Background and aims
Plant and soil microbes can reduce their phosphorus (P)-requirements by replacing phospholipids with non-P containing lipids (e.g., galactolipids, sulfolipids, and betaine lipids). There have been few studies of this process in the field (i.e., in natural ecosystems); thus, it is unclear whether a similar replacement of phospholipids with non-P lipids occurs across natural gradients of soil P-availability.
Methods
We compared the membrane lipid profiles of plant leaves, roots, and soil microbial communities between two adjacent native Australian sclerophyll forest ecosystems—one situated on a severely P-deficient sandstone-derived soil and the other on a comparatively P-rich shale-derived soil. The herbaceous species, Lomandra longifolia, which occurred across both soils was sampled, along with two Myrtaceae tree species associated with each soil type.
Results
The phospholipid content of plant leaves and soil microbes was two- to three-fold greater in the shale site than the sandstone site, but non-P lipid content did not differ between sites.
Conclusion
Our results indicate that plants and soil microbes can have a lower investment of P into phospholipids in response to P-deficiency without a concomitant increase in non-P lipid content. Modulations in phospholipid concentration occurred across all plant- and soil microbial-associated phospholipid classes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The availability of phosphorus (P) in soil is a major driver of biological properties and processes in many terrestrial ecosystems (Elser et al. 2007; Nakamura 2013; Wardle et al. 2004). Soil P concentrations tend to be low in old and/or strongly weathered soils (Kooyman et al. 2017; Orians and Milewski 2007), and organisms associated with such soils (e.g., plants and soil microbes) are often highly specialized, exhibiting various physiological traits associated with conservative use of P. Conservative P-use strategies include having high P-resorption efficiency from senescing tissues (Dalling et al. 2016; Denton et al. 2007; Handreck 1997) and long leaf longevity to increase P residence time (Lambers et al. 2022; Tsujii et al. 2020). The ability to modulate polar membrane lipid composition by degrading phospholipids and synthesising P-free lipids (e.g., galactolipids, sulfolipids, and betaine lipids) as substitutes has emerged as an important P-saving trait in various organisms (Dörmann and Benning 2002; Essigmann et al. 1998; Sebastián et al. 2016; Van Mooy et al. 2009; Warren 2020). Plants and soil micro-organisms with reduced membrane phospholipid content may be able to reduce their overall physiological P requirements in low-P ecosystems and reallocate P to other P-intensive processes (e.g., protein and nucleic acid synthesis) (Andersson et al. 2005; Dörmann and Benning 2002; Essigmann et al. 1998; Härtel et al. 2000; Li et al. 2010; Nakamura 2013; Tjellström et al. 2008; Warren 2020). Substantial reductions in P-requirements could be achieved via phospholipid substitution given that phospholipids account for around one-third of total cellular P in plants (Nakamura 2013), while in micro-organisms phospholipids and nucleic acids collectively account for 60% of cellular P (Bünemann et al. 2011). Therefore, phospholipids play an important role in the internal P economics of plants and the soil microbial biomass (Warren 2020), and the physiological regulation of cellular lipid profiles is likely a key process that enables the persistence of organisms and the sustained productivity of ecosystems on low P soils.
Our understanding of lipid compositional responses to P-availability in plants is largely informed by laboratory- or greenhouse-based experiments investigating the short-term starvation response of a modest number of plant species exposed to incredibly P-deficient conditions. The majority of studies have examined either the model species Arabidopsis thaliana (e.g., Gaude et al. 2008; Härtel et al. 2000; Jouhet et al. 2004; Pant et al. 2015), or crop species from the Poaceae, Brassicaceae, and Fabaceae families (e.g., Andersson et al. 2005, 2003; Gaude et al. 2004; Okazaki et al. 2017; Tjellström et al. 2008). Lab-based manipulative experiments have shown that plants growing under extreme P-deficiency exhibit phospholipid substitution, which is characterised by having lower phospholipid and higher non-P lipid concentrations compared to their counterparts growing under P-replete conditions. These studies have been instrumental in demonstrating the plasticity that exists within plants to remodel their lipid composition in response to variation in P-availability.
By contrast, the lipid composition of vegetation in natural ecosystems has received little attention, with the exception of the study by Lambers et al. (2012), which reported phospholipid substitution during leaf development of six species of Proteaceae in the field. Analyses of foliar P fractions indicate that plants growing on P-poor soils have a lower concentration of phospholipids than those growing on comparatively P-rich soils (Hidaka and Kitayama 2011; 2013; Mo et al. 2019; Suriyagoda et al. 2023; Yan et al. 2019), though it is unclear whether this is facilitated by membrane phospholipids being functionally substituted by non-P containing lipids. Specifically, the paucity of field studies means it remains unclear whether plant species associated with low-P soils have lower phospholipid and higher non-P lipid content than species associated with comparatively higher P soils within the same landscape, as would be predicted from results of lab-based studies.
Modulations in lipid composition has been reported for the roots and leaves of plants, despite intrinsic differences in lipid profiles between organs. Manipulative lab studies have found that there is a greater increase in glycolipids in roots when responding to P-deficient conditions compared to leaves (Pfaff et al. 2020) and shoots (Pant et al. 2015). The functional difference between roots and leaves means that these organs differ markedly in lipid composition. Phospholipids are a key component of extraplastidic membranes, including the plasma, mitochondrial, and tonoplast membranes, but are often scarce in photosynthetic membranes (Andersson et al. 2005; Jouhet et al. 2004; Nakamura 2017; Sadre and Frentzen 2009). By contrast, galactolipids are primarily restricted to photosynthetic membranes (thylakoids) of chloroplasts and account for ~ 80% of membrane lipids in green plant tissue (Härtel et al. 2000; Nakamura 2017; Wahle 1983). That phospholipids comprise a larger proportion of membrane lipids of roots than leaves explains why roots in manipulative lab studies have a greater capacity to reduce P-requirements than leaves (Pant et al. 2015; Pfaff et al. 2020). Currently, there have been no field studies on root lipid composition, nor have there been studies that have comparatively examined the lipid composition of leaves and roots together in a natural context. Thus, studies of field-grown plants are required to contextualise results of lab studies and enable broader generalisation about the capacity of roots versus leaves to lower their P-requirements.
In contrast to plants, less is known about the role of lipids in the P economics of soil microbial biomass. Warren (2020) found that the proportion of phospholipids to total polar (i.e., membrane) lipids declined from 93% in P-rich soils to 61% in P-poor soils, with betaine lipids accounting for a greater proportion of total polar lipids in the P-poor soils. As is also the case for plants, by decreasing their investment of P in phospholipids, soil micro-organisms can improve their P-utilisation efficiency, which is defined as the ability to produce biomass with the available P acquired (Wang et al. 2010), by reducing their cellular P demands and thereby continue growing in P-deficient conditions (Merchant and Helmann 2012). Phospholipid substitution by micro-organisms has implications for ecosystem productivity, as soil P is rendered available for plant uptake through microbially mediated P mineralisation, which itself is regulated by the strength of microbial P-limitation and P demand (Daufresne and Loreau 2001; Hall et al. 2011; Manzoni et al. 2010). Given the profound importance of soil microbes to the soil P cycle and ecosystem function, there is a need for more studies on how soil microbes and plants collectively respond to P-deficiency through regulating their lipid composition.
