Abstract
Purpose
Alfalfa is one of the most valuable forage crops in temperate climate zones. Ensifer meliloti, the endosymbiont of alfalfa, contains all the denitrification genes but the capacity of alfalfa root nodules to produce nitrous oxide (N2O) is not known. In this work, N2O emissions as well as the influence of bacteroidal denitrification on nodulation competitiveness and N2O release from alfalfa nodules has been investigated.
Methods
Medicago sativa cv. Victoria plants were inoculated with E. meliloti 1021, a periplasmic nitrate reductase (Nap) defective mutant, a Nap overexpressing strain and a nitrous oxide reductase defective mutant. Plants were grown in the presence of different nitrate and copper treatments and subjected to flooding during one week before harvesting. N2O production by the nodules was analysed by using gas chromatography. Methyl viologen-dependent nitrate reductase (MV+-NR), nitrite reductase (MV+-NIR) and nitrous oxide reductase (N2OR) enzymatic activities were measured in isolated bacteroids.
Results
Alfalfa root nodules produce N2O in response to nitrate and flooding. Overexpression of Nap improved nodulation competitiveness and induced N2O emissions from nodules. Copper is required for an effective symbiosis as well as triggered a reduction of N2O production due to the induction of the N2OR and a reduction of NIR activities in the bacteroids.
Conclusion
Alfalfa root nodules emit N2O. Nap is involved in nodulation competitiveness and in N2O emissions by the nodules. Bacteroidal N2OR and NIR activities are modulated by Cu and may be considered as effective targets for the mitigation strategies of N2O emissions derived from alfalfa crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrous oxide (N2O) is a potent greenhouse gas and also represents an important ozone layer depleting factor (Ravishankara et al. 2009). According to various studies (Rockström et al. 2009; Steffen et al. 2015) and the last IPCC report (IPCC 2022), agriculture and livestock farming are the main human activities contributing to the increase of the anthropogenic N2O levels in the atmosphere, while fossil fuel combustion, biomass burning and water treatment cause less impact to the environment in terms of N2O emissions. In fact, the non-synchronized application of synthetic nitrogen fertilizers to crops contributes to intensify the biological release of N2O, and has been considered as the main factor causing the remarkably rapid increase of atmospheric N2O concentration occurred over the last century. Therefore, a better understanding of the pathways involved in N2O formation in agricultural soils is essential for the reduction of these emissions to the atmosphere. In this context, several processes involved in N2O formation have been proposed, being nitrification and denitrification the main sources (Butterbach-Bahl et al. 2013; Torres et al. 2016).
Legumes and soil bacteria collectively termed “rhizobia” establish a symbiotic relationship characterized by the formation of new root organs, called nodules, where biological nitrogen fixation takes place (Oldroyd and Downie 2008). Nevertheless, nodulation requires complex chemical and physiological signalling interactions between both partners of the symbiosis (Poole et al. 2018). Either the plant or the endosymbiont are responsible for nodulation competitiveness. In agriculture, microbial interactions are part of a multicomponent equation involving plant genotype, environment and plant and soil microbiomes (Onishchuk et al. 2017). Following this line, genetic features influencing competitiveness are involved in rhizosphere colonization, establishment of an effective symbiosis, or even in plant growth promotion or prevention of the growth of other bacterial cells (reviewed by Mendoza-Suárez et al. 2021). Once nodules are formed, rhizobia differentiate into bacteroids inside the nodules, acquiring the capacity of fixing N2 through nitrogenase activity. However, as nitrogenase is inhibited by oxygen, symbiotic nitrogen fixation (SNF) requires a microaerophilic environment, thus changes in oxygen concentrations from normoxic to microoxic (hypoxic) levels during nodule formation and maturation are required (Rutten and Poole 2019).
Inoculation of legumes with rhizobia has been considered as an environmental-friendly agricultural practice recommended all over the world as part of a strategy for N2O mitigation (Torres et al. 2016). However, it has been reported that legume crops also contribute to N2O production by providing N-rich residues for decomposition (Baggs et al. 2000; Sánchez and Minamisawa 2019), or directly by some free-living or symbiotically-associated rhizobia that are able to denitrify (Bedmar et al. 2005, 2013; Hirayama et al. 2011).
Denitrification is a sequential respiratory process in which nitrate (NO3−) or nitrite (NO2−) is reduced to N2, releasing nitric oxide (NO) and nitrous oxide (N2O) as intermediates, through four steps sequentially catalyzed by a periplasmic (Nap) or a membrane-bound nitrate reductase (Nar), a Cu-containing (NirK) or a cytochrome cd1-containing nitrite reductase (NirS), a c type (cNor), quinol-dependent (qNor) or Cu-containing nitric oxide reductase (CuANor) and a nitrous oxide reductase (Nos). Reviews covering the biochemistry and physiology of denitrification have been published elsewhere (Bueno et al. 2012; Kraft et al. 2011; Richardson 2011; Salas et al. 2021; Torres et al. 2016; van Spanning et al. 2005; 2007). The importance of denitrification in N2O reduction lies in the fact that the Nos enzyme is the only known sink of N2O and, consequently, scientific research has focused on it as a key enzyme in N2O mitigation strategies (Richardson et al. 2009). In this context, it has been reported that inoculation of soybean with N2O-reducing strains of Bradyrhizobium can mitigate N2O emissions (Akiyama et al. 2016; Itakura et al. 2013; Woliy et al. 2019). However, several of the most agronomical interesting rhizobial species do not contain Nos, being unable to reduce N2O to N2 and emitting great levels of N2O as a consequence (Sánchez and Minamisawa 2019; Torres et al. 2016).
N2O emissions from agricultural soils can be influenced by environmental factors such as nitrate or N-derived species, oxygen-limiting conditions, pH or copper concentration among others (Liu et al. 2022; Richardson et al. 2009). With respect to legumes, Tortosa et al. (2015) reported significant increases of N2O emissions from soybean root nodules in response to nitrate and flooding, being the denitrification performed by Bradyrhizobium diazoefficiens bacteroids the main contributor to N2O release. In addition to oxygen and nitrate, another environmental factor that contributes to N2O emissions in soils is copper (Cu) (Liu et al. 2022). In fact, a variety of studies with free-living rhizospheric denitrifying microorganisms have demonstrated that nosZ expression or Nos synthesis and activity decreased when Cu was a limiting nutrient, resulting in notable increases in N2O emissions (Felgate et al. 2012; Pacheco et al. 2022; Sullivan et al. 2013). Recent studies have shown that Cu addition to the plant growth nutrient solution reduced statistically N2O emissions from soybean nodules (Tortosa et al. 2020).
Alfalfa (Medicago sativa L.) is one of the most valuable forage crops and the most productive forage legume in zones with temperate climate (Delgado and Lloveras 2020). This crop does not require nitrogen fertilization, since it is able to fix up to 463 kg of N2 per ha and year, which is mainly used for the synthesis of their own protein. The rest of the nitrogen provided by the nodules is incorporated into the following crops through the remnant plant residues (roots and harvest rests). Because of that reason, alfalfa is also cultivated in crop rotations to improve nitrogen soil enrichment and biomass production of the following crops (Lloveras et al. 2020).
