leaf senescence, delayed reproductive organ development and flower initiation, decreasing plant yield (Shiponi and Bernstein 2021b).
Around 43% of the world’s arable lands is low in P (Chen et al. 2021). This challenges crop production in most highly P-sorbing soils (Richardson et al. 2011), including some of the most ancient and highly weathered soils in south-western Australia (Bolland et al. 2003). While some native vegetations have adapted to low soil P, newly introduced annual crops need an external supply of water-soluble P fertilisers for profitable production (Bolland et al. 2003). However, application of P fertiliser is frequently less efficient in medium to high P-sorbing agricultural soils (Simpson et al. 2011; Tshewang et al. 2020a) as the agronomic efficiency (Hammond et al. 2009) of P fertilisers also depends on the P-sorption capacity of the soils alongside P-use efficiency of the plant species/varieties (Singh and Gilkes 1991).
Several management strategies have been developed to address P-balance problems in high P-sorbing soils and improve agricultural P-use efficiency (Simpson et al. 2011). The strategies mainly include precise and optimum soil fertility management, adaptation of new fertiliser technology, enhanced soil biology and selection of P-efficient cultivar/species with lower external critical-P requirements (Simpson et al. 2011; Tshewang et al. 2020a). Breeding crops with efficient P uptake and/or P utilisation is one strategy to decrease P fertiliser use, producing reasonable yields with lower P inputs or reducing their tissue P requirements and P concentrations (Hammond et al. 2009).
Plants have evolved several morphological and physiological mechanisms to combat P deficiency (Heuer et al. 2017; Liu 2021; Suriyagoda et al. 2012; Tshewang et al. 2020a), such as changing root morphology and root system architecture (e.g. increased root growth, specific root length, root surface area, root hair length and density) and producing highly specialised root structures (e.g. root clusters) (Lambers et al. 2006; Liu 2021; Lyu et al. 2016). These modifications ultimately increase the rhizosphere soil volume explored by root systems to increase P acquisition (Lyu et al. 2016). Furthermore, plant root adaptations triggered by the environment (e.g. induced by nutrient deficiency) can differ among species or genotypes (i.e. variation in root plasticity), which means plants can also evolve naturally or can be bred to better survive and grow under nutrient-limiting conditions (Liu 2021; Marschener 1998). Morphological responses of plant roots to P deficiency have been studied in maize, wheat, canola, lupin, soybean, pea, and chickpea, and perennial pasture grasses (Kidd et al. 2016; Lyu et al. 2016; Schroeder and Janos 2005; Tshewang et al. 2020a; Wang et al. 2008).
Apart from morphological adaptations, plants can respond to P deficiency by altering their root physiology (Suriyagoda et al. 2012). Plant roots release an array of organic acids, phosphatases, and phytases into the rhizosphere to facilitate the mobilisation and solubilisation of ‘sparingly available’ inorganic and organic P (Heuer et al. 2017; Kidd et al. 2016). For example, exudation of carboxylate (low-molecular-weight organic acids) increases soil P solubility and enhances plant P uptake, particularly in low-P-containing or highly P-sorbing soils (Suriyagoda et al. 2012). Carboxylates also influence rhizosphere pH, as evidenced by the increased rhizosphere acidification in P-efficient genotypes/species releasing more carboxylates than P-inefficient genotypes/species (Hinsinger et al. 2003; Iqbal et al. 2020). Rhizosphere carboxylate exudation occurs in many plant species, including rice, wheat, maize, canola, lupin, pasture legumes and grasses (Iqbal et al. 2020; Kidd et al. 2016; Kirk et al. 1999; Pearse et al. 2007, 2006; Tshewang et al. 2020a, b, 2022).
Another vital physiological response of plants under P deficiency is altered photosynthetic activities. In the Calvin cycle, P is required for adenosine triphosphate (ATP) synthesis to regenerate ribulose 1,5 biphosphate (RuBP); however, P deficiency can decrease RuBP regeneration by reducing the activity of Calvin cycle enzymes, particularly the initial activity of ribulose-5-phosphate kinase (Fredeen et al. 1990; Verlinden et al. 2022). Phosphorus deficiency, therefore, decreases photosynthesis and leaf chlorophyll in many crops (Bechtaoui et al. 2021; Chen et al. 2021; Shiponi and Bernstein 2021a; Verlinden et al. 2022). Conversely, some crops increase leaf chlorophyll under limited soil P availability (Bechtaoui et al. 2021; Shiponi and Bernstein 2021b; Wang et al. 2018). However, even though leaf chlorophyll could indicate the extent of damage to photosynthetic apparatus (Zhang et al. 2014), the inhibition of photosynthesis under P deficiency caused by the effect of low-P on photosynthesis rather than on leaf chlorophyll content (Shiponi and Bernstein 2021b).
Despite the well-characterised morphological and physiological responses to P deficiency in many species (i.e., cereals, oilseed crops, legumes, pastures and native Australian species), little information is available for industrial hemp (Cannabis sativa L.), an important multifaceted crop used for food, feed and fibre worldwide (Callaway 2004; Vonapartis et al. 2015). The crop gained particular attention in Western Australia after it became legal to use seeds and oil for human consumption (Crawford et al. 2012). Industrial hemp is mainly grown in south-western Australia, where around 75% of its agricultural soils are acidic, sandy soils, inherently nutrient-poor (Brennan et al. 2004) with low-P availability in their native state (Bolland et al. 2003; Singh and Gilkes 1991). Apart from some morphological and physiological studies on medical cannabis that focused on the effect of P supply on vegetative biomass, photosynthesis and cannabinoids production (Cockson et al. 2020; Shiponi and Bernstein 2021a, b; Veazie et al. 2021), studies on nutrient use efficiency in industrial hemp are limited to growth, biomass, fibre and seed production (Aubin et al. 2015; Deng et al. 2019; Ivanyi and Izsaki 2009).
