Abstract
Background
Despite drought and nitrogen (N) deficit being two of the most important crops’ growth limiting factors, only few studies have explored tomato phenotypic variability in response to both abiotic stresses.
Aims
In this study, we aimed to perform a phenotypic evaluation and an analysis of the growth traits of 40 tomato genotypes (mostly focusing on old cultivars, but also including modern hybrids and wild tomato relatives’ accessions as anchors) grown in pots that were subjected to combined N and water deficit.
Methods
Each genotype was divided into two groups: control (100% N, 100% field capacity) and combined deficit (20% N, 50% field capacity). A total of 14 morpho-physiological traits were evaluated and further analyzed using multivariate statistical methods.
Results
The Principal Component Analyses revealed considerable phenotypical diversity among tomato genotypes, with four principal components explaining 82% of the variability. Data integration on a cluster analysis separated the studied genotypes into three distinct clusters based on their ability to handle the combined deficit. Tolerance was associated with traits such as lower specific leaf area, lower leaf area ratio and higher water use efficiency, comparing to the sensitive genotypes.
Conclusions
This study shows that tomato tolerance to combined N and water deficit largely varies between genotypes and that old cultivars represent a valuable gene pool towards more sustainable food production systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Population growth, especially in light of global climate change, demands a more rational use of agricultural resources (e.g. water, fertilizers and land), which poses several challenges to guarantee global security while promoting sustainable crop production (Fernandes et al. 2022; Kyalo Willy et al. 2019). The agricultural sector uses about 80% of the total available freshwater (which is becoming increasingly scarce) as well as high amounts of N fertilizers that often exceeds crop N requirements (Esteban et al. 2016; Liang et al. 2019; Elbehri 2015). Excessive N fertilization causes environmental problems, such as leaching of nitrate, being worldwide recognized as a serious issue of public and economic concern, due to its potential of increasing the eutrophication of freshwater ecosystems, the acidification of agricultural soils as well as its impact in greenhouse gas emissions (Liang et al. 2018; Esteban et al. 2016; Fernandes et al. 2022). To respond to these and other challenges, the recent European Green Deal - 2020, which strives to make Europe the first climate-neutral continent, has established the need of reducing nutrient losses by at least 50% and fertilizer application by at least 20% by 2030 (Schebesta and Candel 2020). Therefore, it is imperative to increase agricultural production in a sustainable way where the available resources are used more efficiently.
Water and nitrogen (N) are two of the most important resources affecting crop growth and yield (Machado et al. 2022). Several studies demonstrated that drought or N deficit have an impact on a large number of morphological traits (e.g. leaf area, specific leaf area, leaf weight ratio, root growth, root hydraulic conductivity and long-lasting root anatomical changes), as well as dry matter partitioning (promoting root development) (e.g. (Machado et al. 2022; Du et al. 2018; Moles et al. 2018; Wu and Cosgrove 2000). Furthermore, both abiotic stresses are known to have a severe impact on the metabolic pathways, including water relations and photosynthesis impairment, either due to stomatal closure and/or metabolic damage (Ding et al. 2018; Gonzalez-Dugo et al. 2010). Moreover, it is clear that water and N deficits are certainly highly interdependent (Ding et al. 2018; Gonzalez-Dugo et al. 2010; Plett et al. 2020). Despite this, exploring the phenotypic variability and understanding how crops respond to combined N and water deficit is largely unknown.
Tomato plants (Solanum lycopersicum Mill.) are among the most cultivated vegetable crops worldwide (FAO 2020; Heuvelink et al. 2020). Intensive tomato cultivation relies on high fertilizer inputs and regular irrigation for maximum yield (Sandhu et al. 2021). These inputs come at a significant economic cost (Engindeniz 2006) and carbon footprint (Pereira et al. 2021). Thus, considering the increased consumption of fresh tomato and tomato-derived products (Costa and Heuvelink 2018), the development of strategies focused on a rational use of water and fertilizers is required.
Plant breeding can be a powerful tool to enhance crop resilience to these abiotic stresses (Monforte 2020). Nonetheless, for several decades of high-pressure of genotype selection and crop breeding for high yields led to the so-called genetic erosion, and the tomato cultivars had often a narrow genetic basis, with the vast majority of them being sensitive to drought and N deficiency (Esteban et al. 2016; Machado et al. 2022; Shirasawa et al. 2010). To introduce phenotypic variability tomato breeding programs, for fruit quality and environmental adaptation, started to rely on wild tomato relatives (such as Solanum pimpinellifolium) (Conesa et al. 2019). More recently, it has been highlighted that another valuable gene pool for tomato improvement are the old accessions (e.g. landraces and heirlooms) held in germplasm collections. Nonetheless, so far, they represent an untapped genetic resource (Conesa et al. 2019; Roohanitaziani et al. 2020). Therefore, it is important to explore the variability of this genetic resource that might offer opportunities for improving resource use efficiency, thereby increasing the environmental sustainability of production systems (Monforte 2020).
Up to date, the number of studies that have screened a high number of tomato genotypes in response to drought and N deficiency is limited. In this study, we aimed to perform a phenotypic evaluation and analysis of the growth traits of 40 genotypes focusing on old tomato cultivars (but also including, as anchors, four modern hybrids and wild tomato relatives’ accessions) when subjected to combined N and water deficit. To that end, 14 morpho-physiological traits were evaluated and the contribution of the different growth components to the phenotypic variation in growth response of all accessions was used to determine their potential relationships (based on a cluster analysis).
Materials and methods
Plant material and growth conditions
This study included a total of 40 tomato genotypes and wild relatives (34 old cultivars, four modern inbred lines/hybrids and two wild relatives; Table 1) from an existing collection at Wageningen University (EU–SOL tomato core collection) and two Dutch seed companies (Nunhems and Rijk Zwaan). The old cultivars were selected taking into account their interesting phenotypic variability for plant growth and fruit quality-related traits being considered as a relevant gene pool for plant breeding (Consortium et al. 2014; Roohanitaziani et al. 2020, 2022). However, these have never been screened in response to N and water deficit, which is the focus of our study. The modern inbred lines/hybrids genotypes and wild tomato relatives’ accessions were included in this study as anchors.
Seeds were sown in potting soil and approximately two weeks later (corresponding to the third leaf appearance) seedlings from each genotype were selected based on uniformity. These were individually transplanted to pots (10.5 width x 10.5 length x 12 cm height) filled with 110 g of vermiculite (0.1–1.5 mm grade) and were divided into two groups: control plants (100% N requirement; 100% field capacity) and plants subjected to combined deficit (20% N requirement; 50% field capacity). In both cases, just prior to seedling transplanting, each pot was irrigated to 100% field capacity (FC), determined using the soil gravimetric water content method (Machado et al. 2023; Joshi et al. 2021). This led to 550 mL of the respective nutrient solution applied to each pot [Control (100% N): 10 mM NO3−; 1.9 mM H2PO4−; 6.1 mM K+; 3.6 mM Ca2+; 1.9 mM SO42−; 2.5 mM Mg2+; 2.6 mM Cl−; Combined deficit (20% N): 2 mM NO3−; 1.9 mM H2PO4−; 6.2 mM K+; 3.6 mM Ca2+; 4.8 mM SO42−; 2.8 mM Mg; 5.5 mM Cl−], both having the same pH (5.5) and E.C. (2 dSm− 1) as well as the same amount of micronutrients: 35 µM Fe3+; 8 µM Mn2+; 20.1 µM B; 0.5 µM Cu2+; 5.0 µM Zn2+; 0.5 µM MoO42−. The 10 mM NO3− concentration used, in the control nutrient solution, is a common level for guaranteeing an adequate plant growth in several species (Macduff et al. 1993; Lacrampe et al. 2020). Moreover, based on previous studies from our research team (under comparable environmental conditions and using cv. ‘Moneymaker’ as reference cultivar, during vegetative growth stage) it was possible to estimate tomato N needs for the overall experimental period. Briefly, considering the expected dry weight (2.54 g per plant) and an average N concentration in the plant tissues of 3% (Fiebig and Dodd 2016; Sonneveld and Voogt 2009), 76.2 mg N per plant were added in the 550 mL irrigation solution supplied to the control plants. Thus, for simplification, we referred to the N concentration supplied in the combined deficit (2 mM) as representing 20% of this reference optimal concentration (20% N).
Following seedling transplanting all pots were covered to prevent evaporation and no more nutrient solution was added until the end of the experiment. Concerning further irrigation, each pot from control plants was weighted and re-watered (with distilled water on a daily basis) to maintain FC at 100% during the experimental period, whereas in combined deficit no additional irrigation was supplied, resulting in a progressive decrease of substrate FC. Three plants from each genotype and growth condition were randomly collected during the cultivation period. The average fresh weight of these plants (per treatment) was subtracted from the total pot weight (per treatment) to calculate the actual weight of the water/nutrient solution present in the pot (Machado et al. 2023). The experiment ended after 21 to 28 days, depending on genotype, when the latter group of plants reached 53 ± 3% FC. The experiment was carried out during the winter period (Dec/Jan), in a multispan Venlo-type glasshouse compartment (52°N, 6°E; Wageningen University, the Netherlands) set at 25 ºC day/ 23 ºC night and 60% RH. Supplementary light was provided with high sodium pressure lamps (HSP) to extend the natural photoperiod to 16 h. HPS lamps (150 µmol s− 1 m− 2 of photosynthetic photon flux density measured at plant level, HPS, Philips SON-T Agro) were switched on when global radiation was below 300Wm− 2 and switched off when it was above 400Wm− 2. Plants were distributed among eight tables (each representing a block) and each table was split in two halves: in one half the control plants of the 40 genotypes were randomly allocated and in the other half the plants under stress treatment were positioned in a mirrored arrangement. In each table an outer row of plants was placed around the experimental plants to prevent border effects.
Data collection: morphological and physiological measurements
At the end of the experimental period, the stomatal conductance (gs; mmol m− 2 s− 1) was measured between 08:00 (2 h after the beginning of the light period) and 12:00, being carried out randomly among different plants. Measurements were performed on the adaxial side of the youngest fully developed and well-exposed leaf to assure fully active plants (n = 8). The porometer (SC-1 porometer, Decagon Devices Inc., Pullman, Washington, USA) was calibrated and the readings were set at a fixed time-period (30 s) following the manufacturer’s instructions, to ensure that all measurements were comparable and also to avoid the interference of leaving the sensor head on the leaf surface for a long period. Photosynthetic efficiency (Fv/Fm) was measured in 30 min dark-adapted leaves using the same leaf to estimate the photosynthetic capacity (n = 8) (portable minipam, Model PAM 2000. Walz, Effeltrich, Germany). Thereafter several morphological parameters were determined (n = 8): plant height (PH; cm), total leaf number (LN; > 1 cm) and total leaf area (LA; cm2; leaf area meter, Model LI-3100 C, LI-COR, Lincoln, Nebraska, USA). Dry weight (48 h at 105 °C in a ventilated oven; g) of leaves (LDW; g), stems (SDW; g), and roots (RDW; g), the latter after being washed, was determined (n = 8). Specific leaf area (SLA, ratio of leaf area to leaf dry weight; cm2 g− 1 dw), leaf weight ratio (LWR, ratio of leaf dry weight to total dry weight; g g− 1 dw), leaf area ratio (LAR, ratio of leaf area to total dry weight; cm2 g− 1 dw) and shoot-root ratio (S/R, ratio of shoot to root dry weight; g g− 1 dw) were calculated according to the ‘‘classical approach’’ described by Hunt (1990) (n = 8). The water use efficiency (WUE; g mL− 1) was calculated as the ratio between total dry weight (TDW; g) and water consumption per plant (mL) (Supplementary Data, Fig. S1) (n = 8).
Data analysis
For comparing genotypes responsiveness to the combined deficit, all the data was transformed into percentages of decrease, which have been further used as our studied variables. For this, within each table, control plants from a given genotype were paired with their mirrow plant on the opposite side of the table (corresponding to the same genotype but under combined N and water deficit). The difference between the value of the control and the value of the stressed plant was divided by the value of the control plant for each parameter (n = 8). To simplify further analysis and interpretation, the genotypes were then ordered and numbered from low to high percentage of decrease in TDW.
Pearson’s correlation coefficient was used to test associations between the percentage of decrease obtained for each of the 14 morpho-physiological parameters (Fv/Fm_D, gs_D, LN_D, PH_D, LA_D, LDW_D, SDW_D, RDW_D, S/R_D, SLA_D, LWR_D, LAR_D, WUE_D, TDW_D). A Principal Component Analysis (PCA) with Varimax rotation was used to establish the relationship among these quantitative variables with the data set including the percentages of decrease of all traits, as active variables. However, TDW_D was excluded from the PCA as this would be a redundant variable since it results from the sum of the different plant organs that are already taken into account in the analysis. Additionally, TDW of the control plants (TDW) was included as supplementary variable (active observation). After the PCA, a Clustering Analysis was applied to group the genotypes based in the individual coordinates of their projections into the coordinate space, following the k-means clustering with a Wilks’ Lambda criterion (Everitt et al. 2001). Differences in traits among clusters were tested by the non-parametric Kruskal–Wallis test. All statistical analyses were performed using XL-Stat (Addinsoft 2021).
Results
Screening for phenotypic variability
All genotypes showed visual deficiency symptoms when grown under combined N and water deficit. However, some genotypes were more severely affected than others as shown in Supplementary Information (Fig. S2). Actually, already after the second week of the imposed stress, it was visually observed that plants started to present phenotypic variability with regards to the severity of the combined N and water deficit symptoms, with the leaves becoming lighter green/ yellow (particularly the older ones) and often falling at variable degrees amongst genotypes. However, at this timepoint, no leaf rolling and/or wilting were observed.
At harvest (when the stressed plants reached 50% FC), a more detailed analysis of plant responses to the combined N and water deficit, based on 14 morpho-physiological traits, corroborated the observed visual differences between genotypes, with all the studied traits being negatively affected by the applied stress but to a different degree (Fig. 1, Table S1). For instance, this impact was more severe on LA with an average decrease of 77% over all genotypes under combined deficit, followed by the biomass production parameters (TDW, RDW, SDW, LDW) and the gs (all of them with an average decrease larger than 50%) (Fig. 1). On the other hand, Fv/Fm and LWR were the least impacted traits by the combined deficit (5.1 and 11.2% decrease, respectively). Interestingly, in spite of LA being the most sensitive parameter to the combined deficit, all genotypes responded in a similar magnitude resulting in the lowest coefficient of variance (4.5%) (Fig. 1). Regarding LWR, SLA and WUE a high variability was found among genotypes (56–59% CV) (Fig. 1). For SLA and WUE it is important to notice that, in general, a gradient was observed following the genotypes sorting order, which was not observed for LWR (Fig. 1).
Effects of combined nitrogen and water deficit on the percentage of decrease (_D) of total dry weight (TDW), root dry weight (RDW), stem dry weight (SDW), leaf dry weight (LDW), shoot/root ratio (S/R), plant height (PH), leaf number (LN), leaf weight ratio (LWR), leaf area (LA), leaf area ratio (LAR), specific leaf area (SLA), photosynthetic efficiency (Fv/Fm), stomatal conductance (gs) and water use efficiency (WUE) in each of the 40 screened tomato genotypes and wild relatives, and the variation observed across them (minimum and maximum value, mean and standard deviation and the coefficient of variance (%)). Color scale represents the mean of the percentage of decrease (the difference between the mean value of the control plants and mean of the stressed plants divided by the mean value of the control plants, n = 8). Different colors used in the genotype number correspond to a different category: old cultivars (blue); modern inbred lines and hybrids (light orange); wild tomato relatives (green). The genotypes were ordered from low to high percentage decrease in TDW and their identification is given on Table 1
At the end of the experiment, the control plants of the different genotypes had significantly different TDW (p < 0.0001, varying between 1.17 and 3.99 g; Fig. 2). Therefore, we hypothesized that faster growing genotypes could be more prone to the combined deficit as they had access to the same amount of water and N during the experimental period when compared to the smaller genotypes. To clarify this, we determined the correlation between the TDW of the control plants and the percentage of TDW decrease observed under the combined deficit (Fig. 2). This analysis demonstrated a moderate correlation between these variables (R2 = 0.435). Therefore, as plant biomass did not have per se a significant impact on the plant’s response to the combined deficit, all the genotypes were considered for the following analyses.
Linear correlation between the percentage of decrease in total dry weight (TDW_D) after exposure to combined nitrogen and water deficit and the total dry weight of the control plants (TDW_CTR) for the 40 screened tomato genotypes and wild relatives. Different symbols used in the genotype number correspond to a different category: old cultivar (◊); modern inbred lines and hybrids (□); wild tomato relatives (〇)
In order to analyze the relationship among the studied morpho-physiological traits, Pearson’s correlation coefficients were determined (Fig. 3). Most traits showed a positive significant correlation among each other, with the exception of LAR and SLA which mostly showed a significant negative correlation with the other traits. For instance, the percentage of decrease in TDW was positively correlated with the percentage of decrease in RDW, SDW, LDW, PH, LN, LWR, LA, Fv/Fm and WUE but negatively correlated with the percentage of decrease in LAR and SLA (Fig. 3). Additionally, regarding the physiological traits, the percentage of decrease of Fv/Fm was positively correlated with the percentage of decrease in TDW, LDW and PH, whereas gs decrease was not significantly correlated with any of the other analyzed traits (Fig. 3). Finally, the percentage of decrease in WUE, one of the most important traits when particularly coping with water deficit, was positively correlated with the percentage of decrease of all the other evaluated parameters, with the exception of LAR and gs, where no significative correlation was found (Fig. 3).
Pearson’s correlations coefficient for the percentage of decrease (_D) of each evaluated morpho-physiological parameters from the 40 screened tomato genotypes and wild relatives exposed to combined nitrogen and water deficit (* indicates significant correlation at p < 0.05). Green and orange colours represent positive or negative correlations, respectively. Total dry weight = TDW; root dry weight = RDW, stem dry weight = SDW, leaf dry weight = LDW, shoot/root ratio = S/R, plant height = PH, leaf number = LN, leaf weight ratio = LWR, leaf area = LA, leaf area ratio = LAR, specific leaf area = SLA, photosynthetic efficiency = Fv/Fm, stomatal conductance = gs, Water use efficiency = WUE.
Multivariate statistical analyses of the phenotypic variability
Results from the PCA yielded four main factors (those with an Eigenvalue > 1), explaining over 82.4% of the total variance (Table S2, Fig. 4). This result reflects a close relationship between the evaluated traits and the genotype ability to handle with the combined deficit (Fig. 4). Moreover, our study also revealed considerable phenotypical (and presumably genetic) diversity among the screened genotypes with the first two principal components explaining 60.7% of the variability (PC1–42.1% and PC2–18.6%), which demonstrates a different behavior when exposed to the combined N and water deficit (Fig. 5). A further integration of these data in a cluster analysis separated the genotypes into three distinct clusters based on their ability to cope with the combined deficit. Cluster 1 was composed by 15 genotypes (having 13 old cultivars, one modern inbred line and one modern hybrid), cluster 2 held 16 genotypes (13 old cultivars, two modern hybrids and one of the wild relatives) and cluster 3 comprised 9 genotypes (eight old cultivars and one wild relative) (Table 1). According to Fig. 4, clusters 2 and 3 were both correlated with severe decreases in LDW, SDW and RDW, when compared to cluster 1, pointing towards contrasting behavior among these groups. Genotypes from cluster 1, especially genotype 1, 2 and 3 (old cultivars), were the most tolerant genotypes in their responses to combined N and water deficit showing the lowest percentage decrease in TDW (Fig. 1). In general, this cluster was characterized by a marked percentage of decrease in SLA, and LAR but also a higher percentage of decrease in gs (although less pronounced). In all the other morphophysiological traits these genotypes had also a lower percentage of decrease. Cluster 2 mostly included genotypes with a higher TDW under control conditions and genotypes with a higher percentage of decrease in PH, SDW and RDW, as well as genotypes showing a lower percentage of decrease in LAR, SLA and gs, all when grown under combined N and water deficit. Considering cluster 3, the parameters that were associated with their worst performance were the percentage of decrease in traits such as WUE, LWR, LA, S/R, LN and to a lower extent with the percentage of decrease in Fv/Fm.
Principal component analysis (PCA) of the percentage of decrease (_D) of 14 morpho-physiological traits evaluated in a total of 40 screened tomato genotypes and wild relatives subjected to combined nitrogen and water deficit. The two principal components (PC1, PC2) explained 60.7% of the total variance. Different symbols used in the genotype number correspond to a different category: old cultivar (◊); modern inbred lines and hybrids (□); wild tomato relatives (〇). Total dry weight = TDW; root dry weight = RDW, stem dry weight = SDW, leaf dry weight = LDW, shoot/root ratio = S/R, plant height = PH, leaf number = LN, leaf weight ratio = LWR, leaf area = LA, leaf area ratio = LAR, specific leaf area = SLA, photosynthetic efficiency = Fv/Fm, stomatal conductance = gs, Water use efficiency = WUE. The identification of the genotypes is given on Table 1
When characterizing the response of each cluster independently (Table S1), when subjected to the combined deficit, making use of all 14 morpho-physiological traits (Figs. 5, 6 and 7) significantly differences were often found between them. For instance, the genotypes in cluster 1 had a percentage of decrease of TDW of around 58.0 ± 6.4% whereas for the genotypes in clusters 2 and 3 this decrease was significantly higher reaching 69.8 ± 2.6% and 69.3 ± 2.0%, respectively (Fig. 5a). This trend was also found for the biomass of the different plant organs (roots, stem, leaves) with cluster 1 always showing a significantly lower percentage of decrease on organ dry weight, when subjected to the combined deficit, as compared to clusters 2 and 3. These results imply a differential response to the combined deficit conditions in the studied set of genotypes, with the ones from cluster 1 having lower losses of dry weight when exposed to the combined deficit, which suggests a more efficient behavior. All clusters responded with a decrease in S/R (Fig. 5e), reflecting a higher partitioning towards the roots under combined deficit. However, this relative investment in the roots did not significantly vary among clusters.
Box and whisker plots of the different clusters concerning the percentage of decrease in total A root B stem C leaf D dry weight and shoot root ratio E in the 40 screened tomato genotypes and wild relatives subjected to combined nitrogen and water deficit. Boundaries of the boxes represent the 1st (lower limit) and the 3rd quartile (upper limit), while the black line inside the box marks the median. The whiskers, above and below the box, extend to the maximum and the minimum values, respectively. Different letters above box-plots indicate significant statistical differences and n.s. correspond to non-significant differences among clusters according to Kruskal–Wallis test at p < 0.05. Colored points represent the average values for each genotype within the corresponding cluster. Total dry weight = TDW; root dry weight = RDW, stem dry weight = SDW, leaf dry weight = LDW, shoot/root ratio = S/R.
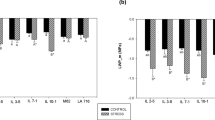
Concerning the percentage of decrease in PH, no statistical differences were found among clusters (Fig. 6a). However, the percentage of decrease in LN was significantly different, with cluster 1 and 2 having lower average values (29.8 ± 8.4 and 31.4 ± 4.4, respectively) than cluster 3 (42.8 ± 7.9) (Fig. 6b). The same pattern was observed for the percentage of decrease in LWR (Fig. 6c) and in LA (Fig. 6d). However, for LAR cluster 2 showed the lowest decrease percentages (21.2 ± 7.1%), being statistically different from cluster 1 (38.3 ± 4.3%) and cluster 3 (38.3 ± 5.4%) (Fig. 6e). Finally concerning the clusters’ response to combined deficit in terms of SLA, cluster 1 showed a significantly higher percentage of decrease (33.2 ± 6.0%) thus demonstrating a better ability to increase leaf thickness under this combined abiotic stress (Fig. 6f).
Regarding the physiological traits, Fv/Fm, only showed a significant difference among clusters 1 and 3, with the later having the higher percentage of decrease (6.5 ± 2.1% in cluster 3 and 4.3 ± 2.1% in cluster 1) (Fig. 7a). For gs significant differences were found among cluster 2 and 3, with the later having again the highest percentage of decrease (57.7 ± 14.0% in cluster 3 and 44.8 ± 14.3% in cluster 2 (Fig. 7b). Finally, significant differences were also found for the percentage of decrease in WUE (Fig. 7c). Cluster 1 had the most efficient genotypes in terms of water use, with the lowest percentages of decrease (11.9 ± 6.6), whereas cluster 2 and 3 were not significantly different among each other (resulting in an average value of 25.3 ± 5.8 and 33.7 ± 15.2, respectively) (Fig. 7c).
Box and whisker plots of the different clusters concerning the percentage of decrease in plant height A leaf number B leaf weight ratio C leaf area D leaf area ratio E and specific leaf area F in the 40 screened tomato genotypes and wild relatives subjected to combined nitrogen and water deficit. Boundaries of the boxes represent the 1st (lower limit) and the 3rd quartile (upper limit), while the black line inside the box marks the median. The whiskers, above and below the box, extend to the maximum and the minimum values, respectively. Different letters above box-plots indicate significant statistical differences and n.s. correspond to non-significant differences among clusters according to a Kruskal–Wallis test at p < 0.05. Colored points represent the average values for each genotype within the corresponding cluster. Plant height = PH, leaf number = LN, leaf weight ratio = LWR, leaf area = LA, leaf area ratio = LAR, specific leaf area = SLA.
Box and whisker plots of the different clusters concerning the percentage of decrease in the photosynthetic efficiency (Fv/Fm,) A stomatal conductance (gs) B water use efficiency (WUE) C in the 40 screened tomato genotypes and wild relatives subjected to combined nitrogen and water deficit. Boundaries of the boxes represent the 1st (lower limit) and the 3rd quartile (upper limit), while the black line inside the box marks the median. The whiskers, above and below the box, extend to the maximum and the minimum values, respectively. Different letters above box-plots indicate significant statistical differences among cluster according to Kruskal–Wallis test at p < 0.05. n.s. = not significant. Colored points represent the average values for each genotype within the corresponding cluster. Photosynthetic efficiency = Fv/Fm, stomatal conductance = gs, water use efficiency = WUE.
Discussion
It is essential to enhance crop resilience to abiotic stresses towards sustainable food production systems (Carvalho and Vasconcelos 2013). Nonetheless, this requires phenotypic variability being also of utmost importance to identify morpho-physiological traits associated with tolerance to these combined stresses. So far, there is limited knowledge on tomato phenotypic variability to combined N and water deficit. Here we have screened the response of a large set of tomato genotypes to this combined deficit. In general, a vast majority of tomato cultivars are frequently referred has sensitive to these abiotic stresses (Machado et al. 2022; Shirasawa et al. 2010). Moreover, old accessions/cultivars (e.g. landraces and heirlooms) represent an underexploited wealth of genetic variation (Roohanitaziani et al. 2020), that has been discarded due to undesired agronomic traits, but might encompass higher NUE and WUE than modern tomato cultivars. From our selection of 40 genotypes (including 34 old cultivars, four modern inbred lines/hybrids and two wild tomato relatives) it was found a large variability in response to combined N and water deficit (Figs. 1 and 4). The combined deficit severely affected all growth parameters, but to a different degree depending on the genotype and trait. Already after the second week of the imposed N and water deficit, it was visually observed that plants started to present phenotypic variability, with regard to the symptoms’ severity. For instance, the older leaves become lighter green/ yellow (reflecting a common N deficiency symptom; (de Bang et al. 2021)) and these have fallen at variable degrees amongst genotypes. Thereafter, at the end of the experimental period, the percentages of decrease in TDW ranged from 42 to 77% (Figs. 1 and 2). Previous studies also found some phenotypic variability in tomato, but they were limited to a much lower number of genotypes and were focused on the plant responses to independent stresses (Abenavoli et al. 2016; Sánchez-Rodríguez et al. 2010).
PCA revealed considerable phenotypical diversity among the studied genotypes with four principal components explaining 82% of the variability (Fig. 4). A further integration of our data, in a cluster analysis, was able to separate the screened genotypes into three distinct clusters based on their ability to handle the combined deficit (Fig. 4). All genotypes responded to the combined deficit by changing the morpho-physiological traits, but genotypes from cluster 1 (composed only by 13 old accessions and two modern inbred lines/hybrids) showed higher tolerance under these conditions, pointing towards a more efficient behavior. In general, these tolerant genotypes showed a lower percentage of decrease in TDW as compared with more sensitive genotypes. Moreover, they were closely associated with traits such as a lower SLA, lower LAR and a higher WUE but also partly associated with lower gs. Different authors have reported a negative effect on the dry weight of leaves, stems, and roots, and consequently on TDW when tomato plants were exposed to one of these abiotic stresses (Okunlola et al. 2015; Al Hassan et al. 2015; Luna et al. 2014; Khan et al. 2015; Zhou et al. 2019). For instance, it has been found that water deficit at the early seedling stage might lead to higher RDW, longer roots and lower S/R, with all these traits being widely accepted as accurate indicators of the plant’s ability to withstand drought stress (Makhadmeh et al. 2022). Tripodi et al. (2022) also demonstrated that root architecture played an important role on tomato plant responses to N starvation or water deficit. In our study, the tomato genotypes from cluster 1 showed lower percentages of decrease in RDW (Figs. 4 and 5b) but no significant differences were found concerning the S/R (Fig. 5e).
Regarding SLA and LAR, the higher percentage of decrease in cluster 1 suggests that increasing leaf thickness (lower SLA) and investing less of their LDW in LA seems to be related to tomato ability to tolerate combined N and water deficit. Moreover, the reduction of SLA is believed to be a possible strategy to improve WUE. This is due to thicker leaves generally having a higher concentration of chlorophyll and proteins per leaf area unit leading to a greater photosynthetic capacity and, consequently, a higher biomass production per unit leaf area than thinner leaves (Chatterjee and Solankey 2015). Higher WUE can be defined as the amount of carbon assimilated as biomass produced per unit of water used (Cantero-Navarro et al. 2016; Ullah et al. 2019). Thus, an improvement in WUE has been related to a higher closing stomata capacity, and lower transpiration rate, with these traits being associated with higher tolerance to drought stress (Galmés et al. 2013; Raja et al. 2020). On the other hand, some studies have shown that a low nutrient availability may reduce WUE and plants’ capacity to adapt to drought (Sardans et al. 2013). Moreover, a screening of tomato landraces for drought tolerance, based on growth and chlorophyll fluorescence analyses, identified Fv/Fm as one of the most sensitive parameters for the detection of drought stress impact on tomato plants (Sousaraei et al. 2021). Indeed, in our study, gs and Fv/Fm were severely affected by the combined deficit, with cluster 3 having the higher decreases (Fig. 7a and b). Although a wide phenotypic variation in the percentage of decrease of these traits was observed (Fig. 1), our results suggest that, for this tomato collection, gs and Fv/Fm were not the key parameters in tomato tolerance response to combined N and water deficit (Fig. 4).
The two accessions of the studied wild tomato relatives S. pimpinellifolium were not part of the tolerant cluster, which was somehow unexpected as wild relatives have been reported as important genetic sources of abiotic tolerance traits (Conesa et al. 2019; Martínez-Cuenca et al. 2020). Recently, Tripodi et al. (2022) studied 42 tomato genotypes (including elite cultivars, heirlooms, landraces, and hybrids) grown in open field under single nitrogen deficit (no-N fertilization) or under single water deficit (30% of the amount given to the control plants). Among the studied genotypes, the two hybrids had the best performance in terms of yield, when grown under N starvation or water deficit, whereas in general the landraces and heirlooms exhibited a better fruit quality. In our study, two of the four screened modern inbred lines/hybrids were also part of the tolerant cluster, but interestingly most of the old accessions from this cluster even presented a better performance for the studied morphophysiological traits when grown under combined deficit (Figs. 1 and 4). Some recent studies have pondered that the physiological and molecular responses of crops to a combination of two stresses are unique, resulting in the deployment of stress-adaptation strategies which sometimes are different and contrasting to those seen under individual stresses, and other times are shared and similar (Hussain et al. 2018; Pandey et al. 2015). This study was a first step to address tomato response to combined N and water deficit and showed that old accessions represent a very interesting wealth of genetic diversity in terms of response to these abiotic stresses, with some of them (Cluster 1) having even a better performance than the two studied wild relative accessions from S. pimpinellifolium (Fig. 4). In future works, the efficiency of these accessions to cope with the combined N and water deficit, should be evaluated in other development stages, especially at the generative phase, as the plant structures have a variable degree of genotypic plasticity to adapt to stressful conditions. Indeed, the flowering and fruit development stages have been described as the most sensitive ones to water deficits in tomato plants (Khapte et al. 2019; Machado et al. 2022). Plus, the impact on yield and fruit quality, at harvest, would give additional relevant information. Moreover, it would also be important to validate our results (performed under controlled conditions) in field trials, preferably including different experimental sites with contrasting pedoclimatic conditions to address possible genotype and environment interactions (G × E). For instance, it would be relevant to design an experiment to investigate the role of soil type [e.g a sandy soil, with high leaching potential (Ayankojo et al. 2020), compared a clay soil, with high water retention capacity (Tripodi et al. 2022)] as well as contrasting climates (e.g. high versus low evaporative demanding conditions) to better analyze the G × E interactions. Indeed, Tripodi et al. (2022) highlighted the importance of exploring such interactions, although from their study the genotype was the main source of variation, with G × T (i.e. single water deficit or no-nitrogen supply) and E × T being less relevant.
Finally, further research on the underlying mechanisms behind tolerance (including single vs. combined stresses) would also be of utmost importance to contribute to sustainable food production. Due to the amount of work involved in those type of analysis, a selection of contrasting genotypes (identified in this study) would be a good approach to explore in-depth these aspects.
Conclusion
This study represents one step forward in the current knowledge since it enabled to explore the tomato phenotypic variability focusing on old cultivars and contributed to a better understanding of plants’ response to combined N and water deficit. The results obtained in this trial, where tomato plants were grown in pots (for a better control over the root environment), have shown for the first time, that tomato has a large phenotypic variability in its response to the combined deficit, and that old cultivars might represent a valuable gene pool in terms of tolerance to these abiotic stresses. Moreover, the data integration in a cluster analysis enabled to separate the 40 screened genotypes into three distinct clusters, based on their ability to handle the combined deficit. All genotypes responded to the combined deficit by adjusting several morpho-physiological traits, but genotypes from cluster 1 showed a more efficient behavior having a significantly lower percentage of decrease in their TDW, but also in the dry weight of each organ type. In general, these more tolerant genotypes were strongly associated with a lower SLA, a lower LAR and higher WUE as compared with less tolerant genotypes. Moreover, they were also associated, but to a lesser extent, with a lower gs. It is concluded that tomato tolerance to combined N and water deficit significantly varied between genotypes, and that old cultivars represent a valuable gene pool towards sustainable food production systems. Additionally, the identified contrasting genotypes might have the potential to be used in future studies, namely, to explore the biochemical and molecular mechanisms behind tolerance to these combined abiotic stresses, or to explore the potential of tolerant genotypes as rootstocks for improved NUE and WUE.
References
Abenavoli MR, Longo C, Lupini A, Miller AJ, Araniti F, Mercati F, Princi MP, Sunseri F (2016) Phenotyping two tomato genotypes with different nitrogen use efficiency. Plant Physiol Biochem 107:21–32
Addinsoft (2021) XLSTAT statistical and data analysis solution. New York, USA. https://www.xlstat.com
Aflitos S, Schijlen E, De Jong H et al (2014) Exploring genetic variation in the tomato (Solanum Lycopersicon) clade by whole-genome sequencing. Plant J 80:136–148. https://doi.org/10.1111/tpj.12616
Al Hassan M, Fuertes MM, Sánchez FJR, Vicente O, Boscaiu M (2015) Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 43(1):1–11
Ayankojo IT, Morgan KT, Kadyampakeni DM, Liu GD (2020) Tomato growth, yield, and root development, soil nitrogen and water distribution as affected by nitrogen and irrigation rates on a Florida sandy soil. HortScience 55(11):1744–1755
Cantero-Navarro E, Romero-Aranda R, Fernández-Muñoz R, Martínez-Andújar C, Pérez-Alfocea F, Albacete A (2016) Improving agronomic water use efficiency in tomato by rootstock-mediated hormonal regulation of leaf biomass. Plant Sci 251:90–100
Carvalho SMP, Vasconcelos MW (2013) Producing more with less: strategies and novel technologies for plant-based food biofortification. Food Res Int 54(1):961–971
Chatterjee A, Solankey S (2015) Functional physiology in drought tolerance of vegetable crops: an approach to mitigate climate change impact. Clim dynamics Hortic Sci 1:149–171
Conesa M, Fullana-Pericàs M, Granell A, Galmés J (2019) Mediterranean long shelf-life landraces: an untapped genetic resource for tomato improvement. Front Plant Sci 10:1651
Consortium TTGS, Aflitos S, Schijlen E, de Jong H, de Ridder D, Smit S, Finkers R, Wang J, Zhang G, Li N, Mao L, Bakker F, Dirks R, Breit T, Gravendeel B, Huits H, Struss D, Swanson-Wagner R, van Leeuwen H, van Ham RCHJ, Fito L, Guignier L, Sevilla M, Ellul P, Ganko E, Kapur A, Reclus E, de Geus B, van de Geest H, te, Lintel Hekkert B, van Haarst J, Smits L, Koops A, Sanchez-Perez G, van Heusden AW, Visser R, Quan Z, Min J, Liao L, Wang X, Wang G, Yue Z, Yang X, Xu N, Schranz E, Smets E, Vos R, Rauwerda J, Ursem R, Schuit C, Kerns M, van den Berg J, Vriezen W, Janssen A, Datema E, Jahrman T, Moquet F, Bonnet J, Peters S (2014) Exploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant J 80(1):136–148
Costa JM, Heuvelink E (2018) The global tomato industry. Tomatoes, vol 27. CABI, Boston, pp 1–26
de Bang TC, Husted S, Laursen KH, Persson DP, Schjoerring JK (2021) The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol 229(5):2446–2469
Ding L, Lu Z, Gao L, Guo S, Shen Q (2018) Is nitrogen a key determinant of water transport and photosynthesis in higher plants upon drought stress? Front Plant Sci 9:1143
Du Y-D, Niu W-Q, Gu X-B, Zhang Q, Cui B-J (2018) Water-and nitrogen-saving potentials in tomato production: a meta-analysis. Agric Water Manage 210:296–303
Elbehri A (2015) Climate change and food systems: global assessments and implications for food security and trade. Food and Agriculture Organization of the United Nations (FAO)
Engindeniz S (2006) Economic analysis of pesticide use on processing tomato growing: a case study for Turkey. Crop Prot 25(6):534–541
Esteban R, Ariz I, Cruz C, Moran J (2016) Review: mechanisms of ammonium toxicity and the quest for tolerance. Plant Sei 248:92–101
Everitt BS, Landau S, Lesse M (2001) Cluster Analyses, 4th edn. Arnold, London
FAO (2020) Statistical Database: Production Statistics. Available online: http://www.fao.org/faostat/en/#data/QC/visualize. Accessed 20 May 2021
Fernandes APG, Machado J, Fernandes TR, Vasconcelos MW, Carvalho SMP (2022) Water and Nitrogen fertilization management in the light of climate change: impacts on food security and product quality. In: Kumar V, Srivastava AK, Suprasanna P (eds) Plant Nutrition and Food Security in the Era of Climate Change. Elsevier, United States, pp 147–164
Fiebig A, Dodd IC (2016) Inhibition of tomato shoot growth by over-irrigation is linked to nitrogen deficiency and ethylene. Physiol Plant 156(1):70–83
Galmés J, Ochogavía JM, Gago J, Roldán EJ, Cifre J, Conesa M (2013) Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ 36(5):920–935
Gonzalez-Dugo V, Durand J-L, Gastal F (2010) Water deficit and nitrogen nutrition of crops. A review. Agron Sustain Dev 30(3):529–544
Heuvelink E, Okello RCO, Peet M et al (2020) Tomato. In: Wien HC, Stützel H (eds) The Physiology of Vegetable Crops. CABI, United Kingdom, p 512
Hunt R (1990) Basic growth analysis. Unwin Hyman, London, p 112. https://doi.org/10.1007/978-94-010-9117-6
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L (2018) Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front Plant Sci 9:393
Joshi S, Thoday-Kennedy E, Daetwyler HD, Hayden M, Spangenberg G, Kant S (2021) High-throughput phenotyping to dissect genotypic differences in safflower for drought tolerance. PLoS ONE 16(7):e0254908
Khan SH, Arsalan K, Uzma L, Shah AS, Khan MA, Muhammad B, Ali MU (2015) Effect of drought stress on tomato cv. Bombino. J Food Process Technol 6(7):465
Khapte P, Kumar P, Burman U, Kumar P (2019) Deficit irrigation in tomato: agronomical and physio-biochemical implications. Sci Hort 248:256–264
Kyalo Willy D, Muyanga M, Jayne T (2019) Can economic and environmental benefits associated with agricultural intensification be sustained at high population densities? A farm level empirical analysis. Land Use Policy 81:100–110
Lacrampe N, Lopez-Lauri F, Lugan R, Colombié S, Olivares J, Nicot PC, Lecompte F (2020) Regulation of sugar metabolism genes in the nitrogen-dependent susceptibility of tomato stems to Botrytis cinerea. Ann Bot 127(1):143–154
Liang S, Li Y, Zhang X, Sun Z, Sun N, Duan Y, Xu M, Wu L (2018) Response of crop yield and nitrogen use efficiency for wheat-maize cropping system to future climate change in northern China. Agric For Meteorol 262:310–321
Liang L, Ridoutt BG, Lal R, Wang D, Wu W, Peng P, Hang S, Wang L, Zhao G (2019) Nitrogen footprint and nitrogen use efficiency of greenhouse tomato production in North China. J Clean Prod 208:285–296
Luna AM, García ER, Servín JLC, Herrera AL, Arellano JS (2014) Evaluation of different concentrations of nitrogen for tomato seedling production (Lycopersicon esculentum Mill). Univ J Agric Res 2(8):305–312
Macduff J, Jarvis S, Larsson C, Oscarson P (1993) Plant growth in relation to the supply and uptake of NO3–: a comparison between relative addition rate and external concentration as driving variables. J Exp Bot 44(9):1475–1484
Machado J, Fernandes APG, Fernandes TR, Heuvelink E, Vasconcelos MW, Carvalho SMP (2022) Drought and nitrogen stress effects and tolerance mechanisms in tomato: a review. In: Kumar V, Srivastava AK, Suprasanna P (eds) Plant Nutrition and Food Security in the Era of Climate Change. Elsevier, United States, pp 315–359
Machado J, Vasconcelos MW, Soares C, Fidalgo F, Heuvelink E, Carvalho SMP (2023) Young tomato plants respond differently under single or combined mild nitrogen and water deficit: an insight into morphophysiological responses and primary metabolism. Plants 12(5):1181
Makhadmeh I, Albalasmeh AA, Ali M, Thabet SG, Darabseh WA, Jaradat S, Alqudah AM (2022) Molecular characterization of tomato (Solanum lycopersicum L.) accessions under drought stress. Horticulturae 8(7):600
Martínez-Cuenca M-R, Pereira-Dias L, Soler S, López-Serrano L, Alonso D, Calatayud Á, Díez MJ (2020) Adaptation to water and salt stresses of Solanum pimpinellifolium and Solanum lycopersicum var. cerasiforme. Agronomy 10(8):1169
Moles TM, Mariotti L, De Pedro LF, Guglielminetti L, Picciarelli P, Scartazza A (2018) Drought induced changes of leaf-to-root relationships in two tomato genotypes. Plant Physiol Biochem 128:24–31
Monforte AJ (2020) Time to exploit phenotypic plasticity. J Exp Bot 71(18):5295–5297
Okunlola O, Olatunji A, Afolabi M, Gbadegesin K (2015) Effects of nitrogen nutritional stress on the morphological and yield parameters of tomato (Solanum lycopersicum L). 寒旱区科学: 英文版 7(2):137–145
Pandey P, Ramegowda V, Senthil-Kumar M (2015) Shared and unique responses of plants to multiple individual stresses and stress combinations: physiological and molecular mechanisms. Front Plant Sci 6:723
Pereira BdJ, Cecílio Filho AB, La Scala N (2021) Greenhouse gas emissions and carbon footprint of cucumber, tomato and lettuce production using two cropping systems. J Clean Prod 282:124517
Plett DC, Ranathunge K, Melino VJ, Kuya N, Uga Y, Kronzucker HJ (2020) The intersection of nitrogen nutrition and water use in plants: new paths toward improved crop productivity. J Exp Bot 71(15):4452–4468
Raja V, Qadir SU, Alyemeni MN, Ahmad P (2020) Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 10(5):1–18
Roohanitaziani R, de Maagd RA, Lammers M, Molthoff J, Meijer-Dekens F, van Kaauwen MPW, Finkers R, Tikunov Y, Visser RGF, Bovy AG (2020) Exploration of a resequenced tomato core collection for phenotypic and genotypic variation in plant growth and fruit quality traits. Genes 11(11):1278
Roohanitaziani R, Lammers M, Molthoff J, Tikunov Y, Meijer-Dekens F, Visser RGF, van Arkel J, Finkers R, de Maagd RA, Bovy AG (2022) Phenotyping of a diverse tomato collection for postharvest shelf-life. Postharvest Biol Technol 188:111908
Sandhu RK, Boyd NS, Zotarelli L et al (2021) Effect of planting density on the yield and growth of intercropped tomatoes and peppers in Florida. HortScience 56:286–290
Sánchez-Rodríguez E, Rubio-Wilhelmi MM, Cervilla LM, Blasco B, Rios JJ, Rosales MA, Romero L, Ruiz JM (2010) Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci 178(1):30–40
Sardans J, Rivas-Ubach A, Estiarte M, Ogaya R, Peñuelas J (2013) Field-simulated droughts affect elemental leaf stoichiometry in Mediterranean forests and shrublands. Acta Oecol 50:20–31
Schebesta H, Candel JJL (2020) Game-changing potential of the EU’s farm to fork strategy. Nat Food 1(10):586–588
Shirasawa K, Isobe S, Hirakawa H, Asamizu E, Fukuoka H, Just D, Rothan C, Sasamoto S, Fujishiro T, Kishida Y, Kohara M, Tsuruoka H, Wada T, Nakamura Y, Sato S, Tabata S (2010) SNP discovery and linkage map construction in cultivated tomato. DNA Res 17(6):381–391
Sonneveld C, Voogt W (2009) Tissue tests. Plant nutrition of greenhouse crops. Springer Netherlands, Dordrecht, pp 83–101
Sousaraei N, Mashayekhi K, Mousavizadeh SJ, Akbarpour V, Medina J, Aliniaeifard S (2021) Screening of tomato landraces for drought tolerance based on growth and chlorophyll fluorescence analyses. Hortic Environ Biotechnol 62(4):521–535
Tripodi P, Figàs MR, Leteo F, Soler S, Díez MJ, Campanelli G, Cardi T, Prohens J (2022) Genotypic and environmental effects on morpho-physiological and agronomic performances of a tomato diversity panel in relation to nitrogen and water stress under organic farming. Front Plant Sci 13
Ullah H, Santiago-Arenas R, Ferdous Z, Attia A, Datta A (2019) Chapter two - improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: a review. In: Sparks DL (ed) Advances in Agronomy, vol 156. Academic Press, pp 109–157
Wu Y, Cosgrove DJ (2000) Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. J Exp Bot 51(350):1543–1553
Zhou R, Kong L, Wu Z, Rosenqvist E, Wang Y, Zhao L, Zhao T, Ottosen C-O (2019) Physiological response of tomatoes at drought, heat and their combination followed by recovery. Physiol Plant 165(2):144–154
Funding
Open access funding provided by FCT|FCCN (b-on). The authors would like to thank to Foundation for Science and Technology (FCT) for funding JM Ph.D. scholarship (SFRH/BD/116147/2016). This research was supported by national funds through FCT within the scope of UIDB/05748/2020, UIDP/05748/2020, UIDB/50016/2020.
Author information
Authors and Affiliations
Contributions
SMP, MWV and EH were responsible for the conception and the experimental design. RF selected and provided the seeds for the trial. JM conducted the experimental work (installing the trial, maintaining the plants and performing the morpho-physiological analyses) and wrote the manuscript. LMC coordinated the multivariate analysis. All authors contributed to data analysis and interpretation, provided critical revision of the manuscript and approved its final version. SMPC, as JM grant supervisor, assumes the responsibility for the integrity of the present work as a whole, from inception to the finished article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Responsible Editor: Ciro A. Rosolem.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 399 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Machado, J., Heuvelink, E., Vasconcelos, M.W. et al. Exploring tomato phenotypic variability under combined nitrogen and water deficit. Plant Soil 496, 123–138 (2024). https://doi.org/10.1007/s11104-023-06023-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06023-5