Ensifer meliloti is a rhizobial species which symbiotically associates with plants of the genera Medicago, Melilotus and Trigonella, and possesses all the denitrification genes: napEFDABC, nirK, norECBQD and nosRZDFYLX, encoding Nap, NirK, cNor and Nos, respectively (Torres et al. 2014). Nevertheless, this bacterium has been considered as a partial denitrifier due to its incapacity to grow with nitrate or nitrite as respiratory substrate under anoxic conditions (Bueno et al. 2015; Torres et al. 2011, 2014). In fact, a recent study reported that nap expression was significantly lower respect to the rest of the denitrification genes when E. meliloti cells were incubated anoxically (Torres et al. 2014). This limitation in the induction of nap genes may be responsible of the incapacity of E. meliloti to respire nitrate in an anoxic environment. In fact, overexpression of nap genes recovered the capacity of E. meliloti to grow anaerobically and to produce N2O under free-living conditions (Torres et al. 2018). However, the capacity of the symbiotic forms of E. meliloti to produce N2O has not been explored so far.
Taking in consideration all this background, the aim of this study was to investigate the capacity of alfalfa root nodules to produce N2O in response to nitrate, flooding and Cu availability. The involvement of E. meliloti denitrification in the symbiotic interaction with alfalfa plants and in N2O emissions from the nodules has also been explored.
Materials and methods
Bacterial strains and growth conditions
Bacterial strains used in this work are listed in Table 1. Escherichia coli S17.1 cells were routinely grown in LB (Luria-Bertani) medium (Miller 1972) at 37ºC and 170 rpm, and were used as donors in plasmid pGUS3 conjugative transfer. Antibiotics were added to E. coli cultures at the following concentrations: streptomycin (Sm), 20 µg∙ml− 1; spectinomycin (Spc), 20 µg∙ml− 1; kanamycin (Km), 25 µg∙ml− 1. E. meliloti cells were routinely cultured in TY (Tryptone-Yeast) medium (Beringer 1974) at 30 ºC and 170 rpm. Then, cells were grown microaerobically in minimal medium (Robertsen et al. 1981) supplemented with 10 mM KNO3. To achieve microaerobic conditions, the headspace atmosphere of 100-ml flasks containing 20 ml of growth medium inoculated at an initial OD600 of 0.1–0.15 was replaced by a gas mixture consisting of 2% O2 and 98% N2. Antibiotics were added to E. meliloti cultures at the following concentrations: Sm, 200 µg∙ml− 1; Km, 200 µg∙ml− 1. For competitivity assays, X-Gluc (5-bromo-4-chloro-3-indolyl β-D-glucuronide) was added to the E. meliloti cultures at a final concentration of 50 µg∙ml− 1.
Plant growth conditions
Alfalfa (Medicago sativa, cv. Victoria) seeds were surface-sterilized by immersion in 2.5% HgCl2 for 9 min. Then, seeds were washed with sterile distilled water and germinated on filter paper discs in Petri dishes in darkness for 2–3 days at 30 ºC. Alfalfa plants were grown using a modified Rigaud and Puppo nutrient solution (1975) (referred as NS throughout the manuscript): K2SO4, 174 mg∙l− 1; KH2PO4, 68 mg∙l− 1; K2HPO4, 44 mg∙l− 1; MgSO4∙7H2O, 123 mg∙l− 1; H3BO3, 2.85 mg∙l− 1; ZnSO4∙7H2O, 0.55 mg∙l− 1; MnSO4∙4H2O, 3.07 mg∙l− 1; Na2MoO4∙2H2O, 0.11 mg∙l− 1; CaSO4∙2H2O, 150 mg∙l− 1; Sequestrene® 138 G100 (6.2 mg chelated Fe), 25 mg∙l− 1. The NS was supplemented with 1, 2, 3, 4 or 10 mM KNO3. The standard Cu concentration of NS was 0.2 mg∙l− 1 (0.8 µM). For studies of the effect of Cu on symbiosis, NS was supplemented with a higher Cu concentration of 5 mg∙l− 1 (20 µM), used in previous studies from our group (Tortosa et al. 2020). Plants in tubes or pots were placed into growth chambers from the Greenhouse and Growth Chamber Service (GGCS) (EEZ, Granada, Spain) with the following parameters: night/day temperature, 24/20 ºC; photoperiod, 16/8 h; photosynthesis photon flux density of 403 µmol photons∙m− 2∙s− 1.
Plant experimental setting
For nodulation kinetics assays, a methodology described by Torres et al. (2013) was used. Basically, germinated seeds were transferred into 43-ml autoclaved glass tubes containing 10 ml water and kept in darkness for approximately 24 h. Then, these tubes were placed into the growth chamber and, after 5 days, water was replaced with 10 ml of NS containing a cell suspension of approximately 108 CFU∙ml− 1. NS was supplemented with 1, 2, 3 or 4 mM KNO3. Anoxic conditions were achieved by sparging NS with N2 gas before adding the inoculum. Finally, tubes were incubated for 30 days, and nodule number was daily counted.
For competitivity assays, seeds were germinated as for nodulation kinetics experiments. After 5 days, water was replaced with 10 ml of NS supplemented with 3 mM KNO3 and seeds were inoculated individually with strains 4002, 4004, 4002-GUS3, or 4004-GUS3 as controls. To determine the competence for nodulation a mix in 1:1 proportion of 4004 and 4002-GUS3 or 4004-GUS3 and 4002 was used at a cellular density of approximately 105 CFU∙ml− 1. Tubes were incubated for 30 days in the controlled environmental chamber with the parameters enumerated above and nodules were revealed with X-Gluc (0.53 mg∙ml− 1) according to Nogales et al. (2006). Nodules produced by 4004 or 4002 strains were revealed after isolation of bacteria from the nodules in plates containing Km and, therefore, carrying the pDS4004 or pDS4002 plasmids.
For plant assays in pots, germinated seeds were transferred into 250-ml autoclaved pots filled with perlite as substrate. The following experiments were carried out (Supplementary Fig. S1):
Experiment 1 (Supplementary Fig. S1A): in order to study the influence of nitrate and flooding in N2O emissions from alfalfa nodules, these pots were placed on 1-l glass jars containing 500 ml of N-free NS. Eight seedlings per pot were inoculated at sowing with a cell suspension of the WT 4004 strain of about 108 CFU∙ml− 1. Three sets were established: the first set, with 20 pots, was watered with N-free NS, and the second and third sets, with 10 pots each, were watered with NS supplemented with 1 mM or 3 mM KNO3, respectively. Plants were watered every two weeks under sterile conditions. Seven days before harvesting (i.e., after 43 days), a nitrate shock of 10 mM KNO3 was applied to 10 pots from the first set. Additionally, at the same time, half of each set was also subjected to flooding conditions, which were achieved by removing alfalfa plants from the pots and transferring them into a glass jar filled with 900 ml NS, thus nodulated roots were completely submerged. After 50-days growth, plants and nodules were harvested.
Experiment 2 (Supplementary Fig. S1B): to investigate the role of Nap in N2O emissions from alfalfa nodules, pots were prepared as described above, and 10 pots with 8 seedlings each were inoculated at sowing with a cell suspension of about 108 CFU∙ml− 1 of the WT 4004, the nap+ strain, or the napA mutant strain (denoted as nap− throughout the manuscript). Plants were grown for 50 days, and watered every two weeks under sterile conditions. Seven days before harvesting, the pots were watered with NS supplemented with 10 mM KNO3 and subjected to flooding conditions as indicated above. After plant growth, the nodules harvested from 5 pots from each treatment were immediately used for N2O emission measurements, whereas the nodules harvested from the remaining 5 pots from each treatment were frozen in liquid nitrogen and stored at -80 ºC for further determinations.