In the previous seed germination and early seedling growth trials of 14 imported and locally available industrial hemp varieties (Islam et al. 2022), we identified two contrasting hemp varieties—locally adapted monoecious Morpeth and dioecious Han FNQ—for seed germination percentage, shoot length, shoot growth rate, seedling vigour index and average coarse root length while Fedora 17—a popular monoecious hemp variety—performed average in between Morpeth and Han FNQ. In the subsequent study (Islam et al. 2023), Morpeth and Han FNQ also differed in shoot length, root length, root diameter, root dry weight, leaf chlorophyll, seed yield and nutrient uptake in shoots and seeds in response to microbial consortium inoculant and rock mineral fertiliser treatments. Therefore, the present study characterised the effect of variable P supply (P starvation to excess P) on growth, physiology, rhizosphere carboxylate exudation and nutrient uptake and evaluated P-use efficiency in three industrial hemp varieties (Morpeth, Han FNQ and Fedora 17) grown in low-P soil. We hypothesised that each variety—depending on photosynthesis, root morpho-physiological adaptations and nutrient uptake—would respond differently to elevated P fertilisation and differ in P use efficiency.
Materials and methods
Plant material
The seeds of the three industrial hemp varieties (Morpeth, Han FNQ and Fedora 17) were obtained from Food, Fibre and Land International Group Pty Ltd (Morpeth), WA Hemp Growers Co-op Ltd (Fedora 17), Premium Hemp Australia, and WA Department of Primary Industries and Regional Development (Han FNQ). Seeds were multiplied in a controlled-environment growth chamber at The University of Western Australia (UWA) site (31°98ʹ S, 115°81ʹ E) and stored in resealable aluminium foil bags in a 15 °C cool room until sowing.
Soil conditions
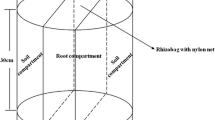
Surface soil (0–10 cm) was collected from UWA Ridgefield Farm, Page Rd, West Pingelly WA 6308 (32° 51ʹ S, 116° 99ʹ E) from a paddock with no history of fertilisation. The loamy sand soil with inherently low phosphorus (6.5 mg kg–1 P) was air-dried and sieved for basic analysis (Table S1). The soil was mixed with RICHGRO® brown river sands (Richgro Garden Products and Amazon Soils, Jandakot WA 6164, Australia.) in a 3:1 ratio to increase soil porosity and water percolation. Table S2 provides the laboratory test results of the sand.
Experimental treatments and growth conditions
The experiment had a randomised complete block design with four replications. The four P treatments (0, 40, 80 and 120 mg kg–1 soil) provided conditions from P starvation to excess (Fig. S1). Preliminary trials have demonstrated that the minimum requirement of P fertiliser for optimum growth of industrial hemp in south-western Australian soils is 40 kg ha–1 (DPIRD-WA 2022). Single superphosphate (SSP) containing (w/w) 9.1% P, 20.0% Ca and 11.5% S was used as the P source. The Ca and S added from SSP were balanced by gypsum and elemental sulphur. Each treatment additionally received a basal dose of N (100 mg kg–1 soil), K (80 mg kg–1 soil) and other essential trace elements (Table S3).
All treatments including basal nutrients were mixed thoroughly in the soil sand mixture. Experimental pots (2.8 L, 180 × 170 mm, black plastic) lined with a polyethylene bag were filled with 3 kg of the soil–sand mixture. Five seeds were sown about 2 cm deep and thinned to two plants per pot 10 days after emergence. Plants were grown in a controlled-environment growth chamber at the UWA site (31°98ʹ S, 115°81ʹ E) with an average temperature, relative humidity and light intensity during a 14/10 h light/dark period of 24.8/21.5 °C, 71.8/70.2% and 5.9/0.0 W m–2. The pots were weighed and hand-watered with deionised H2O daily at 70% field capacity. Re-randomisation of pots was done every week within the blocks to minimise the influence of local environment. Plants were harvested 35 days after sowing at the vegetative growth stage (3rd to nth leaf pair) before the initiation of flowering and seed formation stage (GV point) to achieve higher biomass (Bocsa and Karus 1998; Mediavilla 2001; Mediavilla et al. 1998).
Data collection
We measured several parameters related to plant biomass and growth (shoot and root dry weight, shoot length), physiological functions (leaf chlorophyll content, net photosynthetic rate, stomatal conductance, intercellular CO2 concentration), root morphology (average root length and root surface area, fine and coarse root length), rhizosphere carboxylate exudation (citrate, malate, fumarate, oxalate and pyruvate concentrations, rhizosphere pH), shoot nutrient uptake (NPK concentrations and contents, N:P ratio) and P-use efficiencies (agronomic P-use, P-uptake, P- utilisation) (Table 1).
Carboxylates exudation analyses
Plants were harvested by cutting the stem from the soil surface. After removing the plastic bags from the pots and cutting them open, the roots were gently removed and shaken to remove excess soil. Carboxylate extraction was performed following an established procedure (Kibria et al. 2021; Kidd et al. 2016; Tshewang et al. 2020a, b, 2022). Briefly, the roots with loosely attached rhizosphere soil were transferred into a beaker containing 30–150 mL of 0.2 mM CaCl2 solution, depending on the root surface area. The roots were gently shaken in the solution for about one minute to detach as much rhizosphere soil as possible. The roots were then removed before recording the pH of the solution and filtering 1 mL of the solution through a 0.45 µm syringe filter (Filtropur S) into a 1 mL HPLC vial (Waters™) containing 25 µL of concentrated orthophosphoric acid. Vials were capped and placed onto dry ice before being transferred to − 20 °C until HPLC analysis (600E pump, 717plus auto-injector, 996 photodiode array detectors (PDA); Waters™, Milford, MA 01757, USA). Carboxylates were determined following the improved reversed-phase liquid chromatographic method developed for plant root exudates (Cawthray 2003), except for oxalate, where the method described in Uloth et al. (2015) was followed for oxalate determination.
