Abstract
Background and aims
For invasive plant species that associate with mutualistic symbionts, partner quality can be critical to their invasion success. This might be particularly true for legumes that host nitrogen-fixing bacteria (rhizobia). Here, we examined the relative effectiveness of rhizobial strains on the invasive legume Lupinus polyphyllus.
Methods
We isolated rhizobia from field populations of L. polyphyllus and conducted inoculation experiments in which we quantified plant growth in greenhouse and common-garden conditions.
Results
Differences in nodulation and effectiveness in terms of increasing plant growth among rhizobial strains of the genus Bradyrhizobium were more pronounced in the greenhouse than in the common garden. All six rhizobial strains nodulated the host plant in greenhouse conditions, but one failed to nodulate in the common garden. Under greenhouse conditions, five rhizobial strains increased plant biomass by 66–110%, while one provided negligible benefits compared to control plants without rhizobia, suggesting that rhizobial identity might be critical to the invader’s performance. However, the common-garden experiment revealed no differences in the effectiveness of rhizobial strains in terms of plant biomass, number of leaflets per leaf, height, root:shoot ratio, or survival. Moreover, the performance of rhizobia-inoculated plants in the common garden did not differ from plants without rhizobia, which may call into question the fitness benefits of rhizobia to field populations of this species.
Conclusions
The discrepancies observed between the two environments highlight the importance of considering field-realistic growing conditions and multiple plant traits when assessing the potential growth benefits of symbiotic partners to host plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant growth and survival can greatly benefit from associations with microbial partners, such as mutualistic nitrogen-fixing bacteria known as rhizobia (reviewed in Kebede 2021). Rhizobia live freely in soils and nodulate legumes after germination, with the symbiosis being regulated mainly by the host plant post-establishment (Simms and Taylor 2002; Westhoek et al. 2017; Sachs et al. 2018). Rhizobia fix atmospheric nitrogen for the host plant and in return the plant supplies them with carbon sources and shelter (Lindström and Mousavi 2020). Such symbiotic interactions can be crucial for plants, enhancing their growth (reviewed in Gopalakrishnan et al. 2015; Kebede 2021) as well as resistance to herbivores, diseases, and abiotic stress (e.g., Gopalakrishnan et al. 2015; Kebede 2021; Kalske et al. 2022a). The benefits of rhizobia to the host plant often vary depending on local environmental conditions (Heath et al. 2020), with rhizobia being particularly useful to the plant in poor-quality (low nitrogen) environments (Thrall et al. 2007; Regus et al. 2014). Current theory on plant-symbiont interactions predicts that under high nutrient availability, symbionts are less beneficial to the host plant and may become more parasitic than under poor nutrient conditions (Neuhauser and Fargione 2004; Thrall et al. 2007). Nodulation and nitrogen fixation are also sensitive to temperature, light, and drought (e.g., Montañez et al. 1995; Heath et al. 2020, Hafiz et al. 2021; Lumactud et al. 2022).
As plant-rhizobia interactions are often species-specific (Stępkowski et al. 2018), a lack of suitable partners can be a barrier to plant establishment in new environments (Parker 2001; Simonsen et al. 2017; Harrison et al. 2018). This can be important to invasive species that may not be able to associate with new symbionts encountered in the introduced range (Parker 2001). Moreover, rhizobial strains may differ in their symbiotic effectiveness, i.e. their ability to increase the fitness of a host plant (e.g., Burdon et al. 1999; Sachs et al. 2010; Thrall et al. 2011; Klock et al. 2015; Gano-Cohen et al. 2020), which can retard plant colonisation and spread. As an example, in the tree Acacia decurrens, rhizobial effectiveness varied from an 86% increase in shoot dry weight to no change depending on strain (Burdon et al. 1999). Differences in symbiotic effectiveness are poorly understood, but they can be related to the evolutionary history of the partners, plant community structure, and/or environmental heterogeneity (Thrall et al. 2011). However, potential differences in the effectiveness of rhizobial strains in new environments may not be critical if host plants have adapted to be less dependent on their symbiotic partners in the introduced range (Seifert et al. 2009; terHorst et al. 2018; Kalske et al. 2022a). Such a scenario would be expected if the cost of the interaction, i.e. the investment in nodules, outweighs its benefits for the host plant (Sachs and Simms 2006).
Lupinus polyphyllus (Lindl.) is a short-lived perennial herbaceous legume, 50–100 cm tall, that originates in eastern North America and is invasive in many European countries as well as New Zealand, Chile, Australia, Japan, and western North America (Eckstein et al. 2023). In its introduced range, the species inhabits diverse habitat types, from mountain meadows in central Europe to nutrient-poor road verges and wastelands in Fennoscandia (Eckstein et al. 2023). Due to its rapid growth, L. polyphyllus is a strong competitor and its presence is associated with declines in the diversity of vascular plant species (Valtonen et al. 2006; Ramula and Pihlaja 2012) and arthropods (Ramula and Sorvari 2017), as well as overall changes in plant community composition (Hansen et al. 2021). Individuals in natural populations usually flower in their second year at the earliest (Eckstein et al. 2023). The species is mainly nodulated by bacteria in the genus Bradyrhizobium (reviewed in Stępkowski et al. 2018) and the Lupinus-Bradyrhizobium symbiosis has been described as tolerant to abiotic stress, with increasing soil nitrate levels inhibiting nodulation (Fernández-Pascual et al. 2007). Although Bradyrhizobium spp. are known to nodulate L. polyphyllus in the introduced range (Ryan-Salter et al. 2014; Stępkowski et al. 2018), the effectiveness of different rhizobial strains and their role in the invasion process remain unclear.
Given the context-dependence of the plant-rhizobia symbiosis, the environment in which a study is conducted may be of particular importance. For example, in three soybean varieties tested, the growing environment greatly influenced the nodulation process in rhizobia trapping experiments, with little overlap in findings between natural and greenhouse conditions (Van Dingenen et al. 2022). Indeed, it is possible that greenhouse and field studies may yield different conclusions regarding symbiotic effectiveness, with greenhouse studies potentially overestimating the importance of plant-soil interactions due to their more-homogeneous growing conditions (Schittko et al. 2016, Forero et al. 2019). If so, it could be challenging to translate plant-rhizobia observations obtained from greenhouse experiments - which generally have high humidity and regulated temperature and soil moisture - to real-world conditions in the field. In natural settings, growth benefits provided by rhizobia might be much smaller than expected, which might decrease the dependence of the plant invader on rhizobia.