Experiment 3 (Supplementary Fig. S1C): this experiment was performed to study the involvement of Cu in plant and nodule physiology as well as in N2O emissions from alfalfa nodules. For this goal, pots were placed on glass jars containing 500 ml of N-free NS. Eight seedlings per pot were inoculated at sowing with the WT 1021 or the nosZ mutant strain at a cellular density of about 108 CFU∙ml− 1. For pots inoculated with the WT 1021 strain, three different sets of 10 pots each were established: the first set was watered with NS without Cu added, the second set, with NS supplemented with 0.8 µM CuSO4∙5H2O, and the third set, with NS containing 20 µM CuSO4∙5H2O. The 5 pots inoculated with the nosZ mutant were watered only with 0.8 µM Cu NS. Plants were grown for 43 days and plants and nodules from 5 pots from each set inoculated with the WT were harvested. To induce N2O production, the remaining 5 pots from each WT set were treated with 10 mM KNO3 and subjected to flooding for 7 days. Then, nodules were harvested and N2O emissions from detached nodules were measured.
Plant physiological analyses
Physiological data such as nodule number (NN), nodule fresh weight (NFW), shoot dry weight (SDW) and plant dry weight (PDW) were recorded and expressed per plant. SDW and PDW were determined after 3 days in an oven at 70 ºC.
Prior to analytical determinations (nitrogen and copper content), dry shoots and roots were ground using an IKA A11 mill to less than 0.5 mm according to Tortosa et al. (2020). Seeds and nodules were dried in an oven at 70 ºC for only one day.
Nitrogen content was analysed in dried and ground shoots of alfalfa plants by the N/C Analysis Service of Estación Experimental del Zaidín (EEZ, Granada, Spain) by using an elemental analyser LECO TruSpec® CN (LECO, St Joseph, MI, USA). Briefly, the sample was subjected to complete combustion at 950 ºC in the presence of O2. Then, all the nitrogen oxides formed were converted into N2, and this gas was detected by a thermal conductivity detector. Data were expressed as mg N∙g− 1 of dry sample.
Cu concentration was analysed in dry alfalfa seeds before germination, dry nodules as well as in dry and ground roots and shoots using the Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) available at the Instrumental Technical Service of EEZ (Granada, Spain), model PlasmaQuant® PQ 9000 (Analytik Jena, Jena, Germany). Data were expressed as mg Cu∙kg− 1 of dry sample.
Determination of leghemoglobin content in nodules
Leghemoglobin (Lb) concentration in nodules was determined by fluorimetry according to a method described by Tortosa et al. (2020), which was based on the standard method established by LaRue and Child (1979), but was adapted to alfalfa nodules in the present work. Briefly, 0.125–0.13 g of NFW were crushed and homogenised with a pestle in a cold porcelain mortar by adding 6 ml of buffer solution (50 mM Na2HPO4∙2H2O/NaH2PO4∙H2O, pH 7.4; 0.02% w/v K3Fe(CN)6; 0.1% w/v NaHCO3) and 0.1 g of polyvinyl polypyrrolidone (PVPP). The extract was centrifuged at 12,000x g at 4 ºC for 20 min and 200 µl from the supernatant were transferred into glass tubes containing 3.15 ml of a saturated calcium oxalate solution (66 g∙l− 1) and subsequently autoclaved at 120 ºC during 30 min. Then, samples were cooled down and measured in a Shimadzu spectrofluorometer (Shimadzu Scientific Instruments, Kyoto, Japan), setting λ = 405 and 600 nm as excitation and emission wavelengths, respectively, and compared to non-autoclaved samples as control. Lb concentrations were expressed as mg Lb∙(g NFW)−1 and obtained after extrapolation of the data using a human hemoglobin standard curve built from a stock of 300 mg∙l− 1 and including the following concentrations (mg∙l− 1): 0, 60, 120, 180, 240 and 300.
Nitrous oxide determinations
Detection of N2O emissions was performed according to Tortosa et al. (2020), including certain modifications for alfalfa detached nodules. Briefly, harvested nodules from the same pot (0.2–0.3 g) were immediately transferred to a 10-ml glass vial (SUPELCO®) and a volume of 1 ml or 100 µl NS (with the corresponding nitrate and Cu concentration) was added depending if nodules were isolated from flooded or non-flooded plants, respectively. The vials containing nodules were incubated at 30 ºC. N2O was detected by an HP 4890 gas chromatography instrument provided with an electron capture detector (ECD) as essentially described by Torres et al. (2014). The column was packed with Porapak Q 80/100 mesh. N2 was used as the carrier gas at a flow rate of 23 ml∙min− 1. The injector, column and detector temperatures were 125, 60 and 375 °C, respectively. Gas samples were taken from the headspace of the vials after 3 and 6 h incubation and injected manually by using luer-lock gas-tight syringes BD Microlance™ 3. Peaks corresponding to N2O were integrated by using GC ChemStation Software (Agilent Technologies, Santa Clara, CA, USA) and the values obtained were used to calculate N2O concentration in each sample by extrapolation from a standard curve, performed by using 2% (v/v) N2O standard (Air Liquid, Paris, France) and including the following gas volumes: 0, 0.2, 0.4, 0.6, 0.8 and 1 ml. Total N2O concentration was determined by taking into account both N2O in headspace, and dissolved N2O applying Bunsen solubility coefficient (47.2% at 30 °C). N2O fluxes from alfalfa detached nodules were expressed as nmol N2O∙(g NFW∙h)−1.
Bacteroids isolation
For bacteroids isolation, a method described by Mesa et al. (2004) was used. Basically, bacteroids were isolated by homogenizing 0.25 g of alfalfa nodules with a pestle in a cold porcelain mortar with 7.5 ml of extraction buffer (45.5 g∙l− 1 D-mannitol dissolved in 50 mM Tris-HCl, pH 7.5) previously added. Then, the extract obtained was filtered through a sterile cheesecloth filter and centrifuged at 250x g at 4 ºC for 5 min to remove nodule debris. Subsequently, the supernatant was centrifuged at 12,000x g at 4 ºC during 10 min and pellets were washed twice and resuspended in 0.5 ml of wash buffer (50 mM Tris-HC l, pH 7.5) prior to biochemical determinations.
Protein and nitrite determinations
Total protein concentration was estimated colorimetrically after alkaline lysis (1 N NaOH at 100 °C during 20 min) by using the Bradford reagent (Bio-Rad, Hercules, CA, USA) and extrapolating from a standard curve including 0, 4, 8, 12, 16 and 20 µg∙ml− 1 of bovine serum albumin (BSA) from a stock solution of 100 µg∙ml− 1 (Bradford 1976).
The nitrite concentration present in the bacteroids extract was estimated colorimetrically after diazotisation by adding the sulphanilamide/naphtylethylene diamino dihydrochloride reagent (Hageman and Hucklesby 1971) and extrapolating from a standard curve including 0, 20, 40, 60, 80 and 100 µM NaNO2 from a stock solution of 100 µM.