To investigate the role of lipids in the P-economics of plants and soil microbes we established two adjacent sampling sites that differed in geology and soil P-availability: a low-P sandstone-derived soil and a high-P shale-derived soil. Soil P-availability is a major landscape-level driver of plant species distributions and ecosystem productivity (Beadle 1954; 1962; Condit et al. 2013; Turner et al. 2018), with the relative fitness and growth rates of different species influenced by their respective P acquisition, allocation, and conservation strategies (Chapin 1991; Hidaka and Kitayama 2011). Hence, species occurring on lower-P soils should strongly express traits for using P efficiently. The plant community composition differed markedly between the two sites, though there were some commonalities. The rhizomatous herb Lomandra longifolia was present at both sites, allowing us to compare the lipid composition of the same species growing on two soils differing in P-availability. Additionally, the dominant tree species at both sites were all from the Myrtaceae family. Thus sampling of their leaves and roots afforded an ecologically and phylogenetically controlled contrast.
We hypothesised that organisms associated with the low P sandstone soils will have a lower membrane phospholipid concentration and a greater non-P lipid concentration than those associated with the higher P shale soils, with this general trend being evident across four distinct comparisons: (1) a single species (Lomandra longifolia) growing on the two different soil types; (2) a phylogenetically controlled contrast of the two dominant tree species growing on each soil type; (3) between soil microbial communities associated with each soil type; and (4) the plant and soil microbial communities together between soil types. Additionally, (5) we expected that the capacity to reduce organism P-requirements through modulations in lipid composition would be positively related to the contribution of phospholipids to the total membrane lipid pool in a given organism. Thus, we expected the capacity to reduce organism P-requirements would be greatest in soil microbes, followed by roots, then leaves.
Materials and methods
Site details and experimental design
The study was carried out in Royal National Park, NSW, Australia. Royal National Park has a mosaic of surface geologies that has given rise to spatial heterogeneity in soil fertility and, in particular, P-availability throughout the landscape (Beadle 1962). Two sampling sites were established in the south-west section of Royal National Park in late 2020. The two sites, which are ~ 1.25 km apart, differ in their surface geology, with one site occurring on high P Narabeen shale-derived soil and the other occurring on low P Hawkesbury sandstone-derived soil (Table 1). Within each site, five replicate plots were established, with each plot approximately 50 × 50 m in area and all plots spaced at least 100 m apart from each other.
Sample collection and preparation
At each replicate plot, three soil sub-samples were randomly taken at a depth of 0–15 cm, as microbial biomass generally declines beyond this depth (Thoms et al. 2010). These sub-samples were then pooled together to capture the spatial variation in soil properties of each replicate plot, giving one composite soil sample per plot. Soil samples were mixed well and stored in plastic clip-seal bags and stored at 4 ºC for no longer than 3 h before lipids were extracted.
The native rhizomatous herb Lomandra longifolia was present at both the sandstone and shale sites and was sampled to allow for a direct comparison of the same species between sites. In contrast to the species rich understory, the tree canopy was species poor, with both sites dominated (≥ 90% canopy cover) by two different species from the Myrtaceae family (Beadle 1954; 1962). At the sandstone site, the species sampled were Angophora costata and Eucalyptus piperita. At the shale site, the species sampled were Syncarpia glomulifera, and Eucalyptus pilularis. The Myrtaceae species have a dual Arbuscular Mycorrhiza/Ectomycorrhiza association, while Lomandra longifolia is non-mycorrhizal (Brundrett 2008).
At each replicate plot, plant root and leaf samples were pooled from three individuals of each species. The plant roots sampled were traced outwards from the base of the trunk to ensure that the roots being collected belonged to the individual being sampled and not from surrounding plants. Fully expanded leaves from accessible portions of the canopy were collected using a pole pruner or an arborist throw‐line launcher (Youngentob et al. 2016). Plant root and leaf samples were stored in a plastic clip-seal bag and kept at 4 °C for no more than 2 h before being frozen at -80 °C until lipid extraction. Plant roots were washed using 100 µM CaCl2 and reverse osmosis water to remove soil and organic matter, including much of any potential mycorrhizal sheath. To form a homogenised sample, leaf and root samples were cut up into smaller pieces prior to chemical analyses.
Air-dried soils were used to determine soil total [N] (dry combustion; (Elementar vario MACRO cube, Germany)) and total [P] (HNO3 and H2O2 digestion), pH (2:5, soil:H2O). Plant samples were separated into two subsamples: one frozen subsample was extracted for lipid analysis, and the other subsample was airdried and used to determine total [N] (dry combustion), and FW/DW. For plant total [P], dried leaf samples were digested by a wet acid digestion method using HNO3 and H2O2, and then analysed using ICP-OES (720-ES, Agilent, CA, USA).
Soil microbial biomass C, N, and P
Soil microbial biomass C, N, and P was determined using the fumigation-extraction method (Brookes et al. 1982), with biomass calculated based on the difference between the concentrations of extractable C, N and P in chloroform-fumigated and non-fumigated samples (Cai et al. 2011; Makarov et al. 2016). One subset of fresh fumigated and non-fumigated soils was extracted with 0.5 M NaHCO3 for biomass P analyses, while another subset was extracted with 0.5 M K2SO4 for biomass C and N analyses. Extracted soil samples were digested to determine soil microbial biomass C (dichromate digestion; Cai et al. 2011), N (persulfate digestion; Doyle et al. 2004), and P (potassium persulfate digestion; Berthold et al. 2015). Microbial biomass C and N were calculated based on the equation described by Vance et al. (1987). Microbial biomass P was calculated as per Brookes et al. (1982), with PO43−-P recovery determined by spiking unfumigated sandstone- and shale-derived soil samples with 25 µg PO43−-P per g dry soil (Brookes et al. 1982). The recovery percentage of the spike was 86% in the shale soil, and 65% in the sandstone soil.
Extraction and quantification of lipids
Prior to lipid extraction, plant root and leaf samples were ground to a powder using liquid N2 in a mortar and pestle. Soil and plant samples were extracted for lipids according to the Bligh and Dyer protocol, with water substituted by citrate buffer (0.15 M, pH 4.0) (Frostegård et al. 1991), as previously described by Warren (2018). This method was previously optimised for the extraction of lipids from soil microbial biomass (Warren 2018). Blanks were similarly extracted using the same procedure alongside samples. The lower (organic) phase of the lipid extract was stored at -80 °C until analysis.
LC–MS of intact lipids
Prior to liquid chromatography – mass spectrometry analysis (LC–MS), aliquots of the organic phase of the lipid extracts (plant leaves and roots = 125 μL, soil = 250 μL) were dried with dry N2 gas and then reconstituted in 250 μL of isopropanol:acetonitrile:water (2:1:1 by volume) (Warren 2020). For LC–MS analysis, 190 μL of the reconstituted lipid extract was transferred to a glass vial with an integrated liner, along with 10 μL of internal standard (sphingosyl PE (D17:1/12:0)). Nano-LC-MSn, which provides good coverage of all polar lipid classes, was used to identify and quantify intact polar membrane lipids, as described by Warren (2020). Plant samples of 0.05 μL and soil samples of 0.1 μL were injected into a nano-scale analytical column (150 mm long × 75 μm i.d. column, packed with Acclaim PepMap RSLC C18, 2 μm, 100 Å) and separated by gradient elution with mobile phase A (60% acetonitrile, 40% water, 10 mM ammonium formate, 0.1% formic acid) and mobile phase B (90% isopropanol, 10% acetonitrile, 10 mM ammonium formate, 0.1% formic acid) as described in more detail previously (Warren 2020). Lipids were ionized by nano-ESI and detected in positive mode Auto-MSn and negative mode Auto-MSn by a mass spectrometer (AmaZon, Bruker Daltonics, Germany).
Lipid identification and quantification
Lipid standards were used as references to identify and quantify polar lipid species, as described in Warren (2020). As there are no commercially available lipid standards for GADG, lipid concentration was quantified using the response factor for MGDG (Sebastián et al. 2016).
The presence of specific lipid classes was determined by inspecting the Auto-MSn data in positive and negative mode for diagnostic neutral losses and product ions (Warren 2020). Lipid species were identified to the level of sum composition (i.e., sum of carbon atoms and sum of double bonds on all fatty acyl moieties) according to the lipid species identified in Warren (2018) and the LIPID MAPS database (Fahy et al. 2007). Lipid species were quantified as described in Warren, (2020) by dividing the peak area of a lipid by the peak area of the added internal standard (sphingosyl PE (D17:1/12:0), and then dividing by a response factor describing the relationship between peak area and concentration. Response factors for each lipid class was determined by constructing standard curves for pure lipid standards from each class (Warren 2020).
Statistical analyses
Prior to statistical analyses, the data for the tree species for each organ type (leaves and roots) was averaged across species for each replicate plot. For one replicate plot in the shale site, the negative mode Auto-MSn data for the leaves and roots of E. pilularis and S. glomulifera were unavailable due to instrument failure. As a result, the lipid concentration for the lipid class PC could not be quantified. To remedy this, the lipid concentration for PC for the one replicate plot was substituted with the mean calculated from the species of the four other replicates.
Differences in plant total P, N, N:P and lipid composition were evaluated using two-way ANOVA tests, with site and plant organ type (i.e., leaves and roots) as factors. Where P < 0.05, a post-hoc Tukey HSD test was used to determine the differences between groups. As L. longifolia was the only common species found in both sites, it was analysed separately in parallel to the tree species. Differences in total lipid concentration between leaves and roots, soil pH, total P, N, N:P, microbial biomass C, N, P, and microbial lipid composition were tested with one-way ANOVA, as well as a non-parametric Kruskal Wallis test. The assumptions of normality and homogeneity were tested using the Shapiro-Wilks test and Levene’s test respectively. Data were natural log transformed whenever necessary. Statistical analyses were conducted in R 4.0.4 (R Core Team 2021).
Results
Soil characteristics
Soil pH was similar between the shale and sandstone sites, with the shale site having a mean pH of 5 (± 0.21) and the sandstone site having a mean pH of 4.7 (± 0.19) (F1, 8 = 1.22, P = 0.3). Soil soluble total [P] (i.e., P that can be readily taken up by plants) was four-fold higher in the shale site than in the sandstone site (F1,8 = 22.43, P < 0.01; Table 1). Likewise, soil total [P] in the shale site was roughly double that in the sandstone site (F1,8 = 8.47, P = 0.02; Table 1). Soil total [N] was also significantly higher in the shale site (F1, 8 = 10.56, P = 0.012; Table 1), such that soil total N:P ratios, which were highly variable in the shale site, did not differ significantly between the shale and sandstone sites (F1,8 = 1.47, P = 0.26; Table 1).
Total phosphorus and nitrogen concentration in plant leaves and roots
In Lomandra longifolia leaves and roots, both total [P] and total [N] (Table 2, Fig. 1) were significantly greater in the shale site (leaves: [P] = 0.92 mg g−1, [N] = 10.28 mg g−1; roots: [P] = 0.45 mg g−1, [N] = 4.28 mg g−1) than in the sandstone site (leaves: [P] = 0.59, [N] = 8.05 mg g−1; roots: [P] = 0.31 mg g−1, [N] = 4 mg g−1). Total [P] and total [N] of leaves were greater than roots at both sites. N:P ratios of L. longifolia leaves (shale: 11.98 g g−1; sandstone: 15.09 g g−1) and roots (shale: 10.91 g g−1; sandstone: 13.80 g g−1) were not significantly different between sites or organ (Table 2, Fig. 1).
Total phosphorus, nitrogen concentrations and nitrogen:phosphorus ratios of leaves and roots of Lomandra longifolia (A, B, C; shale: n = 5; sandstone: n = 4) and tree species (B, D, F; n = 10 [2 species × 5 replicate plots]). Square symbol (■) indicates mean concentration. The central box indicates interquartile range and median; whiskers indicate the most extreme values. Circle symbol (○) indicates outliers that are outside the most extreme values. Statistical analyses for the N:P ratios were carried out using natural log-transformed N:P ratios; however untransformed data are presented here for ease of interpretation. Bars denoted by the same letter are not statistically significant (P > 0.05)
In the tree species, leaf total [P] and [N] differed between sites; however, in roots, there was no difference in total [P] or [N] (Table 2, Fig. 1). Leaf total [P] was 50% greater in the shale site (1.15 mg g−1) than in the sandstone site (0.54 mg g−1), while total [N] in leaves was 20% greater in the shale site (10.82 mg g−1) than in the sandstone (8.94 mg g−1). Total [P] of leaves was 50% greater than in roots at the shale site (0.58 mg g−1; P = 0.0047) but not at the sandstone site (0.46 mg g−1). Total [N] was 50% greater in leaves than inroots (shale = 5.3 mg g−1; sandstone = 4.92 mg g−1) at both sites. While leaf N:P ratios tended to be lower in the shale site (N:P = 10.51 g g−1) than in the sandstone site (N:P = 17.33 g g−1), there was no statistically significant difference in N:P between sites or organ (Table 2, Fig. 1).
Soil microbial biomass carbon, nitrogen, and phosphorus
Soil microbial biomass at the shale site contained almost three-times more P (F1,8 = 8.37, P = 0.02) and almost two-times more N (F1,8 = 15.21, P < 0.01) than that of the sandstone site, whereas microbial biomass carbon (C) did not differ between sites (F1,8 = 1.82, P = 0.21; Table 3). Soil microbial N:P (F1,8 = 1.24, P = 0.3), C:P (F1,8 = 2.87, P = 0.13), and C:N (F1,8 = 0.074, P = 0.79) ratios did not differ significantly between sites (Table 3).
Intact polar lipid composition of plant leaves, roots and soil microbes
LC–MS identified 96 polar lipid species in plants and 61 polar lipid species in soil that were quantified at the level of sum composition. In plants, the lipid species identified comprised of phospholipids: 9 phosphatidic acid (PA), 4 phosphatidylinositol (PI), 13 phosphatidylethanolamine (PE), 7 phosphatidylcholine (PC), 9 phosphatidylglycerol (PG), 8 phosphatidylserine (PS); galactolipids: 14 digalactosyldiacylglycerol (DGDG), 11 monogalactosyldiacylglycerol (MGDG), 5 glucuronic acid diacylglycerol (GADG); and sulfolipids: 16 sulfoquinovosyldiacylglycerol (SQDG). In soil, the lipid species identified comprised of phospholipids: 13 PC, 13 PE, 4 PG, 3 PS; betaine lipids: 3 diacylglyceryl-carboxyhydroxymethylcholine (DGCC), 22 diacylglyceryl-N,N,N-trimethylhomoserine (DGTS); and galactolipids: 3 MGDG.
In L. longifolia, phospholipid concentration in both leaves and roots from the shale site was 83% and 64% greater, respectively, than that of the sandstone site (Fig. 2). Phospholipid concentration was greater in leaves than in roots by 71% in the shale and 54% in the sandstone. Non-P lipid concentration (i.e., the sum of galactolipids plus sulfolipids) of L. longifolia did not differ between sites for both leaves and roots (Fig. 2, Table 2). Non-P lipid concentration was six- and eight-fold greater in leaves than in roots in the shale and sandstone site, respectively. Total lipid concentration of L. longifolia leaves was three-fold greater than that in roots (Table 2).
Mean absolute concentration of intact polar lipids in Bligh and Dyer extracts of A) Lomandra longifolia (shale: n = 5; sandstone n = 4) leaves and roots and B) tree species (n = 10 [2 species × 5 replicate plots]), and C) soil microbial community (n = 5) as measured by LC–MS. Data shown are the mean concentration of phospholipids and the mean concentration of the two main non-P lipids identified: galactolipid and sulfolipids/betaine lipids. Error bars are standard error. Bars denoted by the same letter are not statistically significant (P > 0.05)
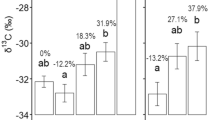
Phospholipid to total lipid concentration was 37% and 21% greater in the shale-associated L. longifolia than in the sandstone for leaves and roots, respectively (Fig. 3). Phospholipids accounted for twice as much of total lipid in roots than in leaves in both sites, while the non-P containing lipid proportion was greater in leaves than in roots by 19% in the shale, and 43% in the sandstone (Fig. 3, Table 2). Notably, considering absolute concentration (Fig. 2) we can see that this was due to a reduced phospholipid concentration in the leaves from the sandstone site compared to those from the shale site, rather than a concomitant increase in non-P lipid concentration with a decrease in phospholipid concentration.
Mean relative intact polar lipid proportion (mole%) in Bligh and Dyer extracts of A) Lomandra longifolia (shale: n = 5; sandstone: n = 4) and B) tree species (n = 10; [2 species × 5 replicate plots]) leaves and roots, and C) soil microbial community. Data shown are the mean relative proportion of phospholipids and the mean relative proportion of the two main non-P lipids identified: galactolipid and sulfolipids/betaine lipids. Bars denoted by the same letter are not statistically significant (P > 0.05)
In the tree species, leaf phospholipid concentration differed between sites, but there was no difference in root phospholipid concentration between sites (Fig. 2). Phospholipid concentration was 70% greater in leaves in the shale site than in the sandstone site (Fig. 2). Leaf phospholipid concentration was around two-fold and three-fold greater than in roots in both the shale and sandstone sites, respectively. Much like L. longifolia, non-P lipid concentration did not differ between sites in either leaves or roots (Fig. 2). Between organs, non-P lipid concentration was seven-fold and ten-fold greater in leaves than in roots in the shale and sandstone sites, respectively. Overall, mean absolute total lipid concentration in leaves was five-times greater than in roots (Table 2).
Differences in the relative proportion of phospholipids and non-P lipids between the sandstone and shale tree communities depended on organ type (Fig. 3). In leaves, the phospholipid proportion was 40% larger in the shale site than in the sandstone. There was no difference in roots between sites. The phospholipid portion in roots was 23% and 58% greater than in leaves in the shale and sandstone site, respectively.
The polar membrane lipid trends found in plants was similar to that found in soil microbes, in which absolute phospholipid concentration was three-fold greater in the soil microbial community associated with the shale-derived soil than those in the sandstone-derived soil (F1,8 = 16.92, P = 0.0034; Fig. 2). There was also no significant difference in absolute concentration of non-P containing lipids (i.e., betaine lipids and galactolipids) between sites (F1,8 = 1.59, P = 0.24). The proportion of phospholipids to total membrane lipids in soil microbial biomass was significantly greater in the shale site by 14% compared to the sandstone site (F1,8 = 13.171, P = 0.0067; Fig. 3).
Discussion
Previous studies have established that plants (Andersson et al. 2005; Essigmann et al. 1998; Härtel et al. 2000; Lambers et al. 2012; Tjellström et al. 2008) and soil microbes (Warren 2020) can alter their polar membrane lipid composition in ways that improve their P use efficiency and contribute to their sustained growth on P-limiting substrates. We build on these earlier studies by simultaneously examining the polar lipid profile of plant leaves, roots, and soil microbial communities in response to local variation in soil parent material in a natural ecosystem. Our findings lend strength to the generality of plants tailoring their phospholipid content to P-availability in two ways: firstly, by showing phospholipid concentration is modulated by small differences in P-availability in the field; and secondly, by extending the taxonomic breadth of previous observations to include the rhizomatous herb L. longifolia (Asparagaceae) and four tree species within the Myrtaceae family.
Multiple independent metrics, including total P, NaHCO3-soluble P, and soil microbial biomass C:P and N:P indicated that the soils of our two study sites were low in P, with the soils at the sandstone site being particularly P-depleted. Both soils are at the lower end of the total [P] spectrum in Australia (NVIS3.0 MVGs mean total P = 27.3 – 301.8 µg g−1; Bui and Henderson 2013) and globally (USDA soil orders mean total P = 400.5 – 1145.8 µg g−1; He et al. 2021). The two-fold difference in total [P] between the sandstone- and shale-derived soils thus provided an opportunity to examine how subtle differences in soil P levels at the low end of the soil P spectrum affects the lipid composition of plants and soil microbes. Notably, according to previously proposed foliar N:P thresholds (mass ratio = 14 – 16 (Koerselman and Meuleman 1996); 10 – 20 (Güsewell 2004); Eucalyptus optimum N:P = 15 (Cromer et al. 1981; Judd 1996)), the N:P lower ratio of the shale associated plant species (foliar N:P = 11) suggests that growth tended towards N-limitation, whereas the growth of the sandstone-associated plant species (foliar N:P = 16) tended towards P-limitation.
Single-species comparison – Lomandra longifolia
The results of our single species comparison of Lomandra longifolia demonstrated that, in a natural context with soils differing two-fold in total [P], the magnitude of difference in phospholipid concentration of leaves (37%) and roots (21%) was consistent with glasshouse studies that have imposed a much wider range of phosphorus treatments (500–1000 fold difference; e.g., Dokwal et al. 2021; Essigmann et al. 1998; Gaude et al. 2008, 2004; Pfaff et al. 2020; Su et al. 2018). This could suggest that membrane phospholipid concentration is tightly controlled by the plant in response to P-availability. Lomandra longifolia is widespread across our study landscape, occurring in low to moderate abundance on most soil types throughout the Royal National Park (DECCW 2011). By contrast, many other plant species – including the tree species we studied – are largely restricted to particular soil types due to varying requirements for P and other mineral nutrients (Beadle 1954; 1962). We suggest that the physiological plasticity of the lipid P pool could be one mechanism that contributes to the persistence of L. longifolia on both the shale and sandstone soils.
Importantly, the plasticity of membrane lipid composition seen in L. longifolia leaves and roots entailed reductions in phospholipids without concomitant increases in non-P lipids (Figs. 2 and 3). This contrasts with how phospholipid substitution has been defined in previous studies, in which reductions in phospholipid concentration are facilitated by – or at least coupled with – increases in non-P lipids (Andersson et al. 2005, 2003; Essigmann et al. 1998; Härtel et al. 2000; Tjellström et al. 2008), such that the total amount of membrane lipid is preserved. Phospholipid substitution has most commonly been reported from controlled laboratory studies with P treatments ranging from deficient to supra-optimal (e.g. 500 fold difference, Härtel et al. 2000; Li et al. 2006; 1000 fold difference), whereas the current study featured sites that differed in P-availability by two-fold and sat on the lower end of soil total [P] spectrum (Bui and Henderson 2013; He et al. 2021; Orians and Milewski 2007). Thus phospholipid substitution as it has been defined in the literature may be evident only across broad ranges in P-availability and/or during aging of leaves (Lambers et al. 2012).
Tree species comparison
Having an ecologically and phylogenetically controlled contrast of Myrtaceae species on each soil type allowed us to examine how plants respond to P-availability through their polar membrane lipid composition in a natural context. The difference in foliar phospholipid concentration between the two sites was proportional to the two-fold difference in total foliar P (Figs. 1 and 2) and soil total [P], but rather modest relative to the four-fold difference in soluble (NaHCO3–extractable) soil [P], which represents our best estimate of the soil PO43− that is immediately available for plant uptake (Table 1). This is in accord with previous foliar P fraction studies that found a comparably modest difference in phospholipid concentration despite the large range in soil P concentrations between their highest and lowest P treatments (e.g., 0.02, 0.12, and 0.19 g m−2 soluble soil P in Hidaka and Kitayama (2011), Hidaka and Kitayama (2013) and Tsujii et al. (2017); 21 – 199 mg kg−1 soil P in Yan et al. (2019)). Taken together, this suggests that the proportion of lipid P to total foliar [P] is tightly controlled by plants, with any variation in lipid P in response to P-availability falling within a certain range. There is likely a minimum amount of phospholipid needed for membrane function (Crous and Ellsworth 2020) and a maximum, with lipid P concentration eventually reaching a plateau at which it is unaffected by inorganic [P] (Suriyagoda et al. 2023).
While our L. longifolia comparison allowed us to examine the plasticity of a single species, the sampling of different species on the two soil types allows us to investigate the adaptation of species that are delineated by soil P-availability. There is some evidence that patterns of total [P] and P allocation into lipids and other P fractions is genetically determined (Tsujii et al. 2023; Yan et al. 2019), with specialised species that are particularly P-efficient having potentially evolved distinct P allocation patterns (Kuppusamy et al. 2014; Yan et al. 2019). For instance, Proteaceae species, such as Hakea prostrata, allocate smaller amounts of P to structural P fractions (i.e., phospholipids and nucleic acids) compared to other co-occurring species while remaining highly photosynthetically productive even in P-deficient conditions (Kuppusamy et al. 2014; Lambers et al. 2012; Tsujii et al. 2023; Yan et al. 2019). However, in the current study, there were only subtle differences in the proportion of total foliar [P] allocated to lipid P, ranging from 33 – 39%, between the tree species on both sites. Previous findings have also shown that various species across a range of soil parent materials and ecosystem types can have similar lipid P fractions despite differences in foliar [P] (Crous and Ellsworth 2020; Hidaka and Kitayama 2013; McQuillan et al. 2020; Zhang et al. 2018). Notably, the magnitude of difference in phospholipid concentration between sites for tree species was comparable to that seen in L. longifolia. Thus, the contribution of plasticity along with genetic adaptation, while difficult to tease apart in the current study, cannot be ruled out.
Leaves vs roots lipid composition
The variable response of root phospholipid concentration to P-availability between L. longifolia and the tree species contrasts with our hypothesis that roots have a greater capacity to reduce plant P-requirements than leaves. The foliar phospholipid concentration of both L. longifolia and the tree species scaled with site differences in soil P-availability; however, for the tree species, the phospholipid concentration of roots did not differ between sites. Consistent with our expectations and previous studies (e.g., Dokwal et al. 2021; Gaude et al. 2008, 2004; Pfaff et al. 2020; Su et al. 2018), phospholipids accounted for a larger proportion of membrane lipids in roots (Tree species = 56 – 64%, L. longifolia = 63 – 79%; Fig. 3) than in leaves (Tree species = 25 – 38%, L. longifolia = 23 – 43%). However, this greater proportion of membrane phospholipids in roots did not translate into a greater capacity to reduce P-requirements through reductions in membrane phospholipid content. In fact, in L. longifolia, the reduction in phospholipid concentration was smaller in roots (39%) than leaves (45%; Fig. 3b). This is in contrast to past laboratory studies that have reported similar or even larger quantitative reductions in root phospholipid concentration than in leaves in response to low P-availability (Gaude et al. 2008; Pfaff et al. 2020).
We speculate that mycorrhizal associations may play a role in determining root phospholipid concentrations, and ultimately explain why root phospholipid concentration was plastic in L. longifolia, and invariant between the tree species. Notably however, the membrane lipids of mycorrhizal fungi likely contribute very little to total root lipid concentration. An independent manipulative experiment with Eucalyptus pilularis seedlings revealed that root phospholipid concentration was unaffected by P-additions while the concentration of other P fractions increased (Mulligan and Patrick 1985). The study suggested that E. pilularis root P is regulated by their ectomycorrhizal symbionts, which store P as polyphosphate in the fungal tissue so that it can be subsequently remobilised to the host tissue when external supplies become limiting (Mulligan and Patrick 1985). We suggest that the roots of the ectomycorrhizal associated Myrtaceae species featured in this study may maintain a minimum phospholipid concentration while the bulk of root P is preferentially partitioned between other P fractions. By contrast, the nonmycorrhizal L. longifolia may use roots as a dynamic P store, such that phospholipid concentration differs with soil P status.
To specifically quantify the potential effect that tailoring phospholipid concentration to P-availability has on an organism’s P requirements, we calculated how much more P the sandstone-associated organisms would hypothetically require if they maintained the same ratio of phospholipid to total membrane lipid as the organisms from the shale site. For the sandstone tree species, they would hypothetically require 24% extra foliar P, while the sandstone-associated L. longifolia would require an extra 11% and 7% in leaf and root P, respectively. These estimates are similar to the estimated 16% of total P spared via phospholipid substitution in P-limited microalgal communities (Van Mooy et al. 2009). Our approach demonstrates that plant P-requirements are likely affected by their capacity to match their phospholipid concentration with P-availability, with leaves having a greater capacity to reduce plant P-requirements than roots.
Soil microbial community comparison
Our results demonstrate that soil microbial phospholipid composition is shaped by the P-availability of their associated soil parent material. However, unlike previous studies (Warren 2020), we did not observe a corresponding increase in non-P-lipids, suggesting a lack of community-level microbial phospholipid substitution. Similarly to the plants we studied, modulations in microbial non-P lipid concentration may be more evident across very broad P-availability gradients (e.g., total [P] from 10 – 3,955 ug g−1 (Warren 2020)). Differences in elemental stoichiometry among microbial communities are likely due in part to physiological flexibility within genotypes (Godwin and Cotner 2015). Although, we know from polar lipid fatty-acid data that the community of soil microbes differs between sandstone and shale soils (Warren 2019), and P-impoverished soils have genetically distinct microbial assemblages (Turner et al. 2019). Thus, community-level adaptation probably plays a substantial role as stoichiometry differs among microbial species (Makino et al. 2003; Quigg et al. 2003). Our results suggest that the shale-and sandstone-associated soil microbial communities likely differ in terms of the amount of P that is invested into phospholipids per unit microbial biomass (Warren 2020). The three-fold difference in microbial phospholipid concentration between the shale and sandstone soils was roughly proportional to the difference in total soil [P] and soluble total [P] between sites (Table 1 and Fig. 2). A similar trend emerged when microbial lipid composition was calculated on the basis of microbial biomass C, although we note that this trend was not statistically significant due to the large variation in microbial C biomass. The greater investment of P into phospholipids could additionally be attributed to the greater abundance of bacterial taxa relative to fungi in shale-derived soils compared to sandstone (Orpheus Butler unpubl. data), considering bacteria typically have low C:P ratios (Reiners 1986).
Contrary to our hypothesis, the substantial difference in soil microbial phospholipid concentration between sites (Fig. 2C) did not translate into a substantial shift in P-requirements and would unlikely substantially alter C and/or P cycling. Phospholipids comprised 72–84% of the total lipid pool (Fig. 3C), but if the sandstone-associated soil microbial community maintained the same phospholipid to total lipid ratio as the shale, they would only require an extra 2% of biomass P. This hypothetically minor change in P-requirements is far smaller than that of the plant organs, and smaller than the estimated 10% P-saving in soil microbial biomass previously proposed by Warren (2020). The small shift in P-requirements here is due to phospholipids accounting for only 16–17% of total soil microbial biomass P (vs leaves where phospholipids are 27–39% of total P), and the ratio of phospholipids to total membrane lipids differing by only 12% between sites (vs 32% difference in Warren (2020)). Changes in P-requirements as a result of modulations in lipid composition notionally affect microbial C:P and N:P ratios (Warren 2020), which could in turn affect rates of nutrient mineralisation and immobilisation (Hall et al. 2011; Manzoni et al. 2008; Mooshammer et al. 2014). However, if the biomass P-requirements of the sandstone soil microbial community were to hypothetically increase by 2%, the minor increase in microbial biomass C:P would unlikely have sufficient influence on substrate C:P ratios to affect the balance between nutrient mineralisation and immobilisation.
Plant and soil microbe comparison
The reduced phospholipid concentration on low-P soil was common to both soil microbial biomass and plant leaves, despite vast differences between microbes and leaves in terms of polar membrane lipid composition. Soil microbial biomass and plant organs notably differ in terms of their phospholipid profiles. In plants, a range of phospholipid classes are unevenly distributed between the various membranes of the cell, including PE, PC, PG, PS, PI, and PA (Reszczyńska and Hanaka 2020). Conversely, the polar lipid composition of soil microbes are dominated by PC and PE (Warren 2020). The non-P lipid profile of soil microbial biomass and plant organs also differ from one another (Figs. 2 and 3), with galactolipids and sulfolipids occurring in plants (Nakamura 2013), while betaine lipids do not occur in higher plants (Dembitsky 1996) but are present in at least some soil microbes (Warren 2018, 2020). These broad differences in membrane lipid composition suggest that declines in phospholipid content under conditions of P-scarcity are not specific to any particular phospholipid class but rather occurs across all phospholipid classes.
The examination of different plant species and soil microbial communities has allowed us to examine how membrane phospholipid concentration is shaped by soil parent material through plasticity and genetic adaptation. We suggest that inherent genetic differences in lipid composition of tree species and soil micro-organisms could underpin some of the difference in phospholipid concentration between the sandstone and shale sites. Previous studies have suggested P-deficient environments favour taxa with reduced P requirements, thus resulting in a shift in plant and microbial community composition along gradients of soil P-availability (Beadle 1954; 1962; Makino et al. 2003; Schade et al. 2005; Warren 2020). For example, extremely P-impoverished soils have genetically distinct assemblages of soil microbes (Turner et al. 2019) and flora that use P with high efficiency (Denton et al. 2007). Disentangling the drivers of differences in phospholipid concentration among plant and soil microbial communities will require additional experiments that contrast different plant species and soil microbial populations and communities under controlled conditions.
Conclusion
Our data suggests that plants and soil microbes occurring in naturally low-P environments can have a reduced phospholipid concentration without a concomitant increase in non-P lipids. We demonstrated that plant leaves, while dominated by non-P lipids, may have a greater capacity than roots and soil microbes to decrease plant P-requirements through tailoring their phospholipid composition to P-availability. Further field-based investigations are needed to tease apart whether soils that encompass a wider range of total [P] give rise to phospholipid substitution in plants and microbes, or if substitution is expressed only during manipulative experiments with lab-grown plants.
Across our three comparisons of L. longifolia, Myrtaceae tree species, and soil microbes between the two different soil types, our results suggest that the phospholipid concentration of distantly related taxa (i.e., plants, soil microbial community) is similarly shaped by the P-availability of their soil and associated parent material. This in turn suggests that differential investments of P into phospholipids occurs at the ecosystem scale and strengthens the case for phospholipids having an important role in the P-economics of plants and soil microbes that helps facilitates persistence in low-P ecosystems.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Andersson MX, Larsson KE, Tjellstrom H, Liljenberg C, Sandelius AS (2005) The plasma membrane and the tonoplast as major targets for phospholipid- to-glycolipid replacement and stimulation of phospholipases in the plasma membrane. J Biol Chem 280:27578–27586. https://doi.org/10.1074/jbc.M503273200
Andersson MX, Stridh MH, Larsson KE, Liljenberg C, Sandelius AS (2003) Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett 537:128–132
Beadle NCW (1954) Soil phosphate and the delimitation of plant communities in eastern Australia. Ecology 35:370–375
Beadle NCW (1962) Soil phosphate and the delimitation on plant communities in eastern Australia II. Ecology 43:281–288
Berthold M, Zimmer D, Schumann R (2015) A simplified method for total phosphorus digestion with potassium persulphate at sub-boiling temperatures in different environmental samples. Rostock Meeresbiologische Beitr 25:7–25
Brookes P, Powlson D, Jenkinson D (1982) Measurement of microbial biomass phosphorus in soil. Soil Biol Biochem 14:319–329
Brundrett MC (2008) Mycorrhizal associations of Australian Plants. In: Mycorrhizal Associations: The Web Resource. https://mycorrhizas.info. Accessed 13 Apr 2023
Bui EN, Henderson BL (2013) C: N: P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 373:553–568
Bünemann EK, Prusisz B, Ehlers K (2011) Characterization of Phosphorus Forms in Soil Microorganisms. In: Bünemann E, Oberson A, Frossard E (eds) Phosphorus in Action: Biological Processes in Soil Phosphorus Cycling. Springer, Berlin Heidelberg, Berlin, Heidelberg
Cai Y, Peng C, Qiu S, Li Y, Gao Y (2011) Dichromate digestion–spectrophotometric procedure for determination of soil microbial biomass carbon in association with fumigation–extraction. Commun Soil Sci Plant Anal 42:2824–2834
Chapin FS III (1991) Integrated Responses of Plants to Stress: A centralized system of physiological responses. Bioscience 41:29–36. https://doi.org/10.2307/1311538
Condit R, Engelbrecht BM, Pino D, Pérez R, Turner BL (2013) Species distributions in response to individual soil nutrients and seasonal drought across a community of tropical trees. Proc Natl Acad Sci 110:5064–5068
Cromer R, Cameron D, Cameron J, Flinn D, Neilsen W, Raupach M, Snowdon P, Waring H (1981) Response of eucalypt species to fertiliser applied soon after planting at several sites. Aust For 44:3–13
Crous KY, Ellsworth DS (2020) Probing the inner sanctum of leaf phosphorus: measuring the fractions of leaf P. Plant Soil 454:77–85
Dalling JW, Heineman K, Lopez OR, Wright SJ, Turner BL (2016) Nutrient availability in tropical rain forests: the paradigm of phosphorus limitation. Springer, Tropical tree physiology
Daufresne T, Loreau M (2001) Ecological stoichiometry, primary producer–decomposer interactions, and ecosystem persistence. Ecology 82:3069–3082
Department of Environment Climate Change and Water (2011) The Vertebrate Fauna of Royal & Heathcote National Parks and Garawarra State Conservation Area. Department of Environment, Climate Change and Water NSW, Hurstville
Dembitsky VM (1996) Betaine ether-linked glycerolipids: chemistry and biology. Prog Lipid Res 35:1–51
Denton MD, Veneklaas EJ, Freimoser FM, Lambers H (2007) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant Cell Environ 30:1557–1565
Dokwal D, Romsdahl TB, Kunz DA, Alonso AP, Dickstein R (2021) Phosphorus deprivation affects composition and spatial distribution of membrane lipids in legume nodules. Plant Physiol 185:1847–1859
Dörmann P, Benning C (2002) Galactolipids rule in seed plants. Trends Plant Sci 7:112–118
Doyle A, Weintraub MN, Schimel JP (2004) Persulfate digestion and simultaneous colorimetric analysis of carbon and nitrogen in soil extracts. Soil Sci Soc Am J 68:669–676
Elser JJ, Bracken ME, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Essigmann B, Güler S, Narang RA, Linke D, Benning C (1998) Phosphate availability affects the thylakoid lipid composition and the expression of SQD1, a gene required for sulfolipid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci 95:1950–1955
Fahy E, Sud M, Cotter D, Subramaniam S (2007) LIPID MAPS online tools for lipid research. Nucleic Acids Res 35:W606–W612
Frostegård Å, Tunlid A, Bååth E (1991) Microbial biomass measured as total lipid phosphate in soils of different organic content. J Microbiol Methods 14:151–163. https://doi.org/10.1016/0167-7012(91)90018-L
Gaude N, Nakamura Y, Scheible WR, Ohta H, Dörmann P (2008) Phospholipase C5 (NPC5) is involved in galactolipid accumulation during phosphate limitation in leaves of Arabidopsis. Plant J 56:28–39
Gaude N, Tippmann H, Flemetakis E, Katinakis P, Udvardi M, Dörmann P (2004) The galactolipid digalactosyldiacylglycerol accumulates in the peribacteroid membrane of nitrogen-fixing nodules of soybean and Lotus. J Biol Chem 279:34624–34630
Godwin CM, Cotner JB (2015) Aquatic heterotrophic bacteria have highly flexible phosphorus content and biomass stoichiometry. ISME J 9:2324–2327
Güsewell S (2004) N: P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266
Hall E, Maixner F, Franklin O, Daims H, Richter A, Battin T (2011) Linking microbial and ecosystem ecology using ecological stoichiometry: a synthesis of conceptual and empirical approaches. Ecosystems 14:261–273
Handreck KA (1997) Phosphorus requirements of Australian native plants. Soil Res 35:241–290
Härtel H, Dörmann P, Benning C (2000) DGD1-independent biosynthesis of extraplastidic galactolipids after phosphate deprivation in Arabidopsis. Proc Natl Acad Sci 97:10649–10654
He X, Augusto L, Goll DS, Ringeval B, Wang Y, Helfenstein J, Huang Y, Yu K, Wang Z, Yang Y (2021) Global patterns and drivers of soil total phosphorus concentration. Earth Syst Sci Data 13:5831–5846
Hidaka A, Kitayama K (2011) Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on Mount Kinabalu, Borneo. J Ecol 99:849–857
Hidaka A, Kitayama K (2013) Relationship between photosynthetic phosphorus-use efficiency and foliar phosphorus fractions in tropical tree species. Ecol Evol 3:4872–4880
Jouhet J, Marechal E, Baldan B, Bligny R, Joyard J, Block MA (2004) Phosphate deprivation induces transfer of DGDG galactolipid from chloroplast to mitochondria. J Cell Biol 167:863–874. https://doi.org/10.1083/jcb.200407022
Judd TS (1996) Nutrient concentrations in Eucalyptus: a synthesis in relation to differences between taxa, sites and components. In: Attiwill PM, Adams MA (eds) Nutrition of Eucalypts. CSIRO Publishing
Koerselman W, Meuleman AF (1996) The vegetation N: P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 1441–1450
Kooyman RM, Laffan SW, Westoby M (2017) The incidence of low phosphorus soils in Australia. Plant Soil 412:143–150
Kuppusamy T, Giavalisco P, Arvidsson S, Sulpice R, Stitt M, Finnegan PM, Scheible W-R, Lambers H, Jost R (2014) Lipid biosynthesis and protein concentration respond uniquely to phosphate supply during leaf development in highly phosphorus-efficient Hakea prostrata. Plant Physiol 166:1891–1911
Lambers H, Cawthray GR, Giavalisco P, Kuo J, Laliberté E, Pearse SJ, Scheible WR, Stitt M, Teste F, Turner BL (2012) Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use-efficiency. New Phytol 196:1098–1108
Lambers H, de Britto CP, Cawthray GR, Denton MD, Finnegan PM, Hayes PE, Oliveira RS, Power SC, Ranathunge K, Shen Q (2022) Strategies to acquire and use phosphorus in phosphorus-impoverished and fire-prone environments. Plant Soil 476:133–160
Li L, Liu C, Lian X (2010) Gene expression profiles in rice roots under low phosphorus stress. Plant Mol Biol 72:423–432. https://doi.org/10.1007/s11103-009-9580-0
Li M, Welti R, Wang X (2006) Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases D ζ1 and D ζ2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol 142:750–761
Makarov M, Malysheva T, Maslov M, Kuznetsova EY, Menyailo O (2016) Determination of carbon and nitrogen in microbial biomass of southern-Taiga soils by different methods. Eurasian Soil Sci 49:685–695
Makino W, Cotner J, Sterner R, Elser J (2003) Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C: N: P stoichiometry. Funct Ecol 17:121–130
Manzoni S, Jackson RB, Trofymow JA, Porporato A (2008) The global stoichiometry of litter nitrogen mineralization. Science 321:684–686
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106
McQuillan M, Smernik RJ, Doolette AL (2020) Partitioning of phosphorus between biochemical and storage compounds in leaves follows a consistent pattern across four Australian genera growing in native settings. Plant Soil 454:57–75
Merchant SS, Helmann JD (2012) Elemental economy: microbial strategies for optimizing growth in the face of nutrient limitation. In: Poole RK (ed) Advances in microbial physiology. Academic Press
Mo Q, Za Li, Sayer EJ, Lambers H, Li Y, Zou B, Tang J, Heskel M, Ding Y, Wang F (2019) Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Funct Ecol 33:503–513
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter AA (2014) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22
Mulligan D, Patrick J (1985) Phosphorus and carbon economies of ectomycorrhizal seedlings of Eucalyptus pilularis Smith. Funct Plant Biol 12:669–679
Nakamura Y (2013) Phosphate starvation and membrane lipid remodeling in seed plants. Prog Lipid Res 52:43–50. https://doi.org/10.1016/j.plipres.2012.07.002
Nakamura Y (2017) Plant phospholipid diversity: emerging functions in metabolism and protein–lipid interactions. Trends Plant Sci 22:1027–1040
Okazaki Y, Takano K, Saito K (2017) Lipidomic analysis of soybean leaves revealed tissue-dependent difference in lipid remodeling under phosphorus-limited growth conditions. Plant Biotechnol 34:57–63
Orians GH, Milewski AV (2007) Ecology of Australia: the effects of nutrient-poor soils and intense fires. Biol Rev 82:393–423
Pant BD, Burgos A, Pant P, Cuadros-Inostroza A, Willmitzer L, Scheible W-R (2015) The transcription factor PHR1 regulates lipid remodeling and triacylglycerol accumulation in Arabidopsis thaliana during phosphorus starvation. J Exp Bot 66:1907–1918
Pfaff J, Denton AK, Usadel B, Pfaff C (2020) Phosphate starvation causes different stress responses in the lipid metabolism of tomato leaves and roots. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids 1865:158763
Quigg A, Finkel ZV, Irwin AJ, Rosenthal Y, Ho T-Y, Reinfelder JR, Schofield O, Morel FM, Falkowski PG (2003) The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 425:291–294
R Core Team (2021) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Reiners WA (1986) Complementary models for ecosystems. Am Nat 127:59–73
Reszczyńska E, Hanaka A (2020) Lipids composition in plant membranes. Cell Biochem Biophys 78:401–414
Sadre R, Frentzen M (2009) Lipids in Plant Mitochondria. In: Wada H, Murata N (eds) Lipids in Photosynthesis Advances in Photosynthesis and Respiration. Springer, Dordrecht, UK
Schade JD, Espeleta JF, Klausmeier CA, McGroddy ME, Thomas SA, Zhang L (2005) A conceptual framework for ecosystem stoichiometry: balancing resource supply and demand. Oikos 109:40–51
Sebastián M, Smith AF, González JM, Fredricks HF, Van Mooy B, Koblížek M, Brandsma J, Koster G, Mestre M, Mostajir B (2016) Lipid remodelling is a widespread strategy in marine heterotrophic bacteria upon phosphorus deficiency. ISME J 10:968–978
Su Y, Li M, Guo L, Wang X (2018) Different effects of phospholipase Dζ2 and non-specific phospholipase C4 on lipid remodeling and root hair growth in Arabidopsis response to phosphate deficiency. Plant J 94:315–326
Suriyagoda LD, Ryan MH, Gille CE, Dayrell RL, Finnegan PM, Ranathunge K, Nicol D, Lambers H (2023) Phosphorus fractions in leaves. New Phytol 237:1122–1135
Thoms C, Gattinger A, Jacob M, Thomas FM, Gleixner G (2010) Direct and indirect effects of tree diversity drive soil microbial diversity in temperate deciduous forest. Soil Biol Biochem 42:1558–1565. https://doi.org/10.1016/j.soilbio.2010.05.030
Tjellström H, Andersson MX, Larsson KE, Sandelius AS (2008) Membrane phospholipids as a phosphate reserve: the dynamic nature of phospholipid-to-digalactosyl diacylglycerol exchange in higher plants. Plant Cell Environ 31:1388–1398
Tsujii Y, Si A, Kitayama K (2020) Phosphorus allocation to and resorption from leaves regulate the residence time of phosphorus in above-ground forest biomass on Mount Kinabalu, Borneo. Funct Ecol 34:1702–1712
Tsujii Y, Fan B, Atwell BJ, Lambers H, Lei Z, Wright IJ (2023) A survey of leaf phosphorus fractions and leaf economic traits among 12 co-occurring woody species on phosphorus-impoverished soils. Plant and Soil 1–18. https://doi.org/10.1007/s11104-023-06001-x
Tsujii Y, Onoda Y, Kitayama K (2017) Phosphorus and nitrogen resorption from different chemical fractions in senescing leaves of tropical tree species on Mount Kinabalu, Borneo. Oecologia 185:171–180
Turner BL, Brenes-Arguedas T, Condit R (2018) Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 555:367–370
Turner BL, Zemunik G, Laliberté E, Drake JJ, Jones FA, Saltonstall K (2019) Contrasting patterns of plant and microbial diversity during long-term ecosystem development. J Ecol 107:606–621
Van Mooy BA, Fredricks HF, Pedler BE, Dyhrman ST, Karl DM, Koblížek M, Lomas MW, Mincer TJ, Moore LR, Moutin T (2009) Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity. Nature 458:69–72
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wahle KW (1983) Fatty acid modification and membrane lipids. Proc Nutr Soc 42:273–287
Wang X, Shen J, Liao H (2010) Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci 179:302–306
Wardle DA, Walker LR, Bardgett RD (2004) Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305:509–513. https://doi.org/10.1126/science.1098778
Warren C (2018) A liquid chromatography–mass spectrometry method for analysis of intact fatty-acid-based lipids extracted from soil. Eur J Soil Sci 69:791–803
Warren CR (2019) Isotope pool dilution reveals rapid turnover of small quaternary ammonium compounds. Soil Biol Biochem 131:90–99
Warren CR (2020) Soil microbial populations substitute phospholipids with betaine lipids in response to low P availability. Soil Biol Biochem 140:107655
Yan L, Zhang X, Han Z, Pang J, Lambers H, Finnegan PM (2019) Responses of foliar phosphorus fractions to soil age are diverse along a 2 Myr dune chronosequence. New Phytol 223:1621–1633
Youngentob KN, Zdenek C, van Gorsel E (2016) A simple and effective method to collect leaves and seeds from tall trees. Methods Ecol Evol 7:1119–1123. https://doi.org/10.1111/2041-210X.12554
Zhang G, Zhang L, Wen D (2018) Photosynthesis of subtropical forest species from different successional status in relation to foliar nutrients and phosphorus fractions. Sci Rep 8:1–12
Acknowledgements
Aleese Gabir, Keshavi Jeyaseelan, Angie John, Lydia Ju, Bridey Lea, and Lillian Zhang are thanked for their assistance in this project. We acknowledge the support of NSW National Parks & Wildlife for providing access to sample plants and soils in Royal National Park.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was supported by a Discovery Grant (DP200102565) from the Australian Research Council.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Grace H. Liang. The first draft of the manuscript was written by Grace H. Liang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Hans Lambers.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, G.H., Butler, O.M. & Warren, C.R. Lipid profiles of plants and soil microbial communities are shaped by soil parent material in Australian sclerophyll forests. Plant Soil 498, 39–55 (2024). https://doi.org/10.1007/s11104-023-06075-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06075-7