Here, we isolated rhizobia from invasive populations of L. polyphyllus in southwestern Finland and characterised the relative effectiveness of six rhizobial strains. We investigated the following two questions: 1) Do rhizobial strains isolated from L. polyphyllus differ in nodulation and symbiotic effectiveness in promoting plant growth? 2) Are nodulation and symbiotic effectiveness affected by growth conditions (greenhouse or common garden)? We predicted that host plants would differ in nodulation and growth depending on the rhizobial strain with which they were inoculated. As plant-soil interactions tend to be weaker in the field than in a greenhouse (Schittko et al. 2016), we also predicted that differences in symbiotic effectiveness of rhizobial strains would be less pronounced under common-garden conditions.
Materials and methods
Rhizobial isolation
We collected rhizosphere samples from ten invasive populations of L. polyphyllus around Turku, Finland, in late April 2021. All populations have existed since at least 2010 (Ramula and Pihlaja 2012) and the distances among them range from 1.7 to 32.7 km, with soil NO3− content ranging from 0 to 8 mg/L based on soil samples collected in 2020 (Table 1). The populations inhabit road verges, forest understoreys, former fields, or wastelands (vacant land/green space in urban environments), with average invader cover being 452.4 m2 (± 401.2 m2 SD; Table 1). The invader is the only species representing the genus Lupinus in the study area. At each site, we collected soil from the rhizosphere of five plants that were at least four metres apart and combined the samples per site.
To trap rhizobia from soil, we grew L. polyphyllus from seeds collected from a local population in 2020. To remove epiphytic microbes, we surface-sterilised the seeds in 96% ethanol for 1 min and 4% NaClO3 for 3 min, followed by five rinses with deionised water. We scarified the seeds by nicking the seed coat with a scalpel to promote germination and grew them on 1.5% water-agar petri dishes for four days. We then planted 40 seedlings into 1-L pots, 2 seedlings per pot and 2 pots per population (20 pots in total). The pots were filled with commercial sand (brand: Kekkilä, sterilised in the oven at 170 °C for 4 h) topped by a 2-cm layer of soil from a single lupine population (as a bacterial inoculant) and a 2-cm layer of sterilised sand. For the negative control, we added sterilised sand to two pots to examine whether they formed nodules without added rhizobia. All the pots were randomised on the benches of a growth chamber (12 h light, 22 °C, and 12 h dark, 18 °C; Panasonic® MLR-352) and were irrigated with either autoclaved Milli-Q water or Jensen’s nitrogen-free solution with macronutrients (following Jensen 1942; Yates et al. 2016). After 45 days when the plants had formed nodules, we chose the largest plant per pot, surface-sterilised the nodules, and crushed one nodule per plant (20 nodules and plants in total). A small loopful of the crushed nodule suspension was streaked on plates containing yeast mannitol agar (YMA), and the plates were incubated at 28 °C (5–7 days) to obtain single colonies. A loopful of well-isolated colonies was suspended in 5 ml tryptone-yeast extract (TY) broth, incubated for up to five days at 28 °C in a shaking incubator (Innova® 4000) at 200 rpm, and subsequently preserved in 20% (v/v) glycerol at −80 °C. From the 20 nodules, we successfully obtained 15 pure cultures (representing 8 populations), resulting in 15 bacterial isolates for our experiment (see the Results).
DNA extraction, PCR, and phylogenetic analysis
DNA extraction was carried out using the NucleoSpin™ Microbial DNA kit (Macherey-Nagel™) following the manufacturer’s protocol. The DNA samples were kept at −20 °C. The recombinase A (recA) gene of all samples was amplified using the primers 41F (5’ TTCGGCAAGGGMTCGRTSATG 3′) and 640R (5’ ACATSACRCCGATCTTCATGC 3′) (59.5 °C) that had been previously applied to Bradyrhizobium (Vinuesa et al. 2005). The amplicons were sequenced using Sanger sequencing by Macrogen Europe (Amsterdam, Netherlands). The recA sequences were analysed and edited manually using BioEdit v.7.2.5 (Hall 1999). The edited sequences were then used as query sequences in nblast in GenBank (https://blast.ncbi.nlm.nih.gov/Blast.cgi) for initial identification of the test isolates. The recA sequences of 15 sequenced test isolates and 119 reference strains belonging to the genera Rhizobium, Bradyrhizobium, Mesorhizobium, Sinorhizobium, Agrobacterium, Neorhizobium, Pararhizobium, and Allorhizobium were aligned using ClustalW in BioEdit (Larkin et al. 2007). The best-fit nucleotide substitution model of the locus was chosen (Tamura 3-parameter model with a discrete Gamma distribution (T92 + G)), and the phylogenetic position of the 15 test strains was estimated based on the maximum likelihood method (1000 bs) using MEGA 11 software (Tamura et al. 2021).
Nodulation test of rhizobia
We tested the nodule formation abilities of the isolated rhizobia in a growth chamber in January 2022. We suspended the 15 test isolates in TY broth and incubated them with agitation (200 rpm) at 28 °C for 3–5 days. Seeds of L. polyphyllus were sterilised and scarified as described above and placed on 1.5% water-agar plates. We inoculated four germinated seeds with each test isolate and placed them on sterilised CYG seed germination pouches (16.50 × 18.00 cm; Mega International Ltd., Minneapolis, MN), two seeds per pouch and two pouches per strain (60 seedlings in total). For the negative control, we “inoculated” six seedlings with autoclaved MilliQ water and placed them on pouches. We kept all plants in a growth chamber for seven weeks (13 h light: 1 h at 20 °C, 11 h at 22 °C, 1 h at 20 °C; and 11 h darkness at 18 °C) and irrigated them as described above. The roots were observed twice per week and nodule formation was recorded. Only seven test strains formed nodules that were all effective when assessed visually by their colour (pink interiors); these all belonged to the genus Bradyrhizobium and represented six different lupine populations (see the results for details). We thus focused on bradyrhizobia in the subsequent experiments and ignored the other non-nodulating isolates. For the sake of simplicity, we chose only one bradyrhizobial strain from each population (i.e. we discarded the second strain (strain R7) obtained from population 2, leaving six strains in total; Table 1).
Soil inoculation experiments in a greenhouse and common garden
To examine the symbiotic effectiveness of the six isolated Bradyrhizobium strains under different growth conditions, we conducted greenhouse and common-garden experiments. In mid-May 2022, we chose 150 fully developed seeds from each of the six populations (the same populations where the rhizobial strains originated, see Table 1). The seeds had been collected in July 2021 from 15 mother plants per population (90 plant in total) and had been stored in paper bags at room temperature until use. To enhance germination, we treated the seeds as described above and placed them on a moist paper towel in plastic containers covered with a lid. The seeds were left in an unheated cottage (+10–20 °C) for ten days. At the end of May (27 May), we planted 576 of the emerged seedlings individually into plastic pots of 8 × 8 cm that were filled with a 1:1 mixture of unfertilised peat and sand (brand: Kekkilä, autoclaved at 120 °C, 1 bar, 2 × 20 mins). The bottom of each pot was covered with two layers of filter cloth to prevent roots growing out of the drainage holes. Three days later (on 30 May), we inoculated the seedlings with 1 ml of rhizobial suspension originating from one of the six rhizobial strains, n = 72 pots per strain. In other words, the seedlings from all populations were inoculated with a strain from its own population as well as with strains from all the other populations. The bacterial density of each strain was determined using a spectrophotometer (Agilent Technologies, Inc.), and the density of each bacterial inoculum was adjusted with broth to a final concentration of approximately 5 × 108 CFU mL−1. For a negative control, we inoculated plants with 1 ml of the growth medium without bacteria (hereafter the control treatment, n = 72 pots). For a positive control, we inoculated plants with the growth medium without rhizobia and then fertilised them with a commercial NPK fertiliser twice during the experiment (on 9 and 26 June), hereafter the nitrogen-addition treatment (n = 72 pots).
The 576 seedlings were then allocated to greenhouse and common garden experiments, with 288 plants in each. Overall, there were 36 pots per inoculation treatment (six rhizobial strains, control and nitrogen-addition treatments without added rhizobia) per experiment. We placed saucers under the pots and added a 1-cm layer of lightweight expanded clay aggregate (LECA) to the surface of each pot to prevent splashing and cross-contamination. In the greenhouse experiment, the pots were grown in an unheated greenhouse (about +22 °C day, +12 °C night) and were connected individually to drip irrigation with tap water. We chose to use tap water as it has been previously used to examine the nodulation of rhizobial strains in the study species (Ryan-Salter et al. 2014). In the common garden experiment, the pots were placed on wooden planks in six rows in a common garden, with each row containing a mixture of all treatments and plant populations. We manually watered the pots with tap water in the saucers when necessary. Unfortunately, plant mortality in the common garden was high after the first week, leaving 15–22 pots per treatment and 150 pots in total for the experiment.
We measured plant height and recorded the number of leaves one week after planting to estimate initial plant size. We repeated these size measurements again every other week during the experiment (i.e. at three, five, and seven weeks after planting). On the last measurement date, we also counted the number of leaflets per leaf and measured the length of the longest leaflet to characterise plant size. Eight weeks after rhizobial inoculation, we washed the roots and counted the number of nodules. We separated shoot, root, and nodule biomass and dried them at +65 °C for 48 h before weighing. For the plants in the greenhouse experiment, we also assessed nodule activity visually based on five dissected nodules per plant (when possible). Nodules with a red or pinkish interior were considered active and N-fixing, while those with a brown or colourless interior were considered inactive (Howieson and Dilworth 2016). For the common-garden experiment, nodule activity was not assessed due to small nodule size. In the greenhouse experiment, some plants in the control and nitrogen-addition treatments produced nodules (9 out of 31 and 5 out of 35 surviving plants, respectively), indicating cross-contamination probably through a leaking roof during a heavy rain or through the irrigation system. In the common-garden experiment, 3 out of 19 surviving plants in the control treatment produced nodules. We excluded these nodulating individuals from all analyses to enable strain comparison with the control and nitrogen-addition treatments without rhizobia.
Statistical analysis
To explore the symbiotic effectiveness of the six rhizobial strains, we used linear mixed models (LMM; lme4::lmer) in R software (R 4.1.3; R Development Core Team 2022) that examined the number of nodules (sqrt(x + 1)-transformed), mean individual nodule mass in mg (total nodule mass/number of nodules, sqrt-transformed), total plant biomass in g after removing nodules (log-transformed), root:shoot ratio (without nodules), and number of leaflets. For nodule activity and plant survival (to the last measurement date), we used a generalised linear mixed model (GLMM; lme4::glmer) with a binomial distribution and logit-link function. We did not consider leaflet length due to its positive correlation with total biomass (rp = 0.92, df = 248 in the greenhouse and rp = 0.85, df = 97 in the field). We conducted all analyses separately for both experiments, with inoculation treatment as a categorical fixed variable with eight levels (six rhizobial strains, control and nitrogen-addition treatments without rhizobia), plant initial height from the first measurement as a continuous covariate, and population (six levels) as a random factor. For the analyses of nodule properties, the inoculation treatment had fewer levels (five or six) because we only compared nodulating rhizobial strains. In addition, we conducted an LMM to explore whether total plant biomass (log-transformed) was associated with nodule number and mean individual nodule mass. For this analysis, we used initial plant height as a continuous covariate and population as a random factor.
To analyse the repeated measurements of plant height (sqrt-transformed) during the experiments, we conducted an LMM with inoculation treatment (eight levels), time (three levels), and their interaction as fixed explanatory variables, and plant initial height as a continuous covariate. Population was again used as a random factor together with plant ID to consider the three sets of repeated measurements taken from each plant.
For all models, we examined whether inoculation treatment affected the six populations differently by fitting different slopes across populations and by comparing model AICs; in the end, different slopes were not supported (∆AIC < 2). We verified model assumptions visually from residual plots for the LMMs, and transformed the response variable when necessary (see above for details). For the GLMMs, we explored the residual plots for potential overdispersion and zero inflation using the DHARMa package (Hartig 2018) and found none. We evaluated the significance of the fixed variables with an F test based on the Kenward-Roger method for LMMs (lmerTest::anova; Kuznetsova et al. 2017) and with a Wald chi-square test for GLMMs (car::Anova; Fox and Weisberg 2019). Pairwise differences in mean values between inoculation treatments were assessed with Tukey’s test (emmeans::emmeans; Lenth 2022).
Results
Phylogeny and nodulation of isolated rhizobia
Based on BLAST analyses and a phylogenetic tree of the recA gene (455 bp), the 15 test strains appeared to represent the genera Bradyrhizobium (7 strains from 6 populations, hereafter named R1-R7), Rhizobium (7 strains), and Allorhizobium (1 strain), with only the strains from Bradyrhizobium forming nodules (data not shown). Six bradyrhizobial test strains (R1, R2, R3, R4, R5, R7) were clustered within a clade representing the species B. barrani in the phylogenetic tree (Fig. 1). Instead, strain R6 appeared to be more distantly related to the other six bradyrhizobial test strains, with affinity to the species B. altum (BLAST: 97.36% identity), B. embrapense (BLAST: 96.92% identity), and B. quebecense (BLAST: 96.26% identity; Fig. 1). In this experiment, none of the plants in the negative control without a rhizobial inoculant formed nodules.
Phylogenetic tree based on recA sequences (455 bp) depicting the relationships between reference species and seven bradyrhizobial strains (R1-R7) isolated from six populations of the invasive herb Lupinus polyphyllus, constructed based on a maximum-likelihood analysis. Strains R2 and R7 were isolated from the same population and strains R1-R6 were used for the inoculation experiments, see the methods. The tree was constructed with MEGA11 software, and the numbers at branch points are the bootstrap values (only values >70% are shown). The genus name Bradyrhizobium is abbreviated as B., and the type strains are shown by a superscript “T” at the end of each strain code
Soil inoculation experiment in the greenhouse
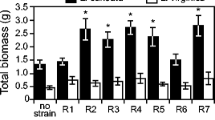
The inoculation treatment affected all plant traits considered except for survival, which was high across treatments (92–98%). In addition, most of the traits measured were positively associated with initial plant size at the beginning of the experiment (Table 2). All six rhizobial strains were successfully able to nodulate the host plant, although plants inoculated with strain R6 produced 63% more nodules than those inoculated with strain R4 (Fig. 2a). Moreover, plants inoculated with strain R2 produced nodules that were 44% and 207% heavier than those inoculated with strain R4 or R6, respectively (Fig. 2b). Nodule activity was generally high across treatments (range: 73–94%), with the exception of rhizobial strain R4, which had lower activity (average 56%) than strains R3 and R6 (Fig. 2c). Plants in the nitrogen treatment produced 258–355% more biomass than plants in the other treatments, and inoculation with all rhizobial strains except for strain R4 resulted in 66–110% greater plant biomass compared to the control treatment without rhizobia, with strain R3 being more efficient than strain R4 (Table 2, Fig. 2d). Total plant biomass was positively associated with both nodule number (F1,127 = 43.96, P < 0.001; intercept = −2.53, slope = 0.08) and mean individual nodule mass (F1,131 = 34.23, P < 0.001; intercept = −2.53, slope = 0.04, LMM) after considering differences in initial plant size. In addition to larger biomass, plants in the nitrogen treatment produced more leaflets per leaf and had a smaller root:shoot ratio (i.e. they invested more biomass in shoots relative to roots) than the plants in the other treatments (Fig. 2e-f).
Effects of inoculation treatments on a–c nodules and d–f other traits of the invasive herb Lupinus polyphyllus under greenhouse conditions (marginal mean ± 95% confidence limits with individual raw data points). C: control treatment without rhizobia, N: nitrogen-addition treatment without rhizobia, R1–R6: six rhizobial strains. Significant differences between treatments are indicated by different letters (p < 0.05, Tukey’s test). Nodule properties in a–c are only compared among rhizobial strains that formed nodules
For plant height, the effect of inoculation varied over time (Table 2), with no differences observed among treatments in the first measurement conducted three weeks after planting (Fig. 3a). In the second measurement, conducted five weeks after planting, plants in the nitrogen-addition treatment and plants inoculated with strain R3 were about 40% taller than those in the control treatment without rhizobia (Fig. 3a). In the third measurement, conducted seven weeks after planting, plants in the nitrogen-addition treatment were 56–105% taller than those in the other treatments (Fig. 3a), while plants inoculated with strain R3 were 46% taller than those inoculated with strain R4 and 56% taller than plants in the control treatment without rhizobia (Fig. 3a). Height during the experiment was positively associated with initial plant height (Table 2).
Effects of inoculation treatments on the height of the invasive herb Lupinus polyphyllus under a greenhouse and b common-garden conditions at different time points after planting. Shown are the medians, the lower and upper quartiles (boxes), the minimum and maximum values (whiskers), and individual raw data points. C: control treatment without rhizobia, N: nitrogen addition treatment without rhizobia, R1–R6: six rhizobial strains. Significant differences between treatments are indicated by different letters (p < 0.05, Tukey’s test)
Soil inoculation experiment in the common garden
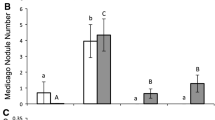
In contrast to the greenhouse experiment, plant survival in the common garden was low (25%) regardless of inoculation treatment (Table 2), and plants inoculated with rhizobial strain R5 produced no nodules under common-garden conditions. Nodulation was observed with the other five strains (Table 2, Fig. 4a), but mean nodule mass did not differ among strains (Table 2, Fig. 4b). In general, nodule production in the common garden was lower than in the greenhouse (mean ± SD calculated across nodulating strains: 1.70 ± 2.82 and 2.79 ± 3.40, respectively; Figs. 2a and 4a). Similar to the greenhouse experiment, plants in the nitrogen treatment were larger than plants in the other treatments in terms of total biomass and the number of leaflets per leaf, and often had a smaller root:shoot ratio (Table 2, Fig. 4c-e). However, the six rhizobial strains did not differ in the three plant traits (total biomass, leaflets per leaf, and root:shoot ratio; Table 2, Fig. 4c-e), with biomass production varying from a 52% increase to a 6% decrease compared to the control treatment without rhizobia (Fig. 4c). As in the greenhouse, total plant biomass in the common garden tended to correlate positively with nodule number (F1,21 = 4.31, P = 0.050; intercept = −3.07, slope = 0.05) and mean individual nodule mass (F1,21 = 10.59, P = 0.006; intercept = −3.07, slope = 0.49, LMM).
Effects of inoculation treatments on a–b nodules and c–e other traits of the invasive herb Lupinus polyphyllus under common-garden conditions (marginal mean ± 95% confidence limits with individual raw data points). C: control treatment without rhizobia, N: nitrogen addition treatment without rhizobia, R1–R6: six rhizobial strains. Significant differences between treatments are indicated by different letters (p < 0.05, Tukey’s test). Nodule properties in a–b are only compared among rhizobial strains that formed nodules
The inoculation treatment had a detectable effect on plant height at the second and third measurements (i.e., a significant treatment × time interaction; Table 2), with plants in the nitrogen-addition treatment being about 55% and 71% taller than plants in the other treatments at those respective times (Fig. 3b). However, height did not differ among plants inoculated with different rhizobial strains (Fig. 3b). Again, height during the experiment was positively associated with initial plant height (Table 2).
Discussion
In this study, we isolated and characterised rhizobia from field populations of the herbaceous invasive legume L. polyphyllus. We discovered that only strains belonging to the genus Bradyrhizobium formed nodules on this plant, while strains belonging to Rhizobium or Allorhizobium did not. Further investigation of six strains of Bradyrhizobium revealed differences in nodulation and effectiveness in terms of plant growth under greenhouse conditions. However, these differences in rhizobial partner quality disappeared in the common garden. While this discrepancy between the two environments supports our second hypothesis on the less-pronounced role of symbiotic interactions under common-garden conditions, it also calls into question the overall fitness benefits of the rhizobial partner to this plant invader in field populations.
A lack of a suitable symbiotic partner can be a barrier to plant invasions in new environments (Parker 2001; Simonsen et al. 2017; Harrison et al. 2018). In the introduced range, L. polyphyllus has been reported to form nodules primarily in symbiosis with members of Bradyrhizobium (reviewed in Stepkowski et al. 2018), and in particular with B. canariense and B. japonicium in Belgium (De Meyer et al. 2011) and with B. canariense, B. japonicium and Bradyrhizobium cytisi in New Zealand (Ryan-Salter et al. 2014). The present study confirmed nodulation with Bradyrhizobium, but interestingly, most of the bradyrhizobial strains isolated here (six out of seven strains) were closely related to an isolate of B. barrani that was recently isolated from the root nodules of soybeans, which had been inoculated with suspensions of root-zone soils of native legumes in Quebec, Canada (Bromfield et al. 2022). In addition to Bradyrhizobium, our test isolates contained other non-nodulating bacteria (Rhizobium and Allorhizobium) whose relationship to the host plant (if any) is not known. However, this observation was not unexpected given a previous report that the root nodules of L. polyphyllus and other legumes can contain several non-N-fixing or non-rhizobial bacterial genera (De Meyer et al. 2015).
In the present study, all six strains of Bradyrhizobium formed effective nodules on the host plant under greenhouse conditions, while one (strain R5) failed to nodulate under common garden conditions. This finding is in line with observations from New Zealand, where L. polyphyllus was able to form nodules in symbiosis with multiple natural strains of Bradyrhizobium under greenhouse conditions (Ryan-Salter et al. 2014). As previously reported from the annual weedy legume Medicago lupulina inoculated with Bradyrhizobium (Batstone et al. 2020), we found that, across rhizobial strains, nodulation was more frequent in the greenhouse than in the common garden. This was probably the result of the more-favourable growth conditions in the greenhouse, as the optimum temperature for rhizobial growth on temperate legumes is +18–30 °C (Kumarasinghe and Nutman 1979). However, nodule formation per se does not necessarily predict rhizobial benefits to the host plant. As an example, in the native annual legume Agmispon trigosus, ineffective Bradyrhizobium strains determined in terms of plant shoot biomass formed more nodules than effective ones (Pahua et al. 2018). In the present study, differences in nodule number and nodule size were generally minor among the six rhizobial strains, although in the greenhouse, inoculation with strain R6 resulted in more nodules than strain R4, and strain R2 was associated with larger nodule size than strains R4 and R6. Nodule activity did not differ among the strains when assessed visually by nodule colour.
Similar to previous studies on various legumes (e.g., Burdon et al. 1999; Sachs et al. 2010; Thrall et al. 2011; Klock et al. 2015; Gano-Cohen et al. 2020), we found considerable variability in rhizobial effectiveness for the invasive L. polyphyllus under greenhouse conditions. Here, five out of six rhizobial strains increased the total biomass of the host plant by 66–110% compared to the control treatment without added rhizobia, while one strain (strain R4) had a negligible effect. We do not have an obvious explanation for the inefficiency of strain R4. However, this strain originated from a declining population of L. polyphyllus that has been overgrown by young aspen trees (Populus tremula) in the past few years (S. Ramula, personal obs.). Changes in light conditions together with intensified interspecific competition with tall vegetation (grasses and young aspen trees) might have weakened the symbiotic interaction between the host plant and its rhizobia in that field population, resulting in poor co-operation if the plant provided less carbon sources to the symbiont. Such poor co-operation might explain the lower efficiency of strain R4 observed in the greenhouse experiment. Total biomass can be expected to reflect a plant’s resource status and, consequently, growth potential, although only one of the strains investigated here (strain R3) tended to also increase plant height under greenhouse conditions. These differences in partner quality among rhizobial strains suggest that rhizobial identity might be crucial to the performance of this plant invader. However, our findings from the common garden challenge this view, as they revealed no differences in total plant biomass or in the other traits considered (number of leaflets per leaf, plant height, root:shoot ratio, survival) among rhizobial strains. Surprisingly, the performance of the rhizobia-inoculated plants was no better than that of the plants in the control treatment without rhizobia, which may mean that all rhizobial strains were ineffective under field conditions. Visual assessment of the plant traits between the two study environments revealed that plant biomass and height were generally smaller in the common garden than in the greenhouse regardless of treatment (Figs. 2d, 3, 4c).
Rhizobial ineffectiveness might be the result of invasive plant species gradually evolving to become less dependent on their symbionts (Seifert et al. 2009; terHorst et al. 2018). Indeed, a previous comparison of native and invasive populations of L. polyphyllus grown with soil microbiota from an invasive population suggested that plants from invasive populations may benefit less from their own soil microbiota than plants from native populations do (Kalske et al. 2022a). Moreover, the fact that plants in the nitrogen-addition treatment in the present study grew better than those inoculated with rhizobia confirmed that the plant invader benefitted from a high-nitrogen environment in both experiments. Therefore, the observed discrepancies between the two different environments (greenhouse and common garden) are probably due to the context-dependent nature of plant-rhizobia interactions and rhizobial benefits to the host plant. For example, rhizobial strains may differ in nodulation ability and effectiveness across environmental conditions, such as temperature or light (e.g., Montañez et al. 1995; Heath et al. 2020; Hafiz et al. 2021). Moreover, abiotic stress in plants usually reduces nodule formation (Miransari et al. 2013; Lumactud et al. 2022) via modifications to signalling molecules that are critical to symbiosis (Miransari et al. 2013). In our case, abiotic conditions were harsher in the common garden, where plants were exposed to wind, lower night temperatures, and stronger solar radiation compared to the greenhouse environment, which might also explain high plant mortality. This higher abiotic variability probably masked any potential differences in the effectiveness of rhizobial strains. Similarly, Schittko et al. (2016) reported that greenhouse and field studies on plant-soil interactions yielded different outcomes for five out of the eight grassland plant species tested; specifically, positive effects found in the greenhouse were absent in the field. Those findings, combined with the results from the current study, collectively highlight the importance of considering field-realistic growth conditions when assessing symbiotic benefits to a host plant. However, it is also possible that a least a part of the discrepancies between our two experiments was due to the smaller sample size in the common garden, which resulted from high plant mortality after they were moved outdoors. Moreover, the plants grown in the common garden could have encountered additional unknown symbionts during the experiment, which might have masked the potential effects of the experimental strains.
Overall, our results call into question the fitness benefits of rhizobia to L. polyphyllus in field conditions, but this comes with several caveats. First, here we inoculated seedlings with a single rhizobial strain, while nodules in nature tend to host multiple strains (e.g., Denison 2000). Although single-strain inoculations without the possibility for partner choice can provide valuable information on differences among strains, they may misestimate partner quality and the long-term benefits to the host plant (Kiers et al. 2013). Second, we considered the performance of host plants during the first two months of growth, which may not adequately reflect the total lifetime fitness benefits of rhizobia to this perennial species. Third, only rhizobial strains that were possible to culture in the lab were isolated. There may have been other strains present in the nodules that might have been beneficial to the host plant in the field, but that we could not be successfully grow and use as inoculants. Fourth, tap water used for watering may have contained some rhizobia (e.g., Paduano et al. 2020), adding extra variation to the inoculation experiments. However, we do not think it would have been critical to our findings because we excluded control plants that formed nodules from the analyses (i.e. we only compared inoculated plants that hosted rhizobia to those without rhizobia). These comparisons revealed no differences in plant growth (total biomass and height) between the two groups in the common garden, suggesting that the effects of the six rhizobial strains (or additional strains received from tap water) on the host plant were minor. Finally, we cannot rule out the possibility that the rhizobia we investigated may be beneficial to the host plant in ways or under circumstances that were not considered here. For example, leaves of L. polyphyllus contain nitrogen-based chemical compounds, alkaloids, which are used as a defence against herbivores (Wink 1984, 2019; Kalske et al. 2022b). Our previous work indicated that soil microbiota can be critical to this plant’s resistance to leaf herbivores (Kalske et al. 2022a). Therefore, we propose that future studies on rhizobial effectiveness should consider additional traits beyond those related to plant growth only (e.g., resistance to herbivores and diseases), to enable a broader examination of potential fitness benefits to the host plant.
Data availability
The datasets generated and analysed during the study are available in the Dryad repository: https://doi.org/10.5061/dryad.2jm63xsv1. The accession numbers of the deposited recA sequences in GenBank are as follows: OQ446412- OQ446425.
References
Batstone RT, Peters MAE, Simonsen AK, Stinchcombe JR, Frederickson ME (2020) Environmental variation impacts trait expression and selection in the legume-rhizobium symbiosis. Am J Bot 107:195–208. https://doi.org/10.1002/ajb2.1432
Bromfield ES, Cloutier S, Wasai-Hara S, Minamisawa K (2022) Strains of Bradyrhizobium barranii sp. nov. associated with legumes native to Canada are symbionts of soybeans and belong to different subspecies (subsp. barranii subsp. nov. and subsp. apii subsp. nov.) and symbiovars (sv. glycinearum and sv. septentrionale). Int J Syst Evol Microbiol 72(10):005549. https://doi.org/10.1099/ijsem.0.005549
Burdon JJ, Gibson AH, Searle SD, Woods MJ, Brockwell J (1999) Variation in the effectiveness of symbiotic associations between native rhizobia and temperate Australian Acacia within-species interactions. J Appl Ecol 36:398–408. https://doi.org/10.1046/j.1365-2664.1999.00409.x
De Meyer SE, Van Hoorde K, Vekeman B, Braeckman T, Willems A (2011) Genetic diversity of rhizobia associated with indigenous legumes in different regions of Flanders (Belgium). Soil Biol Bioch 43:2384–2396. https://doi.org/10.1016/j.soilbio.2011.08.005
De Meyer SE, De Beuf K, Willems A (2015) A large diversity of non-rhizobial endophytes found in legume root nodules in Flanders (Belgium). Soil Biol Bioch 83:1–11. https://doi.org/10.1016/j.soilbio.2015.01.002
Denison RF (2000) Legume sanctions and the evolution of symbiotic cooperation by rhizobia. Am Nat 156:567–576. https://doi.org/10.1086/316994
Eckstein RL, Welk E, Klinger YP, Lennartsson T, Wissma J, Ludewig K, Hansen W, Ramula S (2023) Biological Flora of Central Europe – Lupinus polyphyllus Lindley. Persp Plant Ecol Evol. https://doi.org/10.1016/j.ppees.2022.125715
Fernández-Pascual M, Pueyo JJ, de Felipe MR, Golvano MP, Lucas MM (2007) Singular features of the Bradyrhizobium-Lupinus symbiosis. Dyn Soil Dyn Land 1:1–16
Forero LE, Grenzer J, Heinze J, Schittko C, Kulmatiski A (2019) Greenhouse- and field-measured plant-soil feedbacks are not correlated. Front Environ Sci 7:184. https://doi.org/10.3389/fenvs.2019.00184
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage, Thousand Oaks CA
Gano-Cohen KA, Wendlandt CE, Moussawi KA, Stokes PJ, Quides KW, Weisberg AJ, Chang JH, Sachs JL (2020) Recurrent mutualism breakdown events in a legume rhizobia metapopulation. Proc R Soc B 287:20192549. https://doi.org/10.1098/rspb.2019.2549
Gopalakrishnan S, Sathya A, Vijayabharathi R, Varshney RK, Gowda CLL, Krishnamurthy L (2015) Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech 5:355–377
Hafiz MHR, Salehin A, Itoh K (2021) Growth and competitive infection behaviors of Bradyrhizobium japonicum and Bradyrhizobium elkanii at different temperatures. Horticulturae 7:41. https://doi.org/10.3390/horticulturae7030041
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hansen W, Wollny J, Otte A, Eckstein RL, Ludewig K (2021) Invasive legume affects species and functional composition of mountain meadow plant communities. Biol Invasions 23:281–296. https://doi.org/10.1007/s10530-020-02371-w
Harrison TL, Simonsen AK, Stinchcombe JR, Frederickson ME (2018) More partners, more ranges: generalist legumes spread more easily around the globe. Biol Lett 14:20180616. https://doi.org/10.1098/rsbl.2018.0616
Hartig F (2018) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.0
Heath KD, Podowski JC, Heniff S, Klinger CR, Burke PV, Weese DJ, Yang WH, Lau JA (2020) Light availability and rhizobium variation interactively mediate the outcomes of legume–rhizobium symbiosis. Am J Bot 107:229–238. https://doi.org/10.1002/ajb2.1435
Howieson JG, Dilworth MJ (2016) Working with rhizobia. Australian Centre for International Agricultural Research
Jensen HL (1942) Nitrogen fixation in leguminous plants. I. General characters of root-nodule bacteria isolated from species of Medicago and Trifolium in Australia. Pro Line Soc NSW 57:205–212
Kalske A, Blande JD, Ramula S (2022a) Soil microbiota explain differences in herbivore resistance between native and invasive populations of a perennial herb. J Ecol 110:2649–2660. https://doi.org/10.1111/1365-2745.13975
Kalske A, Luntamo N, Slminen J-P, Ramula S (2022b) Introduced populations of the garden lupine are adapted to local generalist snails but have lost alkaloid diversity. Biol Invasions 24:51–65. https://doi.org/10.1007/s10530-021-02622-4
Kebede E (2021) Competency of rhizobial inoculation in sustainable agricultural production and biocontrol of plant diseases. Front Sust Food Syst 5:728014. https://doi.org/10.3389/fsufs.2021.728014
Kiers ET, Ratcliff WC, Denison RF (2013) Single-strain inoculation may create spurious correlations between legume fitness and rhizobial fitness. New Phytol 198:4–6. https://doi.org/10.1111/nph.12015
Klock MM, Barrett LG, Thrall PH, Harms KE (2015) Host promiscuity in symbionta associations can influence exotic legume establishment and colonization of novel ranges. Diversit Distrib 21:1193–1203. https://doi.org/10.1111/ddi.12363
Kumarasinghe RMK, Nutman PS (1979) The influence of temperature on root hair infection of Trifolium parviflorum and T. glomeratum by root nodule bacteria. I. the effects of constant root temperature on infection and related aspects of nodule development. J Exp Bot 30:515–530
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear -mixed effects models. J Stat Softw 82:1–26
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics. 23(21):2947–2948
Lenth RV (2022) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.8.0. https://CRAN.R-project.org/package=emmeans
Lindström K, Mousavi SA (2020) Effectiveness of nitrogen fixation in rhizobia. Microb Biotechnol 13(5):1314–1335. https://doi.org/10.1111/1751-7915.13517
Lumactud RA, Dollete D, Liyanage DK, Szczyglowski K, Hill B, Thilakarathna MS (2022) The effect of drought stress on nodulation, plant growth, and nitrogen fixation in soybean during early plant growth. J Agro Crop Sci. https://doi.org/10.1111/jac.12627
Miransari M, Riahi H, Eftekhar F, Minaie A, Smith DL (2013) Improving soybean (Glycine max L.) N2 fixation under stress. J Plant Growth Regul 32:909–921. https://doi.org/10.1007/s00344-013-9335-7
Montañez A, Danso SKA, Hardarson G (1995) The effect of temperature on nodulation and nitrogen fixation by five Bradyrhizobium japonicum strains. Appl Soil Ecol 2:165–174. https://doi.org/10.1016/0929-1393(95)00052-M
Neuhauser C, Fargione JE (2004) A mutualism–parasitism continuum model and its application to plant–mycorrhizae interactions. Ecol Model 177:337–352. https://doi.org/10.1016/j.ecolmodel.2004.02.010
Paduano S, Marchesi I, Casali ME, Valeria F, Frezza G, Vecchi E, Sircana L, Spica VR, Borella P, Bargellini N (2020) Characterisation of microbial community associated with different disinfection treatments in hospital hot water networks. Int J Environ Res Public Health 17:2158. https://doi.org/10.3390/ijerph17062158
Pahua VJ, Stokes PJN, Hollowell AC, Regus JU, Gano-Cohen KA, Wendlandt CE, Quides KW, Lyu JY, Sachs JL (2018) Fitness variation among host species and the paradox of ineffective rhizobia. J Evol Biol 31:599–610. https://doi.org/10.1111/jeb.13249
Parker MA (2001) Mutualism as a constraint on invasion success for legumes and rhizobia. Diversity Distrib 7:125–136. https://www.jstor.org/stable/2673345
Ramula S, Pihlaja K (2012) Plant communities and the reproductive success of native plants after the invasion of an ornamental herb. Biol Invasions 14:2079–2090. https://doi.org/10.1007/s10530-012-0215-z
Ramula S, Sorvari J (2017) The invasive herb Lupinus polyphyllus attracts bumblebees but reduces total arthropod abundance. Arthropod-Plant Inter 11:911–918. https://doi.org/10.1007/s11829-017-9547-z
R Development Core Team (2022) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/
Regus JU, Wendlandt CE, Bantay RM, Gano-Cohen KA, Gleason NJ, Hollowell AC, O’Neill MR, Shahin KK, Sachs JL (2014) Nitrogen deposition decreases the benefits of symbiosis in a native legume. Plant Soil 414:159–170. https://doi.org/10.1007/s11104-016-3114-8
Ryan-Salter TP, Black AD, Andrews M, Moot DJ (2014) Identification and effectiveness of rhizobial strains that nodulate Lupinus polyphyllus. Proc New Zealand Grassl Assoc 76:61–66
Sachs JL, Simms EL (2006) Pathways to mutualism breakdown. Trends Ecol Evol 21:585–592. https://doi.org/10.1016/j.tree.2006.06.018
Sachs JL, Ehinger MO, Simms EL (2010) Origins of cheating and loss of symbiosis in wild Bradyrhizobium. J Evol Biol 23:1075–1089. https://doi.org/10.1111/j.1420-9101.2010.01980.x
Sachs JL, Quiedes QW, Wendlandt CE (2018) Legumes versus rhizobia: a model for ongoing conflict in symbiosis. New Phytol 219:1199–1206. https://doi.org/10.1111/nph.15222
Schittko C, Runge C, Strupp M, Wolff S, Wurst S (2016) No evidence that plant–soil feedback effects of native and invasive plant species under glasshouse conditions are reflected in the field. J Ecol 104:1243–1249. https://doi.org/10.1111/1365-2745.12603
Seifert EK, Bever JD, Maron JL (2009) Evidence for the evolution of reduced mycorrhizal dependence during plant invasion. Ecology 90:1055–1062. https://doi.org/10.1890/08-0419.1
Simms EL, Taylor DL (2002) Partner choice in nitrogen-fixation mutualisms of legumes and rhizobia. Integr Comp Biol 42:369–380. https://doi.org/10.1093/icb/42.2.369
Simonsen AK, Dinnage R, Barrett LG, Prober SM, Thrall PH (2017) Symbiosis limits establishment of legumes outside their native range at a global scale. Nat Commun 8:14790
Stępkowski T, Banasiewicz J, Granada CE, Andrews M, Passaglia LMP (2018) Phylogeny and phylogeography of rhizobial symbionts nodulating legumes of the tribe genisteae. Genes 9:163. https://doi.org/10.3390/genes9030163
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Thrall PH, Laine A-L, Broadhurst LM, Bagnall DJ, Brocwell J (2011) Symbiotic effectiveness of rhizobial mutualists varies in interactions with native Australian legume genera. PlosOne 6:e23545. https://doi.org/10.1371/journal.pone.0023545
Thrall PH, Hochberg ME, Burdon JJ, Bever JD (2007) Coevolution of symbiotic mutualists and parasites in a community context. Trends Ecol Evol 22:120–126. https://doi.org/10.1016/j.tree.2006.11.007
terHorst CP, Wirth C, Lau JA (2018) Genetic variation in mutualistic and antagonistic interactions in an invasive legume. Oecologia 188:159–171. https://doi.org/10.1007/s00442-018-4211-6
Valtonen A, Jantunen J, Saarinen K (2006) Flora and lepidoptera fauna adversely affected by invasive Lupinus polyphyllus along road verges. Biol Conserv 133:389–396. https://doi.org/10.1016/j.biocon.2006.06.015
Van Dingenen J, Garcia Mendez S, Beirinckx S, Vlaminck L, De Keyser A, Stuer N, Verschaete S, Clarysse A, Pannecoucque J, Rombauts S, Roldan-Ruiz I, Willems A, Goormachtig S (2022) Flemish soils contain rhizobia partners for northwestern Europe-adapted soybean cultivars. Environ Microbiol 24:3334–3354. https://doi.org/10.1111/1462-2920.15941
Vinuesa P, Silva C, Werner D, Martínez-Romero E (2005) Population genetics and phylogenetic inference in bacterial molecular systematics: the roles of migration and recombination in Bradyrhizobium species cohesion and delineation. Mol Phylogenetic Evol 34:29–54. https://doi.org/10.1016/j.ympev.2004.08.020
Westhoek A, Field E, Rehling F, Mulley G, Webb I, Poole PS, Turnbull LA (2017) Policing the legume-Rhizobium symbiosis: a critical test of partner choice. Sci Rep 7:1419
Wink M (1984) Chemical defense of Leguminosae. Are quinolizidine alkaloids part of the antimicrobial defense system of lupins? Zeitschrift für Naturforschung C 39:548–552. https://doi.org/10.1515/znc-1984-0607
Wink M (2019) Quinolizidine and pyrrolizidine alkaloid chemical ecology – a mini-review on their similarities and differences. J Chem Ecol 45:109–115. https://doi.org/10.1007/s10886-018-1005-6
Yates RJ, Howieson JG, Hungria M, Bala A, O’Hara GW, Terpolilli J (2016) Authentication of rhizobia and assessment of the legume symbiosis in controlled plant growth systems. In: Howieson JG, Dilworth MJ (eds) Working with rhizobia. Australian Centre for International Agricultural Research, pp 73–108
Acknowledgements
We are grateful to the staff of the Ruissalo Botanical Garden, Meri Lindqvist of the Center of Evolutionary Applications at the University of Turku for technical assistance, and three anonymous reviewers for their helpful comments.
Funding
Open Access funding provided by University of Turku (UTU) including Turku University Central Hospital. This work was supported by the Academy of Finland (#331046 to SR).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. The microbial and molecular work, and phylogenetic analyses were conducted by Seyed Abdollah Mousavi, while the experiments were conducted by Satu Ramula and Aino Kalske. Data were analysed and the first draft of the manuscript was written by Satu Ramula, all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no financial or non-financial interests to disclose.
Additional information
Responsible Editor: Stéphane Compant.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramula, S., Mousavi, S.A. & Kalske, A. Rhizobial benefits to an herbaceous invader depend on context and symbiotic strain. Plant Soil 490, 603–616 (2023). https://doi.org/10.1007/s11104-023-06105-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06105-4