Determination of nitrate reductase (NR, EC 1.7.99.4), nitrite reductase (NIR, EC 1.7.2.1) and nitrous oxide reductase (N2OR, EC 1.7.2.4) activities
Methyl viologen (MV+)-dependent nitrate reductase (MV+-NR) and nitrite reductase (MV+-NIR) activities were determined as essentially described by Delgado et al. (2003). The reaction mixtures contained 200 µM methyl viologen, 20–30 µg protein from the cell suspension, 50 µl distilled water, and 10 mM KNO3 for MV+-NR or 100 µM NaNO2 for MV+-NIR assays, adding 50 mM Tris-HCl buffer up to reach a final volume of 450 µl in each reaction tube. Before measurements, a 46 mM sodium dithionite solution was prepared freshly (8 mg∙ml− 1 in 50 mM Tris-HCl buffer, pH 7.5), transferring 50 µl from it to each reaction tube. After incubation for 20–30 min at 30 °C, the reaction was stopped by vigorous shaking until disappearance of blue color from the samples. Control tubes were prepared as the reaction tubes, but these tubes were shaken vigorously immediately after the addition of dithionite. MV+-NR activity was expressed as nmol NO2− produced∙(mg protein∙min)−1. MV+-NIR activity was expressed as nmol NO2− consumed∙(mg protein∙min)−1. Three biological replicates from at least three independent experiments for each treatment were assayed.
Nitrous oxide reductase (N2OR) activity was measured as essentially described by Tortosa et al. (2020), setting some modifications for alfalfa nodules. The assay was performed in 10-ml glass SUPELCO® vials, adding 0.15–0.2 mg protein and 60 mM sodium succinate as electron donor. Then, a mixture of 2% (v/v) N2O and 98% (v/v) N2 (Air Liquid) was injected to reach a final concentration of 0.1% (v/v). To achieve anoxic conditions, the vials were gassed with N2 during 7 min. All the vials were incubated at 30 °C for 1 h. Next, 0.5-ml aliquots were taken from the headspace of each vial. N2O measurements and concentration calculations were performed as described above. N2OR activity was expressed as nmol N2O consumed∙ (mg protein∙h)−1. Three biological replicates from at least three independent experiments for each Cu condition were used.
Statistical analysis
Data were checked for normal distribution according to Kolmogorov-Smirnov and Shapiro-Wilk tests. For data obtained from plant assays in tubes, inferential statistics were performed by applying parametric ANOVA and a post-hoc Tukey HSD test at p ≤ 0.05 with SPSS software. For data obtained from plant assays in pots, inferential statistics to test null hypothesis were performed by applying non-parametric Kruskal-Wallis test for more than two unpaired treatments. Next, a post-hoc U Mann-Whitney test at p ≤ 0.05 with SPSS software was performed.
Results
Periplasmic nitrate reductase has a role in infectivity and competitiveness for nodulation in alfalfa
As a preliminary experiment, we investigated the effect of nitrate on nodulation capacity of E. meliloti by sowing alfalfa seeds in glass tubes and inoculating them with E. meliloti 4004. Plants were grown during 30 days without nitrate or with different nitrate concentrations, ranging from 1mM to 4 mM (Supplementary Fig. S2). In these experiments, nodulation was significantly diminished by 4 mM nitrate, while no differences were observed for the rest of nitrate concentrations (Supplementary Fig. S2).
Next, we were interested in elucidating whether nodulation capacity of E. meliloti was influenced by nap expression. To achieve this goal, seeds were sown in glass tubes with NS supplemented with 3 mM KNO3, and were inoculated with the nap+ strain or the strain 4004 (WT). Before inoculation, NS was subjected or not to anoxic conditions, as described in material and methods. As shown in Fig. 1a, similar nodulation capacity between the WT or nap+ strains inoculated under normal conditions was observed, counting 6 nodules per plant, approximately, after 30 days of plant growth regardless of the strain (p > 0.05). However, the nap+ strain showed a significantly major efficiency for nodulation than the WT under anoxic conditions, counting 8 and 6 nodules per plant, respectively, at the end of the experiment (p < 0.05) (Fig. 1a). These results indicated that nap overexpression might promote nodulation when roots are developed in a hypoxic environment.
Nodulation capacity of a nap overexpressing E. meliloti strain (nap+). a Nodulation kinetics of alfalfa plants inoculated with strain 4004 (wild-type, WT, circles) or the nap + strain 4002 (squares) and grown during 30 days with nutrient solution supplemented with 3 mM KNO3. Half of the tubes containing nutrient solution were sparged with N2 gas during 10 min before inoculation (anoxic conditions, white symbols) and the other half were not fluxed with N2 (oxic conditions, black symbols) b Nodule competition assays. Data represent the percentage of nodules occupied by the nap+, WT-GUS3, nap+-GUS3 or WT strains inoculated separately as control of the experiments or after co-inoculation (ratio 1:1) under anoxic conditions. In a and b, data represent means with standard error bars using a Tukey HSD test at p ≤ 0.05 from three independent experiments assayed by using ten plant replicates
The next step was to analyse the competitiveness of the nap+ strain for nodulation. We performed experiments with alfalfa plants grown in glass tubes with 3 mM nitrate and inoculated with a mixture (1:1 ratio) of the WT 4004-GUS3 and the nap+ strain (4002), or the WT 4004 and the nap+-GUS3 strain. Plants inoculated only with the WT or the nap+ strain (harbouring pGUS3 or not) were used as control of the experiments. Additionally, stability of pGUS3, pDS4002 or pDS4004 was checked in plates as described in Material and Methods. As shown in Fig. 1b, the nap+ strain produced 59% of the total number of nodules, while the WT 4004-GUS3 generated the remaining 41%. Moreover, the nap+-GUS3 strain produced 61% of the total number of nodules, while the WT 4004 elicited the remaining 39%. Therefore, these results confirm the previous results on nodulation kinetics (Fig. 1a) and suggest that E. meliloti nap overexpression improves competitiveness for nodulation of alfalfa plants.
Periplasmic nitrate reductase is involved in N2O emissions from alfalfa root nodules
To investigate the capacity of alfalfa root nodules to produce N2O, seeds were inoculated with E. meliloti 4004 and N2O emissions were measured after growing the plants in pots containing NS with different nitrate concentrations. As shown in Fig. 2, N2O emissions were not detected in nodules from plants grown without nitrate independently of the application of flooding conditions or not. A weak induction of N2O production was observed in non-flooded nodules and in the presence of 1 mM nitrate in the growth medium. The addition of 3 mM to NS or the treatment of the plants with 10 mM nitrate 7 days before harvesting slightly induced N2O release from non-flooded nodules compared to 1 mM treatment (Fig. 2). Interestingly, flooding triggered a significant increase in N2O emissions from nodules of plants subjected to 1 mM, 3 mM or 7-day 10 mM nitrate, compared to non-flooded nodules (Fig. 2). It is worth mentioning that no significant differences in N2O emission levels under flooding conditions were found between 1 and 3 mM nitrate for 50 days, and between 3 mM and the application of a nitrate shock of 10 mM, 7 days before harvesting. However, N2O levels from flooded nodules of 7-day treated plants with 10 mM nitrate were significantly higher compared to those from flooded plants grown in the presence of 1 mM (p < 0.05; Fig. 2).
Nitrous oxide emissions from detached nodules elicited by E. meliloti 4004. Alfalfa plants were grown without nitrate (0 mM), with 1 or 3 mM KNO3 during 50 days, or treated with 10 mM KNO3 7 days before harvesting. Flooding conditions (black bars) were applied or not (white bars) during one week before harvesting. Data represent means with standard error bars from three independent experiments assayed by using ten pot replicates containing 8 plants each. Lower-case letters indicate comparisons between plants subjected to flooding and nitrate treatments (1 or 3 mM KNO3 for 50 days or 10 mM KNO3 for 7 days). Same lower-case letters are not statistically significant according to U Mann-Whitney test at p ≤ 0.05. NFW, nodule fresh weight
As shown in Table 2, NFW per plant significantly decreased when plants were grown with 1 or 3 mM KNO3 for 50 days in comparison to those plants treated with 10 mM KNO3 during 7 days before harvesting. However, nodule number (NN) per plant was not affected by any nitrate treatment. It is also important to mention that the application of 1 or 3 mM nitrate during 50 days as well as 10 mM nitrate treatment for 7 days caused a major impact on leghemoglobin content in nodules obtaining a decrease of 1.9, 1.7 and 1.5-fold respectively, compared to that observed in nodules from plants grown without nitrate. With respect to PDW per plant, the treatment of 3 mM nitrate during 50 days increased PDW significantly compared to plants grown without nitrate or with 1 mM nitrate or treated with 10 mM nitrate, where no differences were observed. These results indicate that the increase in PDW of plants grown with 3 mM is possibly due to the nitrogen uptake by plant roots rather than the SNF, since nodule growth and physiology were severely affected under these conditions compared to those grown without nitrate (Table 2).
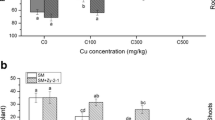
To investigate the involvement of Nap in N2O emissions, alfalfa plants were grown in pots and, a week before harvesting, they were subjected to 10 mM KNO3 and flooding conditions, since these were the conditions where the highest N2O emission levels were found and nodule biomass was not affected (Fig. 2, Table 2). Plants were inoculated with the WT strain 4004, the nap+, or the nap− strain (Table 1, Supplementary Fig. S1B). As shown in Fig. 3a, MV+-NR activity from bacteroids of the WT strain was 2.4-fold higher compared to that from nap− bacteroids. Inoculation of the plants with the nap+ strain induced about 1.8-fold MV+-NR activity of the bacteroids compared to those from plants inoculated with the WT strain. When N2O emissions from the nodules were analysed, N2O levels decreased 3.5-fold in the nodules produced by the nap mutant compared to the WT nodules. Interestingly, inoculation of the plants with the nap+ strain resulted in a large increase of N2O emissions (about 6.7-fold) from these nodules compared to those from plants inoculated with the WT strain (Fig. 3b). Collectively, these results indicate that E. meliloti Nap is clearly involved in N2O emissions from alfalfa nodules.
a Methyl viologen-dependent (MV+) nitrate reductase (MV+-NR) activity from bacteroids isolated from nodules elicited by E. meliloti 4004 (WT), the napA::Ω mutant (nap-) or the nap + strain. b N2O emissions from the nodules. In a and b, plants were subjected to flooding and 10 mM KNO3, both applied 7 days before harvesting. Data represent means with standard error bars using a Tukey HSD test at p ≤ 0.05 assayed by using three biological replicates from three independent experiments. NFW, nodule fresh weight
Copper modulates N2O emissions from alfalfa nodules and bacteroidal nitrous oxide reductase activity
In order to investigate the effect of Cu on the alfalfa-E. meliloti symbiotic interaction, plants were grown during 43 days in pots containing NS without nitrate and without Cu2+ addition (0 µM) or supplemented with 0.8 µM or 20 µM Cu2+ (Table 3). Supplementary Fig. S3C shows the experimental setting for these experiments.
As shown in Table 3, NFW, SDW, PDW and N content significantly decreased in plants grown without Cu2+ added compared to those grown in the presence of Cu2+. No differences in those parameters were found between plants grown with 0.8 or 20 µM Cu2+. Moreover, plants grown without Cu2+ added displayed a pale green tone in their leaves, while they were dark green in the other treatments (Supplementary Fig. S3). These results indicate a negative effect of Cu-limitation on alfalfa-E. meliloti SNF. Leghemoglobin values support this idea, since nodules from plants grown without Cu2+ contained 1.8-fold less leghemoglobin than those from plants grown with 0.8 µM Cu2+ (Table 3). As observed in Table 3, Cu concentration was higher in roots and nodules comparing to shoots, especially in plants grown with 20 µM Cu2+, where we found 5.5-fold and 7.6-fold more Cu in nodules and roots, respectively, compared to shoots. According to these results, Cu may be primarily accumulated in the roots, and only a minimal proportion would be transferred to shoot and leaves. Similar results were obtained by using E. meliloti 2011 as WT (data not shown).
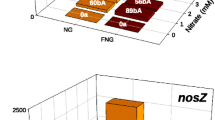
In order to elucidate the contribution of Nos to N2O emissions from alfalfa nodules, bacteroids were isolated from nodules elicited by the WT 1021 or a nosZ mutant. These nodules were collected from plants grown for 50 days and subjected to 10 mM nitrate and flooding during 7 days before harvesting (Supplementary Fig. S1C). Bacteroids from the nosZ mutant showed 5.9-fold less N2OR activity than bacteroids from the WT strain. On the contrary, N2O emission rates by nosZ − nodules were 5.3-fold higher compared to those by nodules from plants inoculated with the WT strain (Table 4). Similar results were obtained by using E. meliloti 2011 as WT (data not shown). These results demonstrate the involvement of Nos in N2O reduction in alfalfa nodules. Finally, another set of alfalfa pots was inoculated with the WT strain and grown without Cu added (0 µM), or in the presence of 0.8 µM or 20 µM Cu2+. Seven days before harvesting (i. e., after 43 days), plants were treated with 10 mM KNO3 and subjected to flooding in order to induce N2O emissions (Supplementary Fig. S1C). As shown in Table 4, Cu2+ accumulation in nodules was correlated with the Cu concentration added to NS. Moreover, while MV+-NR activity from the bacteroids was not significantly influenced by Cu availability, MV+-NIR activity was significantly reduced in bacteroids from 0 or 20 µM Cu2+ treatments comparing with 0.8 µM Cu2+. N2OR activity increased in parallel with the Cu concentration provided. Conversely, N2O emission rate decreased with Cu concentration. These results suggest that environmental Cu concentration plays an essential role in modulating bacteroidal NIR and N2OR activities and consequently in the decrease of N2O emissions by alfalfa nodules.
Discussion
E. meliloti is unable to grow under free-living anaerobic conditions with nitrate as sole electron acceptor. This incapacity is due to the very low expression of nap genes compared to nirK, nor and nos denitrification genes (Torres et al. 2014). In fact, an overexpressing nap mutant (nap+) recovered the ability to grow anaerobically with nitrate as well as the capacity to produce N2O (Torres et al. 2018). In this context, it would be appealing to explore if the capacity to grow anoxically from nitrate respiration confers to E. meliloti an advantage to infect and nodulate alfalfa roots under anoxic conditions, as this question has not been yet addressed for endosymbionts. Our results demonstrate that overexpression of Nap increases the fitness of E. meliloti-alfalfa symbiotic interaction, since plants inoculated with the nap+ strain showed a higher capacity for nodulation than those inoculated with the WT under low oxygen conditions. Similar to our observations, Lecomte et al. (2021) also found that nap genes play an important role in root colonization efficiency of the plant-associated microorganism Agrobacterium fabrum. Supporting our findings, previous studies demonstrated that B. diazoefficiens nirK or norC mutants showed a reduced ability for nodulation in soybean plants grown with nitrate (Mesa et al. 2004). These authors proposed that denitrification enzymes played a role in nodule formation rather than in nodule function. On the contrary, no significant differences in competitiveness for nodulation were observed between WT or a napA E. meliloti mutant in M. truncatula, suggesting that Nap is not involved in the early steps of the interaction (Ruiz et al. 2022). The apparent discrepancy between both sets of results can be explained by the difference in the strains used. While in Ruiz et al. (2022), a mutant lacking nap was used, in our work we have used a strain overexpressing nap that confers to E. meliloti the ability to grow under anoxic conditions by nitrate respiration in contrast to the WT, which is not able to respire nitrate anoxically. Another important difference between our work and Ruiz et al. (2022) results is that in our experiments nutrient solution was fluxed with N2 for 10 min before inoculation in order to provoke low oxygen conditions during the first steps of infection. Our results do not invalidate those obtained by Ruiz et al. (2022), since we do not suggest that nap is important for nodulation, but it might help when nap is overexpressed and roots are subjected to low oxygen conditions during the first steps of the interaction.
In soybean plants, it has been reported that 4 mM nitrate and flooding conditions induce N2O emissions from nodules and that the bacteroidal denitrification is the main process involved (Tortosa et al. 2015). Since E. meliloti lacks the ability to grow and produce N2O under free-living anoxic conditions, we were interested in investigating the capacity of E. meliloti to produce N2O under symbiotic conditions as well as the involvement of bacteroidal denitrification. To achieve this goal, we cultivated alfalfa plants in the presence of nitrate. In contrast to soybeans, which are very tolerant to nitrate, 4 mM nitrate is excessive for alfalfa plants, since the number of nodules elicited by E. meliloti was strongly diminished under this concentration. Even more, although 1 or 3 mM nitrate present in the growth NS did not affect nodule number, these nitrate levels caused the reduction of nodule biomass. In order to investigate the effect of nitrate in N2O emissions, we have selected a 7-day 10 mM treatment together with flooding, since this nitrate treatment did not affect nodule growth and had a small effect on nodule functionality. This parameter was determined by analysing leghemoglobin content of the nodules, which is directly related with their capacity to fix N2. While 7-day 10 mM nitrate treatment provoked a slight decrease in Lb content compared to non-nitrate treatment (7.38 mg versus 10.9 mg), addition of 1 or 3 mM nitrate to the nutrient solution during the entire growth period (50 days) severely diminished Lb content (around 6 mg) indicating a more severe effect on nodule fitness. Nitrate and flooding drastically induced N2O emissions, as it was previously reported in soybeans (Tortosa et al. 2015). Regarding the involvement of Nap in N2O release from the nodules, we found a correlation between MV+-NR activity and N2O emissions either in the nap− or the nap+ strains, indicating that Nap has an important role on N2O released from alfalfa nodules in response to nitrate and flooding. In this context, Brambilla et al. (2020) isolated novel E. meliloti strains which produced lower N2O emissions comparing to the model strain 1021 or the commercial strain B399, and reported that all these isolates harboured spontaneous mutations in napC gene, which encodes NapC, a c-type cytochrome required for Nap activity. Our results complement previous studies where the involvement of bacteroidal nitrate reduction in NO synthesis in Medicago truncatula nodules was reported (Horchani et al. 2011).
Cu is an important micronutrient involved in many physiological plant processes (Nagajyoti et al. 2010; Yruela 2009). In the present work, we have shown the negative effect of Cu limitation in symbiotic nitrogen fixation and a consequent incapacity of the nodules to provide all the N demands required by the alfalfa plant. In fact, non-Cu treated plants displayed a pale green tone in shoot and leaves indicating that Cu-limitation would affect chlorophyll synthesis causing a drastic diminution in plant biomass and chlorosis in leaves. Similarly, in a recent study, Printz et al. (2016) reported leaf chlorosis and a lower leaf density in alfalfa plants inoculated with a commercial peat-based inoculant and grown with 3 and 30 nM Cu. Therefore, the present work highlights that an adequate Cu supply is essential for a proper SNF. With respect to Cu accumulation, it is known that the interaction of Cu with amino and carboxyl ligands reduces its translocation to the shoots (Nikolaevna et al. 2016). Supporting this assertion, Printz et al. (2016) found that Cu accumulation in roots was 325-fold higher in the presence of 10 µM Cu comparing to 3 nM Cu (i.e., the highest and the lowest Cu concentrations assayed in that study), whereas this ratio only reached 50- and 22-fold in stems and leaves, respectively. Our results are coherent with these observations, since shoots displayed a significantly lower Cu concentration in both Cu treatments, 0.8 and 20 µM Cu2+, comparing to roots and nodules. In a recent study, Tortosa et al. (2020) demonstrated that 20 µM Cu2+ treatment was the maximal Cu concentration that soybean plants could bear before suffering Cu stress. Our studies in alfalfa support this idea, since SNF was not affected by 20 µM.
Lastly, NR, NIR and N2OR activities were analysed in bacteroids from nodules of alfalfa plants grown in the presence of different Cu levels. Since Nap was involved in N2O emissions from alfalfa nodules, as discussed above, the influence of Cu on its activity and on the following denitrification enzyme, NirK, was interesting to be explored. Nevertheless, the results obtained showed that MV+-NR activity was not significantly influenced by Cu at the concentration range assayed in the present work. Similarly, previous studies showed that NR activity of B. diazoefficiens bacteroids was not affected by 20 µM Cu2+ (Tortosa et al. 2020). The notable decrease in MV+-NIR activity observed in bacteroids under 0 µM Cu2+ comparing with 0.8 µM Cu2+ highlights that NirK is a Cu-dependent enzyme. Furthermore, MV+-NIR activity was also significantly diminished in the presence of 20 µM Cu2+. In a previous work, Tortosa et al. (2020) found a significant reduction in this activity when soybean bacteroids were subjected to 10, 20 40 and 60 µM Cu. Therefore, a Cu concentration of 20 µM affects NirK function either in bacteroids from soybean or alfalfa nodules. In B. diazoefficiens, NirK requires a cytochrome c550 (CycA) as an essential electron donor for its activity (Bueno et al. 2008). In this context, it has been reported that biogenesis of cytochromes c can be blocked by an excess of Cu (Durand et al. 2015). Thus, the sensitivity of NirK to high Cu levels in soybean or alfalfa nodules may be due to a negative effect on the periplasmic cytochrome c550 (CycA) biogenesis. The contribution of Nos to N2O reduction in alfalfa nodules has also been demonstrated, since N2OR activity was significantly higher in WT bacteroids comparing to nosZ mutant bacteroids. On the contrary, N2O emissions were notably reduced in the WT nodules compared to the levels obtained in the nosZ − nodules. Similar results were reported by Tortosa et al. (2015) for soybean nodules, highlighting the role of Nos as the sole enzyme able to reduce N2O to N2 and confirming the role of Nos as a key enzyme in N2O mitigation strategies. In this context, it has been proposed that Cu concentration in agricultural soils may importantly affect N2O emissions from microbial processes, especially denitrification and nitrification (Li et al. 2019). Furthermore, Tortosa and colleagues (2020) demonstrated that the decrease in N2O emissions was concomitant with the increase in the Cu concentration added to NS, and concluded that Cu was a relevant factor involved in N2O reduction in soybean nodules. Following the same line, the results displayed in the present work suggest that Cu bioavailability significantly induces N2OR activity and, by extension, reduces N2O emissions from nodules elicited by E. meliloti.
Taken together, the results from this work suggest a controversial advantage of nap overexpression, since it improves nodulation capacity under oxygen-limiting conditions, but contributes to N2O emissions at the same time. In this regard, a strategy for an effective E. meliloti-alfalfa symbiotic interaction and N2O mitigation from legume crops might be the selection of inoculants with an adequate Nap expression and high N2OR activity. In this context, it has been recently shown that N2O emissions from soybean crops can be reduced at the field scale by inoculation with a mixed culture of indigenous strains of B. diazoefficiens isolated from agricultural fields that show high N2OR activity levels (Akiyama et al. 2016).
Conclusion
The present work reports for the first time the capacity of alfalfa nodules to emit N2O in response to nitrate, flooding and copper limitation. Furthermore, the involvement of E. meliloti Nap in the competence for nodulation and infectivity effectiveness, as well as in N2O emissions from alfalfa nodules has also been demonstrated. Finally, we also report the capacity of Cu to modulate bacteroidal NIR and N2OR activity and consequently N2O emissions from alfalfa nodules.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Akiyama H, Hoshino YT, Itakura M, Shimomura Y, Wang Y, Yamamoto A, Tago K, Nakajima Y, Minamisawa K, Hayatsu M (2016) Mitigation of soil N2O emission by inoculation with a mixed culture of indigenous Bradyrhizobium diazoefficiens Sci Rep 6:32869. https://doi.org/10.1038/srep32869
Baggs EM, Rees RM, Smith KA, Vinten AJA (2000) Nitrous oxide emission from soils after incorporating crop residues. Soil Use Manag 16(2):82–87. https://doi.org/10.1111/j.1475-2743.2000.tb00179.x
Bedmar EJ, Bueno E, Correa D, Torres MJ, Delgado MJ, Mesa S (2013) Ecology of denitrification in soils and plant-associated bacteria. In: Rodelas B, Gonzalez-López J (eds) Beneficial plant-microbial interactions: Ecology and applications, 1st edn. CRC Press, Florida, pp 164–182. https://doi.org/10.1201/b15251
Bedmar EJ, Robles EF, Delgado MJ (2005) The complete denitrification pathway of the symbiotic, nitrogen-fixing bacterium Bradyrhizobium japonicum Biochem Soc Trans 33:141–144. https://doi.org/10.1042/BST0330141
Beringer JE (1974) R factor transfer in Rhizobium leguminosarum J Gen Microbiol 84(1):188–198. https://doi.org/10.1099/00221287-84-1-188
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Brambilla S, Soto G, Odorizzi A, Arolfo V, McCormick W, Primo E, Giordano W, Jozefkowicz C, Ayub N (2020) Spontaneous mutations in the Nitrate reductase gene napC drive the emergence of eco-friendly Low-N2O-Emitting Alfalfa Rhizobia in regions with different climates. Microb Ecol 79(4):1044–1053. https://doi.org/10.1007/s00248-019-01473-w
Bueno E, Bedmar EJ, Richardson DJ, Delgado MJ (2008) Role of Bradyrhizobium japonicum cytochrome c550 in nitrate and nitrite respiration. FEMS Microbiol Lett 279:188–194. https://doi.org/10.1111/j.1574-6968.2007.01034.x
Bueno E, Mania D, Frostegård Ã, Bedmar EJ, Bakken LR, Delgado MJ (2015) Anoxic growth of Ensifer meliloti 1021 by N2O-reduction, a potential mitigation strategy. Front Microbiol 6:537. https://doi.org/10.3389/fmicb.2015.00537
Bueno E, Mesa S, Bedmar EJ, Richardson DJ, Delgado MJ (2012) Bacterial adaptation of respiration from oxic to microoxic and anoxic conditions: redox control. Antioxid Redox Signal 16(8):819–852. https://doi.org/10.1089/ars.2011.4051
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci 27:368. https://doi.org/10.1098/rstb.2013.0122
Casse F, Boucher C, Julliot JS, Michel M, Dénarié J (1979) Identification and characterization of large plasmids in Rhizobium meliloti using agarose gel electrophoresis. J Gen Microbiol 113(2):229–242. https://doi.org/10.1099/00221287-113-2-229
Delgado MJ, Bonnard N, Tresierra-Ayala A, Bedmar EJ, Müller P (2003) The Bradyrhizobium japonicum napEDABC genes encoding the periplasmic nitrate reductase are essential for nitrate respiration. Microbiology 149:3395–3403. https://doi.org/10.1099/mic.0.26620-0
Delgado I, Lloveras J (2020) Historia y distribución de la alfalfa. In: Lloveras J, Delgado I, Chocarro C (eds) La alfalfa: agronomía y utilización, 2nd edn. Edicions de la Universitat de Lleida, Lleida, pp 21–31
Durand A, Azzouzi A, Bourbon ML, Steunou AS, Liotenberg S, Maeshima A, Astier C, Argentini M, Saito S, Ouchane S (2015) c-type cytochrome assembly in a key target of copper toxicity within the bacterial periplasm. mBio 6(5):e01007-15. https://doi.org/10.1128/mBio.01007-15
Felgate H, Giannopoulos G, Sullivan MJ, Gates AJ, Clarke TA, Baggs E, Rowley G, Richardson DJ (2012) The impact of copper, nitrate and carbon status on the emission of nitrous oxide by two species of bacteria with biochemically distinct denitrification pathways. Environ Microbiol 14(7):1788–1800. https://doi.org/10.1111/j.1462-2920.2012.02789.x
Garcia-Rodriguez FM, Toro N (2000) Sinorhizobium meliloti nfe (nodulation formation efficiency) genes exhibit temporal and spatial expression patterns similar to those of genes involved in symbiotic nitrogen fixation. Mol Plant Microbe Interact 13(6):583–591. https://doi.org/10.1094/MPMI.2000.13.6.583
Hageman RHG, Hucklesby DP (1971) Nitrate reductase from higher plants. In: San Pietro A (ed) Methods in Enzymology. Academic, London, pp 491–503. https://doi.org/10.1016/S0076-6879(71)23121-9
Hirayama J, Eda S, Mitsui H, Minamisawa K (2011) Nitrate-dependent N2O emission from intact soybean nodules via denitrification by Bradyrhizobium japonicum bacteroids. Appl Environ Microbiol 77(24):8787–8790. https://doi.org/10.1128/AEM.06262-11
Horchani F, Prevot M, Boscari A, Evangelisti E, Meilhoc E, Bruand C, Raymond P, Boncompagni E, Aschi-Smiti S, Puppo A, Brouquisse R (2011) Both plant and bacterial nitrate reductases contribute to nitric oxide production in Medicago truncatula nitrogen-fixing nodules. Plant Physiol 155(2):1023–1036. https://doi.org/10.1104/pp.110.166140
IPCC (2022) Summary for policymakers. In: Shukla PR, Skea J, Slade R, Al Khourdajie A, van Diemen R, McCollum D, Pathak M, Some S, Vyas P, Fradera R, Belkacemi R, Hasija A, Lisboa G, Luz S, Malley J (eds) Climate change 2022: mitigation of climate change. contribution of working group iii to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge and New York. https://doi.org/10.1017/9781009157926.001
Itakura M, Uchida Y, Akiyama H, Hoshino YT, Shimomura Y, Morimoto S, Tago K, Wang Y, Hayakawa C, Uetake Y, Sánchez C, Eda S, Hayatsu M, Minamisawa K (2013) Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation. Nat Clim Change 3:208–212. https://doi.org/10.1038/nclimate1734
Kraft B, Strous M, Tegetmeyer HE (2011) Microbial nitrate respiration - genes, enzymes and environmental distribution. J Biotechnol 155(1):104–117. https://doi.org/10.1016/j.jbiotec.2010.12.025
LaRue TA, Child JJ (1979) Sensitive fluorometric assay for leghemoglobin. Anal Biochem 92(1):11–15. https://doi.org/10.1016/0003-2697(79)90618-3
Lecomte SM, Nesme X, Franzino T, Villard C, Pivard M, Vial L, Doré J, Hommais F, Haichar FEZ (2021) Agrobacterium fabrum C58 involved nitrate reductase NapA and antisense RNA NorR to denitrify. FEMS Microbiol Ecol 97(1):fiaa233. https://doi.org/10.1093/femsec/fiaa233
Li S, Yang XR, Buchner D, Wang HT, Xu HJ, Haderlein SB, Zhu YG (2019) Increased copper levels inhibit denitrification in urban soils. Earth Environ Sci Trans R Soc Edinb 109:421–427. https://doi.org/10.1017/S1755691018000592
Liu H, Li Y, Pan B, Zheng X, Yu J, Ding H, Zhang Y (2022) Pathways of soil N2O uptake, consumption, and its driving factors: a review. Environ Sci Pollut Res 29(21):30850–30864. https://doi.org/10.1007/s11356-022-18619-y
Lloveras J, Delgado I, Chocarro C (2020) Fertilización. In: Lloveras J, Delgado I, Chocarro C (eds) La alfalfa: agronomía y utilización, 2nd edn. Edicions de la Universitat de Lleida, Lleida, pp 79–92
Meade HM, Long SR, Ruvkun GB, Brown SE, Ausubel FM (1982) Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol 149(1):114–122. https://doi.org/10.1128/jb.149.1.114-122.1982
Mendoza-Suárez M, Andersen SU, Poole PS, Sánchez-Cañizares C (2021) Competition, nodule occupancy, and persistence of inoculant strains: key factors in the Rhizobium-Legume Symbioses. Front Plant Sci 12:690567. https://doi.org/10.3389/fpls.2021.690567
Mesa S, Alché JDD, Bedmar EJ, Delgado MJ (2004) Expression of nir, nor and nos denitrification genes from Bradyrhizobium japonicum in soybean root nodules. Physiol Plant 120(2):205–211. https://doi.org/10.1111/j.0031-9317.2004.0211.x
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, New York
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Nikolaevna G, Sergeevna M, Fjodorovna N, Anatoljevna N (2016) Resistance of plants to Cu stress: transgenesis. In: Ahmad P (ed) Plant metal interaction. Emerging remediation techniques. Elsevier, Amsterdam, pp 69–114. https://doi.org/10.1016/B978-0-12-803158-2.00004-7
Nogales J, Muñoz S, Olivares J, Sanjuán J (2006) Sinorhizobium meliloti genes involved in tolerance to the antimicrobial peptide protamine. FEMS Microbiol Lett 264(2):160–167. https://doi.org/10.1111/j.1574-6968.2006.00445.x
Oldroyd GE, Downie JA (2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59:519–546. https://doi.org/10.1146/annurev.arplant.59.032607.092839
Onishchuk OP, Vorobyov NI, Provorov NA (2017) Nodulation competitiveness of nodule bacteria: genetic control and adaptive significance: review. Appl Biochem Microbiol 53(2):131–139. https://doi.org/10.1134/S0003683817020132
Pacheco PJ, Cabrera JJ, Jiménez-Leiva A, Bedmar EJ, Mesa S, Tortosa G, Delgado MJ (2022) Effect of copper on expression of functional genes and proteins Associated with Bradyrhizobium diazoefficiens Denitrification. Int J Mol Sci 23(6):3386–3407. https://doi.org/10.3390/ijms23063386
Pobigaylo N, Wetter D, Szymczak S, Schiller U, Kurtz S, Meyer F, Nattkemper TW, Becker A (2006) Construction of a large signature-tagged mini-Tn5 transposon library and its application to mutagenesis of Sinorhizobium meliloti Appl Environ Microbiol 72(6):4329–4337. https://doi.org/10.1128/AEM.03072-05
Poole P, Ramachandran V, Terpolilli J (2018) Rhizobia: from saprophytes to endosymbionts. Nat Rev Microbiol 16(5):291–303. https://doi.org/10.1038/nrmicro.2017.171
Printz B, Guerriero G, Sergeant K, Audinot J-N, Guignard C, Renaut J, Lutts S, Hausman J-F (2016) Combining -omics to unravel the impact of Copper Nutrition on Alfalfa (Medicago sativa) stem metabolism. Plant Cell Physiol 57(2):407–422. https://doi.org/10.1093/pcp/pcw001
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326(5949):123–125. https://doi.org/10.1126/science.1176985
Richardson DJ (2011) Redox complexes of the nitrogen cycle. In: Moir JWB (ed) Nitrogen cycling in bacteria. Caister Academic Press, Norkfolk, pp 23–39
Richardson D, Felgate H, Watmough N, Thomson A, Baggs E (2009) Mitigating release of the potent greenhouse gas N2O from the nitrogen cycle - could enzymic regulation hold the key? Trends Biotechnol 27(7):388–397. https://doi.org/10.1016/j.tibtech.2009.03.009
Rigaud J, Puppo A (1975) Indole-3-acetic acid catabolism by soybean bacteroids. J Gen Microbiol 88:223–228. https://doi.org/10.1099/00221287-88-2-223
Robertsen BK, Aman P, Darvill AG, McNeil M, Albersheim P (1981) Host-symbiont interactions: V. The structure of acidic extracellular polysaccharides secreted by Rhizobium leguminosarum and Rhizobium trifolii Plant Physiol 67(3):389–400. https://doi.org/10.1104/pp.67.3.389
Rockström J, Steffen W, Noone K, Persson A, Chapin FS III, Lambin EF, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ et al (2009) A safe operating space for humanity. Nature 461(7263):472–475. https://doi.org/10.1038/461472a
Ruiz B, Sauviac L, Brouquisse R, Bruand C, Meilhoc E (2022) Role of nitric oxide of bacterial origin in the Medicago truncatula-Sinorhizobium meliloti symbiosis. Mol Plant Microbe Interact 35(10):887–892. https://doi.org/10.1094/MPMI-05-22-0118-SC
Rutten PJ, Poole PS (2019) Oxygen regulatory mechanisms of nitrogen fixation in rhizobia. Adv Microb Physiol 75:325–389. https://doi.org/10.1016/bs.ampbs.2019.08.001
Salas A, Cabrera JJ, Jiménez-Leiva A, Mesa S, Bedmar EJ, Richardson DJ, Gates AJ, