Abstract
Aims
Domestication and crop breeding has improved plants for human use, but may have unintended consequences for traits that are not selected upon. Disruption in the mycorrhizal mutualism has been observed in crops; possibly due to breeding in fertilised soil. Little is known about whether the legume-rhizobia mutualism can be disrupted by domestication and crop evolution, despite the importance of symbiotic nitrogen fixation. We aimed to identify differences in mutualistic outcomes between five legume cultivars and their wild progenitors in terms of their reactions across varying nitrogen levels.
Methods
With five greenhouse experiments, we characterised symbiosis traits in chickpea, lentil, pea, peanut, and soybean across a gradient of N fertilisation to characterise whether symbiosis traits differ context specifically between wild and domesticated lines.
Results
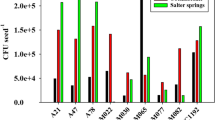
At lower levels of N addition, wild soybean benefited more from rhizobia than domesticated soybean. Chickpea cultivars abandoned symbiosis at lower N than wild chickpea accessions. Chickpea cultivars reduced per-nodule colony forming units (CFU) in response to N addition more than wild chickpeas, but lentil cultivars reduced CFU less than lentil accessions. The lentil, pea, and peanut cultivars did not differ from their wild relatives in rhizobial benefit, nor in nodulation response to N addition.
Conclusions
Differences in the regulation of the root nodule symbiosis are evident between domesticated and wild chickpea and soybean, but not lentil, pea, or peanut. This indicates that mutualism disruption—a decrease in the magnitude of the mutually symbiotic interaction—is a possible, but not necessary, consequence of domestication.



Similar content being viewed by others
Data availability
The data presented in this manuscript will be archived with Dryad upon acceptance.
References
Abbo S, Berger J, Turner NC (2003) Evolution of cultivated chickpea: Four bottlenecks limit diversity and constrain adaptation. Funct Plant Biol 30:1081–1087
Abbo S, Pinhasi van-Oss R, Gopher A, Saranga Y, Ofner I, Peleg Z (2014) Plant domestication versus crop evolution: a conceptual framework for cereals and grain legumes. Trends Plant Sci 19:351–360
Abruña F, Vicente-Chandler J, Silva S, Gracia W (2022) Productivity of nine coffee varieties growing under intensive management in full sunlight and partial shade in the coffee region of Puerto Rico. J Agric Univ Puerto Rico 49:244–253
Aerts R, Verhoeven JTA, Whigham DF (1999) Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology 80:2170–2181
Alo F, Furman BJ, Akhunov E, Dvorak J, Gepts P (2011) Leveraging genomic resources of model species for the assessment of diversity and phylogeny in wild and domesticated lentil. J Hered 102:315–329
Bennett AE, Daniell TJ, White PJ (2013) Benefits of breeding crops for yield response to soil organisms. In: Molecular microbial ecology of the rhizosphere, vol 1. John Wiley & Sons, Inc., Hoboken, NJ, USA, pp 17–27
Bertioli DJ, Cannon SB, Froenicke L, Huang G, Farmer AD, Cannon EKS, Liu X, Gao D, Clevenger J, Dash S et al (2016) The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat Genet 48(4):438–446
Bertioli DJ, Seijo G, Freitas FO, Valls JFM, Leal-Bertioli SCM, Moretzsohn MC (2019) An overview of peanut and its wild relatives
Bever JD, Richardson SC, Lawrence BM, Holmes J, Watson M (2009) Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol Lett 12:13–21
Bobrecka-Jamro D, Jarecki W, Buczek J (2018) Response of soya bean to different nitrogen fertilization levels. J Elem 23:559–568
Botelho DMS, Pozza EA, Alves E, Botelho CE, Pozza AA, Ribeiro Júnior PM, de Souza PE (2011) Efeito do silício na intensidade da cercosporiose e na nutrição mineral de mudas de cafeeiro. Arq Inst Biol 78:23–29
Brooks MEJKK, van Benthem K, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Brozynska M, Furtado A, Henry RJ (2016) Genomics of crop wild relatives: expanding the gene pool for crop improvement. Plant Biotechnol J 14:1070–1085
Campitelli BE, Stinchcombe JR (2013) Natural selection maintains a single-locus leaf shape cline in Ivyleaf morning glory, Ipomoea hederacea. Mol Ecol 22:552–564
Carter TE, Boerma HR, Specht JE, Nelson RL, Sneller CH, Cui Z (2004) Genetic diversity in soybean. In: Soybeans: Improvement, production, and uses. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America, pp 303–416
Chen YH, Gols R, Benrey B (2015) Crop domestication and its impact on naturally selected trophic interactions. Annu Rev Entomol 60:35–58
Córdova-Campos O, Adame-Álvarez RM, Acosta-Gallegos JA, Heil M (2012) Domestication affected the basal and induced disease resistance in common bean (Phaseolus vulgaris). Eur J Plant Pathol 134:367–379
De Carvalho Moretzsohn M, Hopkins MS, Mitchell SE, Kresovich S, Montenegro Valls JF, Ferreira ME (2004) Genetic diversity of peanut (Arachis hypogaea L.) and its wild relatives based on the analysis of hypervariable regions of the genome. BMC Plant Biol 4:11
de Oliveira SM, Pierozan Junior C, Lago BC, de Almeida REM, Trivelin PCO, Favarin JL (2019) Grain yield, efficiency and the allocation of foliar N applied to soybean canopies. Sci Agric 76:305–310
Denison RF (2015) Evolutionary tradeoffs as opportunities to improve yield potential. Field Crop Res 182:3–8
Denison RF, Kiers ET (2011) Life histories of symbiotic rhizobia and mycorrhizal fungi. Curr Biol 21(18)
Diamond J (2002) Evolution, consequences and future of plant and animal domestication. Nature 418:700–707
Dillehay TD, Rossen J, Andres TC, Williams DE (2007) Preceramic adoption of peanut, squash, and cotton in northern Peru. Science (New York, N.Y.) 316:1890–3
Drew EA, Ballard RA (2010) Improving N2 fixation from the plant down: Compatibility of Trifolium subterraneum L. cultivars with soil rhizobia can influence symbiotic performance. Plant Soil 327:261–277
Egerton-Warburton LM, Johnson NC, Allen EB (2007) Mycorrhizal community dynamics following nitrogen fertilization : A cross-site test in five grasslands. Ecol Monogr 77:527–544
Ellis THN, Poyser SJ, Knox MR, Vershinin AV, Ambrose MJ (1998) Polymorphism of insertion sites of Ty1-copia class retrotransposons and its use for linkage and diversity analysis in pea. MGG - Mol Gen Genet 260:9–19
Escuredo PR, Minchin FR, Gogorcena Y, Iturbe-Ormaetxe I, Klucas RV, Becana M (1996) Involvement of activated oxygen in nitrate-induced senescence of pea root nodules. Plant Physiol 110:1187–1195
Foster SD, Bravington MV (2013) A Poisson-Gamma model for analysis of ecological non-negative continuous data. Environ Ecol Stat 20:533–552
Fox J (2003) Effect displays in R for generalised linear models. J Stat Softw 8(15):1–27
Friel CA, Friesen ML (2019) Legumes modulate allocation to rhizobial nitrogen fixation in response to factorial light and nitrogen manipulation. Front Plant Sci 10:1316
Fujikake H, Yamazaki A, Ohtake N, Sueyoshi K, Matsuhashi S, Ito T, Mizuniwa C, Kume T, Hashimoto S, Ishioka NS et al (2003) Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J Exp Bot 54:1379–1388
Gan Y, Stulen I, Posthumus F, Van Keulen H, Kuiper P (2002) Effects of N management on growth, N2 fixation and yield of soybean. Nutr Cycl Agroecosyst 62:163–174
Glyan’ko AK, Vasil’eva GG, Mitanova NB, Ishchenko AA (2009) The influence of mineral nitrogen on legume-rhizobium symbiosis. Biol Bull 36:250–258
Goyal RK, Mattoo AK, Schmidt MA (2021) Rhizobial–host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front Microbiol 12:1290
Griesmann M, Chang Y, Liu X, Song Y, Haberer G, Crook MB, Billault-Penneteau B, Lauressergues D, Keller J, Imanishi L et al (2018) Phylogenomics reveals multiple losses of nitrogen-fixing root nodule symbiosis. Science 361:eaat1743
Grman E, Kellogg WK (2012) Plant species differ in their ability to reduce allocation to non-beneficial arbuscular mycorrhizal fungi. Ecology 93:711–718
Guo J, Wang Y, Song C, Zhou J, Qiu L, Huang H, Wang Y (2010) A single origin and moderate bottleneck during domestication of soybean (Glycine max): Implications from microsatellites and nucleotide sequences. Ann Bot 106:505–514
Herridge DF, Peoples MB, Boddey RM (2008) Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311:1–18
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Hetrick BAD, Wilson GWT, Cox TS (1993) Mycorrhizal dependence of modern wheat cultivars and ancestors: a synthesis. Can J Bot 71:512–518
Hoeksema JD, Chaudhary VB, Gehring CA, Johnson NC, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC et al (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407
Husted L (1936) Cytological studies on the peanut, Arachis. II. CYTOLOGIA 7:396–423
Hyten DL, Song Q, Zhu Y, Choi I-Y, Nelson RL, Costa JM, Specht JE, Shoemaker RC, Cregan PB (2006) Impacts of genetic bottlenecks on soybean genome diversity. Proc Natl Acad Sci USA 103:16666–16671
Jing R, Johnson R, Seres A, Kiss G, Ambrose MJ, Knox MR, Ellis THN, Flavell AJ (2007) Gene-based sequence diversity analysis of field pea (Pisum). Genetics 177:2263–2275
Jing R, Vershinin A, Grzebyta J, Shaw P, Smýkal P, Marshall D, Ambrose MJ, Ellis TN, Flavell AJ (2010) The genetic diversity and evolution of field pea (Pisum) studied by high throughput retrotransposon based insertion polymorphism (RBIP) marker analysis. BMC Evol Biol 10:44
Johnson NC, Angelard C, Sanders IR, Kiers ET (2013) Predicting community and ecosystem outcomes of mycorrhizal responses to global change. Ecol Lett 16:140–153
Kazmierczak T, Nagymihaly M, Lamouche F, Barriére Q, Guefrachi I, Alunni B, Ouadghiri M, Ibijbijen J, Kondorosi É, Mergaert P et al (2017) Specific host-responsive associations between Medicago truncatula accessions and Sinorhizobium strains. Mol Plant Microbe Interact 30:399–409
Kiers ET, Rousseau RA, West SA, Denlson RF (2003) Host sanctions and the legume-rhizobium mutualism. Nature 425:78–81
Kiers ET, Hutton MG, Denison RF (2007) Human selection and the relaxation of legume defences against ineffective rhizobia. Proc Ro Soc B: Biol Sci 274:3119–3126
Kim MY, Lee S, Van K, Kim T-H, Jeong S-C, Choi I-Y, Kim D-S, Lee Y-S, Park D, Ma J et al (2010) Whole-genome sequencing and intensive analysis of the undomesticated soybean (Glycine soja Sieb. and Zucc.) genome. Proc Natl Acad Sci 107:22032–22037
Kim MY, Van K, Kang YJ, Kim KH, Lee S-H (2012) Tracing soybean domestication history: From nucleotide to genome. Breed Sci 61(5):445–452
Kono TJY, Fu F, Mohammadi M, Hoffman PJ, Liu C, Stupar RM, Smith KP, Tiffin P, Fay JC, Morrell PL (2016) The role of deleterious substitutions in crop genomes. Mol Biol Evol 33:2307–2317
Kosterin OE, Zaytseva OO, Bogdanova VS, Ambrose MJ (2010) New data on three molecular markers from different cellular genomes in Mediterranean accessions reveal new insights into phylogeography of Pisum sativum L. subsp. elatius (Bieb.) Schmalh. Genet Resour Crop Evol 57:733–739
Koutroubas SD, Papakosta DK, Gagianas AA (1998) The importance of early dry matter and nitrogen accumulation in soybean yield. Eur J Agron 9:1–10
La T, Large E, Taliercio E, Song Q, Gillman JD, Xu D, Nguyen HT, Shannon G, Scaboo A (2019) Characterization of select wild soybean accessions in the USDA germplasm collection for seed composition and agronomic traits. Crop Sci 59:233–251
Lammerts van Bueren ET, Struik PC, van Eekeren N, Nuijten E (2018) Towards resilience through systems-based plant breeding A review. Agron Sustain Dev 38(5):1–21
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Lebrón L, Lodge DJ, Bayman P (2012) Differences in arbuscular mycorrhizal fungi among three coffee cultivars in Puerto Rico. ISRN Agron 2012:1–7
Lecomte J-B, Benoît HP, Ancelet S, Etienne M-P, Bel L, Parent E (2013) Compound Poisson-gamma vs. delta-gamma to handle zero-inflated continuous data under a variable sampling volume (RB O’Hara, Ed.). Methods Ecol Evol 4:1159–1166
Lewis F, Butler A, Gilbert L (2011) A unified approach to model selection using the likelihood ratio test. Methods Ecol Evol 2:155–162
Liber M, Duarte I, Maia AT, Oliveira HR (2021) The history of lentil (Lens culinaris subsp. culinaris) domestication and spread as revealed by genotyping-by-sequencing of wild and landrace accessions. Front Plant Sci 12:355
Liu J, Yu X, Qin Q, Dinkins RD, Zhu H (2020) The impacts of domestication and breeding on nitrogen fixation symbiosis in legumes. Front Genet 11:973
Liu X, Ju X, Zhang Y, He C, Kopsch J, Fusuo Z (2006) Nitrogen deposition in agroecosystems in the Beijing area. Agr Ecosyst Environ 113:370–377
Lu J, Tang T, Tang H, Huang J, Shi S, Wu C-I (2006) The accumulation of deleterious mutations in rice genomes: a hypothesis on the cost of domestication. Trends Genet 22:126–131
Makino T, Rubin CJ, Carneiro M, Axelsson E, Andersson L, Webster MT (2018) Elevated proportions of deleterious genetic variation in domestic animals and plants. Genome Biol Evol 10:276–290
Mandic V, Simic A, Krnjaja V, Bijelic Z, Tomic Z, Stanojkovic A, Ruzic-Muslic D (2015) Effect of foliar fertilization on soybean grain yield. Biotechnol Anim Husb 31(1):133–143
Marques E, Krieg CP, Dacosta-Calheiros E, Bueno E, Sessa E, Penmetsa RV, von Wettberg E (2020) The impact of domestication on aboveground and belowground trait responses to nitrogen fertilization in wild and cultivated genotypes of chickpea (Cicer sp.). Front Genet 11:1506
Martín-Robles N, Lehmann A, Seco E, Aroca R, Rillig MC, Milla R (2018) Impacts of domestication on the arbuscular mycorrhizal symbiosis of 27 crop species. New Phytol 218:322–334
McGoey BV, Stinchcombe JR (2009) Interspecific competition alters natural selection on shade avoidance phenotypes in Impatiens capensis. New Phytol 183:880–891
Milla R, Matesanz S (2017) Growing larger with domestication: a matter of physiology, morphology or allocation? Plant Biol 19:475–483
Moyers BT, Morrell PL, McKay JK (2018) Genetic costs of domestication and improvement. J Hered 109:103–116
Muñoz N, Qi X, Li M-W, Xie M, Gao Y, Cheung M-Y, Wong F-L, Lam H-M (2016) Improvement in nitrogen fixation capacity could be part of the domestication process in soybean. Heredity 117:84–93
Ndungu SM, Messmer MM, Ziegler D, Gamper HA, Mészáros É, Thuita M, Vanlauwe B, Frossard E, Thonar C (2018) Cowpea (Vigna unguiculata L. Walp) hosts several widespread bradyrhizobial root nodule symbionts across contrasting agro-ecological production areas in Kenya. Agric Ecosyst Environ 261:161
Nishida H, Suzaki T (2018a) Nitrate-mediated control of root nodule symbiosis. Curr Opin Plant Biol 44:129–136
Nishida H, Suzaki T (2018b) Two negative regulatory systems of root nodule symbiosis: How are symbiotic benefits and costs balanced? Plant Cell Physiol 59:1733–1738
Nishida H, Nosaki S, Suzuki T, Ito M, Miyakawa T, Nomoto M, Tada Y, Miura K, Tanokura M, Kawaguchi M et al (2021) Different DNA-binding specificities of NLP and NIN transcription factors underlie nitrate-induced control of root nodulation. Plant Cell 33:2340–2359
Oono R, Muller KE, Ho R, Jimenez Salinas A, Denison RF (2020) How do less-expensive nitrogen alternatives affect legume sanctions on rhizobia? Ecol Evol 10:10645–10656
Pampana S, Masoni A, Mariotti M, Ercoli L, Arduini I (2018) Nitrogen fixation of grain legumes differs in response to nitrogen fertilisation. Exp Agric 54:66–82
Pannecoucque J, Goormachtigh S, Ceusters N, Bode S, Boeckx P, Roldan-Ruiz I (2022) Soybean response and profitability upon inoculation and nitrogen fertilisation in Belgium. Eur J Agron 132:126390
Parvin S, Van Geel M, Ali MM, Yeasmin T, Lievens B, Honnay O (2021) A comparison of the arbuscular mycorrhizal fungal communities among Bangladeshi modern high yielding and traditional rice varieties. Plant Soil 462:109–124
Porter SS, Sachs JL (2020) Agriculture and the disruption of plant–microbial symbiosis. Trends Ecol Evol 35:426–439
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Ramoneda J, Le Roux J, Stadelmann S, Frossard E, Frey B, Gamper HA (2021) Soil microbial community coalescence and fertilization interact to drive the functioning of the legume–rhizobium symbiosis. J Appl Ecol 58(11):2590–2602
Regus JU, Wendlandt CE, Bantay RM, Gleason NJ, Hollowell AC, Shahin KK, Sachs JL (2017) Nitrogen deposition decreases the benefits of symbiosis in a native legume. Plant Soil 414(1/2):159–170
Renaut S, Rieseberg LH (2015) The accumulation of deleterious mutations as a consequence of domestication and improvement in sunflowers and other Compositae crops. Mol Biol Evol 32:2273–2283
Rigg JL, Webster AT, Harvey DM, Orgill SE, Galea F, Dando AG, Collins DP, Harris CA, Newell MT, Badgery WB et al (2021) Cross-host compatibility of commercial rhizobial strains for new and existing pasture legume cultivars in south-eastern Australia. Crop Pasture Sci 72:652–665
Rose TJ, Julia CC, Shepherd M, Rose MT, Van Zwieten L (2016) Faba bean is less susceptible to fertiliser N impacts on biological N2 fixation than chickpea in monoculture and intercropping systems. Biol Fertil Soils 52:271–276
Ryan MH, Kidd DR, Sandral GA, Yang Z, Lambers H, Culvenor RA, Stefanski A, Nichols PGH, Haling RE, Simpson RJ (2016) High variation in the percentage of root length colonised by arbuscular mycorrhizal fungi among 139 lines representing the species subterranean clover (Trifolium subterraneum). Appl Soil Ecol 98:221–232
Samago TY, Anniye EW, Dakora FD (2018) Grain yield of common bean (Phaseolus vulgaris L.) varieties is markedly increased by rhizobial inoculation and phosphorus application in Ethiopia. Symbiosis 75:245–255
Sawers RJH, Gebreselassie MN, Janos DP, Paszkowski U (2010) Characterizing variation in mycorrhiza effect among diverse plant varieties. Theor Appl Genet 120:1029–1039
Sawers RJH, Ramírez-Flores MR, Olalde-Portugal V, Paszkowski U (2018) The impact of domestication and crop improvement on arbuscular mycorrhizal symbiosis in cereals: insights from genetics and genomics. New Phytol 220:1135–1140
Seijo G, Lavia GI, Fernandez A, Krapovickas A, Ducasse DA, Bertioli DJ, Moscone EA (2007) Genomic relationships between the cultivated peanut (Arachis hypogaea, Leguminosae) and its close relatives revealed by double GISH. Am J Bot 94:1963–1971
Seijo JG, Lavia GI, Fernández A, Fernández F, Krapovickas A, Ducasse D, Moscone EA (2004) Physical mapping of the 5S and 18S–25S rRNA genes by FISH as evidence that Arachis duranensis and A. ipaensis are the wild diploid progenitors of A. hypogaea (Leguminosae) 1; Physical mapping of the 5S and 18S–25S rRNA genes by FISH as evidence that Arachis duranensis and A. ipaensis are the wild diploid progenitors of A. hypogaea (Leguminosae) 1
Sessitsch A, Mitter B (2015) 21st century agriculture: Integration of plant microbiomes for improved crop production and food security. Microb Biotechnol 8:32–33
Singh R, Sharma P, Varshney RK, Sharma SK, Singh NK (2008) Chickpea improvement: role of wild species and genetic markers. Biotechnol Genet Eng Rev 25:267–314
Singleton PW, AbdelMagid HM, Tavares JW (1985) Effect of phosphorus on the effectiveness of strains of Rhizobium japonicum. Soil Sci Soc Am J 49:613–616
Siol M, Jacquin F, Chabert-Martinello M, Smýkal P, Le Paslier M-C, Aubert G, Burstin J (2017) Patterns of genetic structure and linkage disequilibrium in a large collection of pea germplasm
Skovbjerg CK, Knudsen JN, Füchtbauer W, Stougaard J, Stoddard FL, Janss L, Andersen SU (2020) Evaluation of yield, yield stability, and yield–protein relationship in 17 commercial faba bean cultivars. Legume Science 2:e39
Smith BD (2016) Neo-Darwinism, niche construction theory, and the initial domestication of plants and animals. Evol Ecol 30:307–324
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, New York
Smýkal P, Coyne C, Redden R, Maxted N (2013) Peas. In: Singh M, Upadhyaya HD, Singh Bish ISB (eds) Genetic and genomic resources of grain legume improvement. Elsevier, pp 41–80
Smýkal P, Hradilová I, Trněný O, Brus J, Rathore A, Bariotakis M, Das RR, Bhattacharyya D, Richards C, Coyne CJ et al (2017) Genomic diversity and macroecology of the crop wild relatives of domesticated pea. Sci Rep 7:17384
Smýkal P, Nelson M, Berger J, von Wettberg E (2018) The impact of genetic changes during crop domestication. Agronomy 8:119
Somasegaran P, Hoben HJ (1994) Handbook for Rhizobia. Springer, New York
Sonnante G, Hammer K, Pignone D (2009) From the cradle of agriculture a handful of lentils: History of domestication. Rendiconti Lincei 20:21–37
Sprent JI, Ardley JK, James EK (2013) From North to South: A latitudinal look at legume nodulation processes. S Afr J Bot 89:31–41
Sprent JI, Ardley J, James EK (2017) Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytol 215:40–56
Stalker HT (2017) Utilizing wild species for peanut improvement. Crop Sci 57:1102–1120
Sudupak MA, Akkaya MS, Kence A (2002) Analysis of genetic relationships among perennial and annual Cicer species growing in Turkey using RAPD markers. Theor Appl Genet 105:1220–1228
Tanno K, Willcox G (2006) The origins of cultivation of Cicer arietinum L. and Vicia faba L.: early finds from Tell el-Kerkh, north-west Syria, late 10th millennium b.p. Veg Hist Archaeobotany 15:197–204
Terpolilli JJ, O’Hara GW, Tiwari RP, Dilworth MJ, Howieson JG (2008) The model legume Medicago truncatula A17 is poorly matched for N2 fixation with the sequenced microsymbiont Sinorhizobium meliloti 1021. New Phytol 179:62–66
Terpolilli JJ, Hood GA, Poole PS (2012) What determines the efficiency of N2-Fixing rhizobium-legume symbioses? Adv Microb Physiol 60:325–389
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Turrini A, Giordani T, Avio L, Natali L, Giovannetti M, Cavallini A (2016) Large variation in mycorrhizal colonization among wild accessions, cultivars, and inbreds of sunflower (Helianthus annuus L.). Euphytica 207:331–342
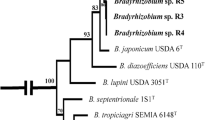
USDA Agricultural Research Service (n.d.) NRRL medium no. 46 modified arabinose gluconate agar. https://webcache.googleusercontent.com/search?q=cache:OiydKkZO9vwJ:https://nrrl.ncaur.usda.gov/cgi-bin/usda/medium/46/nrrl_medium_46.pdf&cd=9&hl=en&ct=clnk&gl=us
Valluru R, Gazave EE, Fernandes SB, Ferguson JN, Lozano R, Hirannaiah P, Zuo T, Brown PJ, Leakey ADB, Gore MA et al (2019) Deleterious mutation burden and its association with complex traits in sorghum (Sorghum bicolor). Genetics 211:1075–1087
van der Maesen LJG, Maxted N, Javadi F, Coles S, Davies AMR (2009) Taxonomy of the genus Cicer revisited. In: Chickpea breeding and management, pp 14–46
Venado RE, Liang J, Marín M (2020) Rhizobia infection, a journey to the inside of plant cells. Adv Bot Res 94:97–118
Vincent JM (1970) A manual for the practical study of root-nodule bacteria. Blackwell Scientific Publications, Oxford
Weese DJ, Heath KD, Dentinger BTM, Lau JA (2015) Long-term nitrogen addition causes the evolution of less-cooperative mutualists. Evolution 69:631–642
Wendlandt CE, Regus JU, Gano-Cohen KA, Hollowell AC, Quides KW, Lyu JY, Adinata ES, Sachs JL (2019) Host investment into symbiosis varies among genotypes of the legume Acmispon strigosus, but host sanctions are uniform. New Phytol 221:446–458
Westhoek A, Clark LJ, Culbert M, Dalchau N, Griffiths M, Jorrin B, Karunakaran R, Ledermann R, Tkacz A, Webb I et al (2021) Conditional sanctioning in a legume-rhizobium mutualism. Proc Natl Acad Sci USA 118(19)
Xing X, Koch AM, Jones AMP, Ragone D, Murch S, Hart MM (2012) Mutualism breakdown in breadfruit domestication. Proc R Soc B: Biol Sci 279:1122–1130
Zeder MA (2017) Domestication as a model system for the extended evolutionary synthesis. Interface Focus 7(5)
Zhang S, Lehmann A, Zheng W, You Z, Rillig MC (2019) Arbuscular mycorrhizal fungi increase grain yields: a meta-analysis. New Phytol 222:543–555
Zohary D (1999) Monophyletic vs. polyphyletic origin of the crops on which agriculture was founded in the Near East. Genet Resour Crop Evol 46:133–142
Zohary D, Hopf M (1973) Domestication of pulses in the old world. Science 183(4115):887–894
Acknowledgements
We thank those who provided germplasm: soybean cultivar MN1312CN (Aaron Lorenz, Department of Agronomy and Plant Genetics, University of Minnesota), peanut cultivars GA06G and Jupiter (Ricky Hartley, Golden Peanut Company and Jill Manahan, Oklahoma Foundation State Seed Stocks, respectively), pea cultivar Serge and lentil cultivar Pardina (Lyndon Porter, USDA), chickpea cultivar Sawyer and the lentil cultivar Avondale (Clarice Coyne, USDA), chickpea cultivar Sierra (Tiffany Fields, USDA), and wild peanut seeds (Shyamalrau Tallury, USDA). All other wild seeds were provided by GRIN’s U.S. National Plant Germplasm System (see Table 2). All rhizobia were provided by Patrick Elia, USDA Germplasm Resource Collection.
Funding
This research was funded by the National Science Foundation (DEB- 1943239 to SSP) as well as by Washington State University, Vancouver in the form of a Mini-Grant to SSP and a Natural Sciences Graduate Fellowship to NM.
Author information
Authors and Affiliations
Contributions
Niall Millar (NM) conducted the experiment and wrote the manuscript. Jonah Piovia-Scott assisted with the data analysis. Stephanie Porter (SSP) supervised the practical work and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest statement
The authors have no conflicts of interest to declare.
Additional information
Responsible Editor: Katharina Pawlowski.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11104_2023_6128_MOESM2_ESM.docx
Supplementary file2 (DOCX 104 KB) Fig. S1 Individual plant lines of domesticated field pea (Pisum sativum) and wild Pisum sativum subsp. elatius differ in their nodule/shoot mass response to N fertiliser addition. Means and standard errors of nodule/shoot mass of domesticated and wild Pisum across levels of N fertilisation. Cultivars shown in grey, wild accessions shown in black. Fig. S2 Individual plant lines of domesticated chickpea (Cicer arietinum) and wild Cicer reticulatum differ in their number of viable rhizobia per nodule in response to N fertiliser addition. Means and standard errors of colony forming units (CFU) of domesticated and wild Cicer across levels of N fertilisation. CFU per nodule is a measure of rhizobial symbiont abundance (i.e., viable bacterial cells) per nodule. Cultivars shown in grey, wild accessions shown in black.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Millar, N., Piovia-Scott, J. & Porter, S.S. Impacts of domestication on the rhizobial mutualism of five legumes across a gradient of nitrogen-fertilisation. Plant Soil 491, 479–499 (2023). https://doi.org/10.1007/s11104-023-06128-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06128-x