Shoot NPK measurements
Tissue NPK concentrations were measured in oven-dried shoot samples (70 °C for 72 h). Shoot N concentrations were measured by elemental analysis using an Elementar (Model No. Vario MACRO cube, Elementar Analysensysteme GmbH, Langenselbold, Hesse, Germany) via high temperature gaseous combustion and thermal conductivity. Shoot P and K concentrations were measured using inductively coupled plasma optical emission spectroscopy (ICP-OES, Model No. Optima 5300DV, Perkin Elmer®, Norwalk, CT 06851, USA) after digesting ∼200 mg of finely ground plant material in concentrated HNO3 and HClO4 as described by Simmons (1978). NPK contents were derived from NPK concentrations multiplied by their corresponding shoot dry biomass.
Leaf chlorophyll and leaf gas exchange measurements
Leaf chlorophyll was expressed as SPAD readings obtained from a Soil Plant Analysis Development (SPAD) chlorophyll meter (Model No. SPAD-502 Plus, Konica Minolta Inc., Tokyo, Japan). In addition, other photosynthetic and leaf gas exchange data (net photosynthetic rate, stomatal conductance and intercellular CO2 concentration) were recorded using a portable photosynthesis system (Model No. LI-6400, LI-COR Biosciences, Lincoln, NE, USA).
Root morphology analyses
Root morphology analyses (average root length, root surface area, fine root length, coarse root length) were performed via root scanner (Epson Perfection V800 Photo Scanner® to generate image files at 400 dpi). The images were cropped and evaluated in ‘batch mode’ in WinRHIZO™ software version 2009 (Regent Instruments Inc., Quebec City, QC, Canada), with the cut-off threshold values set to ≤ 0.2 mm for fine roots and > 0.2 mm for coarse roots.
Calculation of P-use efficiencies
P-use efficiencies were calculated at harvest following the formulas suggested by Hammond et al. (2009). Agronomic P-use efficiency (APUE) was measured as the increase in shoot dry matter per unit of added P fertiliser, P-uptake efficiency (PUpE) as the increase in shoot P content per unit of added P fertiliser and P-utilisation efficiency (PUtE) as the increase in shoot dry matter per unit of increased shoot P content.
Statistical analyses
Analysis of variance (ANOVA) was executed to determine the effect of three hemp varieties, four P treatments, and their interactions on the measured parameters (Table 1). In case of no significant interaction, main effects (P ≤ 0.05) were shown by pooling the data across varieties and/or four P rates. The post hoc Tukey’s test was used for multiple comparisons and to guess significant differences among means (P ≤ 0.05).
Results
Plant growth and biomass
Variety and P rate had significant main effects (P ≤ 0.05) on shoot dry weight (Table 1) with Fedora 17 producing higher shoot dry biomass than Han FNQ. All P rates (P40, P80 and P120) produced significantly higher shoot dry biomass than the control (P0) (Table 2). Additionally, P80 and P120 produced significantly higher shoot dry biomass than P40 (Table 2). Phosphorus rate significantly affected root dry weight, with P40, P80 and P120 producing higher root dry biomass than the control (P0) across all varieties. The variety × P rate interaction significantly influenced shoot length, with higher shoot lengths for Fedora 17 at P120 than Morpeth and Han FNQ at all P levels and Fedora 17 at P0 and P40, but similar to Fedora 17 at P80 (Fig. 1a). This incremental shoot length pattern of Fedora 17 to elevated P supply was not identical to Morpeth and Han FNQ.
Root morphology
Variety and P rate had significant effects on root length, fine root length and root surface area (P ≤ 0.05), while the variety × P rate interaction affected coarse root length (Table 1). Morpeth had significantly higher root lengths and fine root lengths than Han FNQ, but they were similar to Fedora 17. Morpeth and Fedora 17 had higher root surface areas than Han FNQ (Table 3). Morpeth treated with P120 had significantly higher coarse root lengths than Morpeth, Han FNQ and Fedora 17 at P0 and P40, and this incremental coarse root length pattern of Morpeth with increasing P rates was not similar to Han FNQ and Fedora 17. Coarse root lengths did not significantly differ between the three varieties at P80 (Fig. 1b).
Plant physiological functions
Variety and P rate significantly influenced (P ≤ 0.05) leaf chlorophyll content (SPAD reading), with higher values in Morpeth than Han FNQ and Fedora 17. Hemp plants grown at P80 had higher leaf chlorophyll contents than the controls (P0) (Table 4). Phosphorus rate affected the net photosynthetic rate and stomatal conductance, with a higher net photosynthetic rate at P0 and higher stomatal conductance at P120 than at P80. Variety significantly affected intercellular CO2 concentration, with Fedora 17 higher than Morpeth (Table 4).
Rhizosphere carboxylates and pH
Variety and P rate governed citrate concentration, P rate influences malate concentration and the variety × P rate interaction affected rhizosphere soil pH (Table 1). Across all P rates, Han FNQ had significantly higher citrate concentrations (P ≤ 0.05) in rhizosphere soil than Morpeth. Across the three varieties, P0 had higher citrate concentrations than the other P treatments (P40, P80 and P120). P40 had significantly higher malate exudation in the rhizosphere soil than P0, but they were statistically similar to P80 and P120 (Table 5). Fedora 17 at P0 had significantly higher rhizosphere pH than Fedora 17 at higher P rates (P40, P80 and P120), Morpeth at P80 and P120 and Han FNQ at P0 and P80, but it was statistically similar to Morpeth and Han FNQ at P40, Morpeth at P0 and Han FNQ at P120 (Fig. 2). However, the decremental pattern of rhizosphere pH to elevated P supply in Fedora 17 was not similar to Morpeth and Han FNQ.
Shoot N, P and K accumulation and N:P ratio
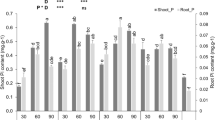
Variety and P rate significantly affected (P ≤ 0.05) shoot P concentration and shoot N:P ratio, while P rate affected shoot N and K concentrations and N, P and K contents (Table 1). Han FNQ had higher shoot P concentrations than Morpeth, but Morpeth had higher N:P ratios than Fedora 17 (Table 6). Shoot N and K concentrations of all three varieties decreased, and P concentration increased with increasing P supply (Table 6). P120 produced higher shoot P concentrations than P0 and P40, but it was statistically similar to P80. Shoots at P0 had higher N:P ratios than the other P rates (P0, P40 and P80). P0 produced significantly higher shoot N and K concentrations than other P rates, except for similar shoot K concentrations at P40. P120 produced the highest shoot P content, followed by P80, P40 and P0 (Table 6). P80 and P120 produced significantly higher shoot N contents than P0 and P40. P80 produced significantly higher shoot K contents than P0 and P40, but they were statistically similar to P120. P0 produced significantly higher N:P ratios than P40, P80 and P120.
P-use efficiencies
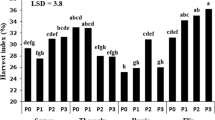
Both variety and P rates significantly affected agronomic P-use (APUE) and P-utilisation (PUtE) efficiencies, whereas the variety × P rate interaction influenced P-uptake efficiency (PUpE) (Table 1). Fedora 17 had significantly higher APUE than Han FNQ, and Morpeth and Fedora 17 had significantly higher PUtE than Han FNQ (Table 7). The P40 produced higher APUE and PUtE than P80 and P120 (Table 7). Han FNQ at P40 had significantly higher PUpE than Morpeth in all treatments and Han FNQ and Fedora 17 at P80 and P120, but it was similar to Fedora 17 at P40 (Fig. 3).
Discussion
This study investigated the differential responses to P fertilisation on the growth, physiology, rhizosphere carboxylate activity and nutrient uptake of three industrial hemp varieties (Morpeth, Han FNQ and Fedora) grown in low-P soil (6.5 mg P kg–1 dry soil) in a controlled-environment growth chamber. Phosphorus fertilisation rates (P40, P80 and P120) produced significantly higher shoot and root dry biomasses of industrial hemp than the control (i.e., no added P) (Table 2). Generally, additional P fertilisation does not affect hemp crops when soil P concentrations are adequate (Aubin et al. 2015; Vera et al. 2010, 2004). However, with low initial soil fertility, yield responses to added P have been reported in industrial hemp (Black and Vessel 1945). In addition, adding P fertiliser, particularly with N, can increase hemp shoot height (Coffman and Gentner 1977; Vera et al. 2004). Like N, industrial hemp responses to P fertilisation could be cultivar and/or environment-dependent (Wylie et al. 2021). For example, Fedora 17 at P120 had a higher shoot length than Morpeth and Han FNQ at all P levels and Fedora 17 at P0 and P40, and this response pattern of Fedora 17 was not similar to Morpeth and Han FNQ (Fig. 1a). Fedora 17 also produced significantly higher (26%) shoot dry biomass than Han FNQ but was statistically similar to Morpeth.
Plant responses to P fertilisation and the efficiency of P acquisition depend on root system architecture and root morphology (e.g., root length, diameter, surface area) (Liu 2021; Marschener 1998). The expression of such traits is genotype-specific and influenced by environmental conditions such as P availability (Tshewang et al. 2020a). In our study, Morpeth at P120 had significantly higher coarse root lengths than Morpeth, Han FNQ and Fedora 17 at P0 and P40, and this response pattern of Morpeth was not similar to Han FNQ and Fedora 17 (Fig. 1b). Morpeth also had significantly higher root lengths and fine root lengths (58%) than Han FNQ, and Morpeth and Fedora 17 produced significantly higher root surface areas (74% and 60%, respectively) than Han FNQ (Table 3). Plant responses to P deficiency generally include increased root length and decreased root diameter, among others (Lambers et al. 2006; Tshewang et al. 2020a; Wang et al. 2008). However, in this experiment, root diameter did not differ significantly between varieties or P rates (data not presented), suggesting that the trait is not associated with hemp root responses to low-P supply and that the hemp varieties might adapt their root morphology by increasing root length and root surface area.
Plant physiology is also affected by P deficiency and P fertilisation under low-P soil conditions. In this study, leaf chlorophyll increased with elevated P rate, with 22% higher values at P80 than P0, and 18% and 13% higher in Morpeth than Han FNQ and Fedora 17, respectively (Table 4). SPAD readings positively correlate with leaf N concentration and reflect leaf chlorophyll concentration (Burgel et al. 2020; Pagnani et al. 2018). Studies have reported reduced chlorophyll concentrations in medical cannabis under P starvation, attributed to reduced leaf N as an indirect effect of P on chlorophyll production (Shiponi and Bernstein 2021a, b). Compared with P0, high P fertilisation (P80) adversely affected the net photosynthetic rate (decreased by 55%) and stomatal conductance (decreased by 63%) of industrial hemp (Table 4). A similar reduction in net photosynthesis under high P-application was reported in medical cannabis (Fredeen et al. 1990; Shiponi and Bernstein 2021a) and attributed to P toxicity with no effect on biomass production, indicating that carbon fixation was not a limiting factor (Shiponi and Bernstein 2021a). In our study, intercellular CO2 concentration varied among hemp varieties, with 11% higher CO2 assimilation in Fedora 17 than in Morpeth. Higher intercellular CO2 concentration increases carboxylation and water use efficiency, reduces stomatal conductance, decreases photorespiration, and promotes photosynthesis and plant growth (Liu et al. 2019) which can be cultivar/species-specific and environment-dependent (Wang et al. 2018).
Plant adaptation to adequate P acquisition under low soil P availability depends on physiological root traits, such as the exudation of carboxylates and phosphatases into the rhizosphere (Kidd et al. 2016; Tshewang et al. 2020a). Plant roots’ capacity to exudate more carboxylates is a primary factor in solubilising and mobilising poorly available soil P (Pearse et al. 2007, 2006; Richardson et al. 2011). Variability in rhizosphere carboxylate exudation in P-impoverished soil has been noted in many crops (Iqbal et al. 2020; Roelofs et al. 2001; Suriyagoda et al. 2012). In the current study, we detected five carboxylates (citrate, malate, fumarate, oxalate and pyruvate) in the rhizosphere of industrial hemp grown under low soil P, of which only citrate and malate concentrations varied significantly (Table 5). Similarly, citrate and malate accounted for most (~ 40–50%) of the total carboxylates in perennial grass species [Harding grass (Phalaris aquatica) and cat grass (Dactylis glomerata)] (Kidd et al. 2016). These two compounds are also common in exudates of Australian Proteaceae (Banksia, Hakea and Dryanda species) (Roelofs et al. 2001).
We recorded almost double (98% higher) the concentration of citrate exudation in Han FNQ than Morpeth across all treatments (Table 5). Citrate is more effective than other carboxylates in mobilising P in the soil solution (Pearse et al. 2007) and reportedly increases P uptake in rice (Oryza sativa L.) (Kirk et al. 1999). In addition, genotypic and species variability in citrate exudation has been reported in wheat and maize genotypes under P adequate and deficient conditions (Iqbal et al. 2020). In our study, citrate exudation was highest under P-deficient conditions (i.e., P0), declining 81%, 93% and 93% in P40, P80 and P120, respectively. In wheat, citrate was released in higher proportions than other carboxylates under P-deficient conditions, which was attributed to decreased activity of the enzyme ribulose-5-phosphate kinase responsible for the conversion of citrate to iso-citrate in the tricarboxylic acid cycle of C3 plants (Iqbal et al. 2020).
In contrast, the P-fertilised treatments had much higher malate exudation than P0 across all hemp varieties, being 1538%, 1379% and 1075% higher in P40, P80 and P120, respectively. Wheat genotypes also exuded more malate under adequate P conditions than P deficit, while maize genotypes predominantly released malate under P-deficient conditions (Iqbal et al. 2020), suggesting that C3 (wheat) and C4 (maize) metabolism is associated with changes in carboxylate exudation. A significant shift from citrate (tricarboxylic acid) to malate (dicarboxylic acid) release has been observed in other C3 crops, including some lupin and canola species (species that predominantly release citrate under P deficit) with increasing P supply (Pearse et al. 2007, 2006).
P deficiency and P fertilisation in low-P soils also influence rhizosphere pH. Alteration in rhizosphere pH affects root physiology, rhizosphere microorganisms, toxic elements and nutrient bioavailability (Pearse et al. 2006). In this study, the variety and P rate interaction significantly influenced rhizosphere pH (Fig. 2). The low-P soil used in this study had an initial pH (H2O) of around 7.4 (Table S1). At P0, rhizosphere pH decreased in all varieties (average pH 6.0), with Han FNQ significantly lower (by 5.8%) than Fedora 17 (Fig. 2). Under P deficiency, more cations are taken up by root cells than anions, inducing carboxylate release by root systems to maintain cellular homeostasis (i.e., cation–anion balance) (Hinsinger et al. 2003; Iqbal et al. 2020). Carboxylate exudation is also accompanied by proton (H+) release to balance charges, ultimately reducing rhizosphere pH (Iqbal et al. 2020). The significantly high amount of citrate released by Han FNQ acidified the rhizosphere at P0. A high amount of citrate release had been associated with strong rhizosphere acidification in cluster roots of white lupin (Hinsinger et al. 2003). However, in the present study, as P rate increased, rhizosphere pH decreased below than P0, with Fedora 17 significantly lower than the control at P40, P80 and P120 (by 5.8%, 7.6% and 7.7%, respectively) (Fig. 2). This could be related to the higher amount of malate released by hemp roots with increasing P supply, further acidifying the rhizosphere due to the accompanying proton exudation.
The N:P ratio is used to assess constraints in plant productivity associated with N and P availability (Cernusak et al. 2010; Garrish et al. 2010; Zhang et al. 2014). In this study, variety and P rate influenced the shoot N:P ratios in industrial hemp. An N:P ratio < 14 indicates N limitation to biomass production, whereas N:P ratio > 16 indicates P limitation (Koerselman and Meuleman 1996). Han FNQ and Fedora 17 had N:P ratios < 14, indicating N limitation, while Morpeth had an N:P ratio > 14 (Table 6), indicating that N was not a limiting factor, as reflected in the higher leaf chlorophyll content of Morpeth than Han FNQ and Fedora 17 (Table 4). The N:P ratios were > 16 at P0 but decreased to < 14 at P40, P80 and P120, indicating that P fertilisation inhibits N uptake, which can be attributed to nutrient uptake and nutrient availability antagonism (Veazie et al. 2021). However, the N:P ratio, leaf chlorophyll and gas exchange data (Table 4) coupled with shoot and coarse root length data (Fig. 1) suggests that the morpho-physiological adaptations used to maintain optimum photosynthesis and growth differ between Morpeth and Fedora 17, with Morpeth having increasing leaf chlorophyll production and coarse root length and Fedora 17 having increasing intercellular CO2 concentration and shoot length.
Plants with higher Agronomic P-use efficiency (APUE) tend to produce more biomass with increasing P supply (Tshewang et al. 2020a) either by increasing P-uptake efficiency (PUpE) or optimising P-utilisation efficiency (PUtE), depending on soil fertility (Neto et al. 2016). However, in this study, APUE and PUtE decreased with increasing P supply (Table 7), as reported for grass species (Tshewang et al. (2020a) and medical cannabis (Shiponi and Bernstein (2021a). Fedora 17 had significantly higher APUE (27%) and PUtE (24%), and Morpeth had 31% higher PUtE than Han FNQ. Considering PUpE, influenced by the variety × P rate interaction, Han FNQ and Fedora 17 had decreased PUpE at P80 and P120 and Morpeth at P120. Similarly, species and genotypic variability in P-use efficiency have been reported in grass species (APUE varied) (Tshewang et al. 2020a) and coffee (Neto et al. 2016) and Brassica oleracea (Hammond et al. 2009) ( PUpE and PUtE varied).
Conclusions
Morpeth and Fedora 17 had improved shoot biomass, root length and root surface area, with high agronomic P-use and P-utilisation efficiencies in response to increasing P fertility, while Han FNQ had moderate shoot biomass and root growth, high citrate exudation and tissue P concentration with high P uptake efficiency. Hemp roots predominantly released citrate in P-deficient conditions and gradually shifted to malate exudation with increasing P supply. The N:P ratio, leaf chlorophyll and gas exchange data coupled with shoot and coarse root length data suggest that morpho-physiological adaptations to maintain photosynthesis and growth differ between Morpeth and Fedora 17. The study also revealed that P80 is the optimum P rate for increasing hemp aboveground biomass and growth while P40 is the agronomically efficient P rate for maximum P-use efficiency with improved belowground biomass and growth in low-P soil.
Data Availability
All data generated or analysed during this study are included in this published article [and its supplementary information files]. However, more detailed information corresponding to this current study are available from the corresponding author on reasonable request.
References
Agren GI (2008) Stoichiometry and nutrition of plant growth in natural communities. Annu Rev Ecol Evol Syst 39:153–170. https://doi.org/10.1146/annurev.ecolsys.39.110707.173515
Aubin MP, Seguin P, Vanasse A, Tremblay GF, Mustafa AF, Charron JB (2015) Industrial hemp response to nitrogen, phosphorus, and potassium fertilization. Crop Forage Turfgrass Manage 1:1–10. https://doi.org/10.2134/cftm2015.0159
Bechtaoui N, Rabiu MK, Raklami A, Oufdou K, Hafidi M, Jemo M (2021) Phosphate-dependent regulation of growth and stresses management in plants. Front Plant Sci 12:679916–679916. https://doi.org/10.3389/fpls.2021.679916
Black CA, Vessel AJ (1945) The response of hemp to fertilizers in Iowa. Soil Sci Soc Am J 9:179–184. https://doi.org/10.2136/sssaj1945.036159950009000C0029x
Bocsa I, Karus M (1998) The cultivation of hemp: botany, varieties, cultivation and harvesting. Hemptech
Bolland MD, Allen DG, Barrow NJ (2003) Sorption of phosphorus by soils: how it is measured in Western Australia. Department of Primary Industries and Regional Development, Perth, Western Australia. https://library.dpird.wa.gov.au/cgi/viewcontent.cgi?article=1035&context=bulletins. Accessed: 19 Nov 2022
Brennan RF, Bolland MDA, Bowden JW (2004) Potassium deficiency, and molybdenum deficiency and aluminium toxicity due to soil acidification, have become problems for cropping sandy soils in south-western Australia. Aust J Exp Agric 44:1031–1039. https://doi.org/10.1071/EA03138
Burgel L, Hartung J, Graeff-Hönninger S (2020) Impact of different growing substrates on growth, yield and cannabinoid content of two Cannabis sativa L. genotypes in a pot culture. Horticulturae 6:1–14. https://doi.org/10.3390/horticulturae6040062
Callaway JC (2004) Hempseed as a nutritional resource: An overview. Euphytica 140:65–72. https://doi.org/10.1007/s10681-004-4811-6
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr A 1011:233–240. https://doi.org/10.1016/S0021-9673(03)01129-4
Cernusak LA, Winter K, Turner BL (2010) Leaf nitrogen to phosphorus ratios of tropical trees: experimental assessment of physiological and environmental controls. New Phytol 185:770–779. https://doi.org/10.1111/j.1469-8137.2009.03106.x
Chen G, Li Y, Jin C, Wang J, Wang L, Wu J (2021) Physiological and morphological responses of hydroponically grown pear rootstock under phosphorus treatment. Front Plant Sci 12:696045–696045. https://doi.org/10.3389/fpls.2021.696045
Cockson P, Schroeder-Moreno M, Veazie P, Barajas G, Logan D, Davis M, Whipker BE (2020) Impact of phosphorus on Cannabis sativa reproduction, cannabinoids, and terpenes. Appl Sci 10:1–19. https://doi.org/10.3390/app10217875
Coffman CB, Gentner WA (1977) Responses of greenhouse-grown Cannabis sativa L. to nitrogen, phosphorus, and potassium. Agron J 69:832–836. https://doi.org/10.2134/agronj1977.00021962006900050026x
Crawford F, Deards B, Moir B, Thompson N (2012) Human consumption of hemp seed: prospects for consumption and production, ABARES report to client prepared for FSANZ. Australian Bureau of Agricultural and Resource Economics and Sciences (ABARES), Canberra, Australia. https://www.foodstandards.gov.au/code/applications/Documents/A1039_SD2_a.pdf. Accessed: 31 Oct 2022
Deng G, Du G, Yang Y, Bao Y, Liu F (2019) Planting density and fertilization evidently influence the fiber yield of hemp (Cannabis sativa L.). Agronomy 9:368. https://doi.org/10.3390/agronomy9070368
DPIRD-WA (2022) Growing industrial hemp (Cannabis sativa) in southern Western Australia. Department of Primary Industries and Regional Development (DPIRD), Western Australia. https://www.agric.wa.gov.au/sites/gateway/files/Growing%20Hemp%20Southern%20WA.pdf. Accessed: 28 Mar 2023
Fredeen AL, Raab TK, Rao IM, Terry N (1990) Effects of phosphorus nutrition on photosynthesis in Glycine max (L.) Merr. Planta 181:399–405. https://doi.org/10.1007/BF00195894
Garrish V, Cernusak LA, Winter K, Turner BL (2010) Nitrogen to phosphorus ratio of plant biomass versus soil solution in a tropical pioneer tree, Ficus insipida. J Exp Bot 61:3735–3748. https://doi.org/10.1093/jxb/erq183
Hammond JP, Broadley MR, White PJ, King GJ, Bowen HC, Hayden R, Meacham MC, Mead A, Overs T, Spracklen WP, Greenwood DJ (2009) Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J Exp Bot 60:1953–1968. https://doi.org/10.1093/jxb/erp083
Heuer S, Gaxiola R, Schilling R, Herrera-Estrella L, López-Arredondo D, Wissuwa M, Delhaize E, Rouached H (2017) Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant J 90:868–885. https://doi.org/10.1111/tpj.13423
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59. https://doi.org/10.1023/A:1022371130939
Iqbal S, Akhtar J, Saqib ZA, Ahmad R (2020) Genotypic and species variability in carboxylate exudation of wheat (Triticum aestivum L.) and maize (Zea mays L.) in phosphorus deficiency. Pak J Agric Sci 57:665–674. https://doi.org/10.21162/PAKJAS/20.9794
Islam MM, Rengel Z, Storer P, Siddique KHM, Solaiman ZM (2022) Industrial hemp (Cannabis sativa L.) varieties and seed pre-treatments affect seed germination and early growth of seedlings. Agronomy 12:6. https://doi.org/10.3390/agronomy12010006
Islam MM, Rengel Z, Storer P, Siddique KHM, Solaiman ZM (2023) Microbial consortium inoculant and rock mineral fertiliser differentially improved yield and nutrient uptake of industrial hemp (Cannabis sativa L.) varieties. Ind Crop Prod 197:116599. https://doi.org/10.1016/j.indcrop.2023.116599
Ivanyi I, Izsaki Z (2009) Effect of nitrogen, phosphorus, and potassium fertilization on nutrional status of fiber hemp. Commun Soil Sci Plant Anal 40:974–986. https://doi.org/10.1080/00103620802693466
Kibria MG, Barton L, Rengel Z (2021) Applying foliar magnesium enhances wheat growth in acidic soil by stimulating exudation of malate and citrate. Plant Soil 464:621–634. https://doi.org/10.1007/s11104-021-04984-z
Kidd DR, Ryan MH, Haling RE, Lambers H, Sandral GA, Yang Z, Culvenor RA, Cawthray GR, Stefanski A, Simpson RJ (2016) Rhizosphere carboxylates and morphological root traits in pasture legumes and grasses. Plant Soil 402:77–89. https://doi.org/10.1007/s11104-015-2770-4
Kirk GJD, Santos EE, Santos MB (1999) Phosphate solubilization by organic anion excretion from rice growing in aerobic soil: rates of excretion and decomposition, effects on rhizosphere pH and effects on phosphate solubility and uptake. New Phytol 142:185–200. https://doi.org/10.1046/j.1469-8137.1999.00400.x
Koerselman W, Meuleman AFM (1996) The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33:1441–1450. https://doi.org/10.2307/2404783
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Liu D (2021) Root developmental responses to phosphorus nutrition. J Integr Plant Biol 63:1065–1090. https://doi.org/10.1111/jipb.13090
Liu X, Zhang H, Wang J, Wu X, Ma S, Xu Z, Zhou T, Xu N, Tang X, An B (2019) Increased CO2 concentrations increasing water use efficiency and improvement PSII function of mulberry seedling leaves under drought stress. J Plant Interact 14:213–223. https://doi.org/10.1080/17429145.2019.1603405
Lyu Y, Tang H, Li H, Zhang F, Rengel Z, Whalley WR, Shen J (2016) Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Front Plant Sci 7:1939–1939. https://doi.org/10.3389/fpls.2016.01939
Marschener H (1998) Role of root growth, arbuscular mycorrhiza, and root exudates for the efficiency in nutrient acquisition. Field Crop Res 56:203–207. https://doi.org/10.1016/S0378-4290(97)00131-7
Mediavilla V (2001) Influence of the growth stage of industrial hemp on the yield formation in relation to certain fibre quality traits. Ind Crops Prod 13:49–56. https://doi.org/10.1016/S0926-6690(00)00052-2
Mediavilla V, Jonquera M, Schmid-Slembrouck I, Soldati A (1998) Decimal code for growth stages of hemp (Cannabis sativa L.). J Int Hemp Assoc 5:65–68
Neto AP, Favarin JL, Hammond JP, Tezotto T, Couto HTZ (2016) Analysis of phosphorus use efficiency traits in coffea genotypes reveals Coffea arabica and Coffea canephora have contrasting phosphorus uptake and utilization efficiencies. Front Plant Sci 7:408–408. https://doi.org/10.3389/fpls.2016.00408
Pagnani G, Pellegrini M, Galieni A, D’Egidio S, Matteucci F, Ricci A, Stagnari F, Sergi M, Lo Sterzo C, Pisante M, Del Gallo M (2018) Plant growth-promoting rhizobacteria (PGPR) in Cannabis sativa ‘Finola’ cultivation: an alternative fertilization strategy to improve plant growth and quality characteristics. Ind Crops Prod 123:75–83. https://doi.org/10.1016/j.indcrop.2018.06.033
Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H (2006) Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 288:127–139. https://doi.org/10.1007/s11104-006-9099-y
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MDA, Lambers H (2007) Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol 173:181–190. https://doi.org/10.1111/j.1469-8137.2006.01897.x
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156. https://doi.org/10.1007/s11104-011-0950-4
Roelofs RFR, Rengel Z, Cawthray GR, Dixon KW, Lambers H (2001) Exudation of carboxylates in Australian Proteaceae: chemical composition. Plant Cell Environ 24:891–904. https://doi.org/10.1046/j.1365-3040.2001.00741.x
Schroeder MS, Janos DP (2005) Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza 15:203–216. https://doi.org/10.1007/s00572-004-0324-3
Shiponi S, Bernstein N (2021a) The highs and lows of P supply in medical cannabis: effects on cannabinoids, the ionome, and morpho-physiology. Front Plant Sci 12:657323–657323. https://doi.org/10.3389/fpls.2021.657323
Shiponi S, Bernstein N (2021b) Response of medical cannabis (Cannabis sativa L.) genotypes to P supply under long photoperiod: functional phenotyping and the ionome. Ind Crop Prod 161:113154. https://doi.org/10.1016/j.indcrop.2020.113154
Simmons WJ (1978) Background absorption error in determination of copper in plants by flame atomic absorption spectrometry. Anal Chem 50:870–873. https://doi.org/10.1021/ac50029a014
Simpson RJ, Oberson A, Culvenor RA, Ryan MH, Veneklaas EJ, Lambers H, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Richardson AE (2011) Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant Soil 349:89–120. https://doi.org/10.1007/s11104-011-0880-1
Singh B, Gilkes RJ (1991) Phosphorus sorption in relation to soil properties for the major soil types of south-western Australia. Aust J Soil Res 29:603–618. https://doi.org/10.1071/SR9910603
Suriyagoda LDB, Lambers H, Renton M, Ryan MH (2012) Growth, carboxylate exudates and nutrient dynamics in three herbaceous perennial plant species under low, moderate and high phosphorus supply. Plant Soil 358:105–117. https://doi.org/10.1007/s11104-012-1311-7
Tshewang S, Rengel Z, Siddique KHM, Solaiman ZM (2020a) Growth, rhizosphere carboxylate exudation, and arbuscular mycorrhizal colonisation in temperate perennial pasture grasses varied with phosphorus application. Agronomy 10:2017. https://doi.org/10.3390/agronomy10122017
Tshewang S, Rengel Z, Siddique KHM, Solaiman ZM (2020b) Nitrogen and potassium fertilisation influences growth, rhizosphere carboxylate exudation and mycorrhizal colonisation in temperate perennial pasture grasses. Agronomy 10:1878. https://doi.org/10.3390/agronomy10121878
Tshewang S, Rengel Z, Siddique KHM, Solaiman ZM (2022) Microbial consortium inoculant increases pasture grasses yield in low-phosphorus soil by influencing root morphology, rhizosphere carboxylate exudation and mycorrhizal colonisation. J Sci Food Agric 102:540–549. https://doi.org/10.1002/jsfa.11382
Uloth MB, You MP, Cawthray G, Barbetti MJ (2015) Temperature adaptation in isolates of Sclerotinia sclerotiorum affects their ability to infect Brassica carinata. Plant Pathol 64:1140–1148. https://doi.org/10.1111/ppa.12338
Veazie P, Cockson P, Kidd D, Whipker B (2021) Elevated phosphorus fertility impact on Cannabis sativa ‘BaOx’ growth and nutrient accumulation. Int J Sci Eng Technol 8. https://ijiset.com/vol8/v8s2/IJISET_V8_I02_32.pdf. Accessed 18 May 2023
Vera CL, Malhi SS, Raney JP, Wang ZH (2004) The effect of N and P fertilization on growth, seed yield and quality of industrial hemp in the Parkland region of Saskatchewan. Can J Plant Sci 84:939–947. https://doi.org/10.4141/p04-022
Vera C, Malhi S, Phelps S, May W, Johnson E (2010) N, P, and S fertilization effects on industrial hemp in Saskatchewan. Can J Plant Sci 90:179–184. https://doi.org/10.4141/CJPS09101
Verlinden MS, Abdelgawad H, Ven A, Verryckt LT, Wieneke S, Janssens IA, Vicca S (2022) Phosphorus stress strongly reduced plant physiological activity, but only temporarily, in a mesocosm experiment with Zea mays colonized by arbuscular mycorrhizal fungi. Biogeosciences 19:2353–2364. https://doi.org/10.5194/bg-19-2353-2022
Vonapartis E, Aubin M-P, Seguin P, Mustafa AF, Charron J-B (2015) Seed composition of ten industrial hemp cultivars approved for production in Canada. J Food Compos Anal 39:8–12. https://doi.org/10.1016/j.jfca.2014.11.004
Wang B, Shen J, Tang C, Rengel Z (2008) Root morphology, proton release, and carboxylate exudation in lupin in response to phosphorus deficiency. J Plant Nutr 31:557–570. https://doi.org/10.1080/01904160801895084
Wang J, Chen Y, Wang P, Li YS, Wang G, Liu P, Khan A (2018) Leaf gas exchange, phosphorus uptake, growth and yield responses of cotton cultivars to different phosphorus rates. Photosynthetica 56:1414–1421. https://doi.org/10.1007/s11099-018-0845-1
Wylie SE, Ristvey AG, Fiorellino NM (2021) Fertility management for industrial hemp production: Current knowledge and future research needs. Glob Change Biol Bioenergy 13:517–524. https://doi.org/10.1111/gcbb.12779
Zhang S, Jiang H, Zhao H, Korpelainen H, Li C (2014) Sexually different physiological responses of Populus cathayana to nitrogen and phosphorus deficiencies. Tree Physiol 34:343–354. https://doi.org/10.1093/treephys/tpu025
Acknowledgements
Mohammad Moinul Islam thanks Australian Government for awarding him International RTP Fees Offset Scholarship and The University of Western Australia’s UPA Scholarship to complete this research. In addition, the authors thank Western Australia’s Department of Primary Industries and Regional Development, and Food, Fibre and Land International Group Pty Ltd, Premium Hemp Australia and WA Hemp Growers' Co-op Ltd for supplying seeds. We also thank Greg Cawthray for root exudation analyses.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michel-Pierre Faucon.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Islam, M.M., Rengel, Z., Storer, P. et al. Phosphorus fertilisation differentially influences growth, morpho-physiological adaptations and nutrient uptake of industrial hemp (Cannabis sativa L.). Plant Soil 492, 301–314 (2023). https://doi.org/10.1007/s11104-023-06171-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06171-8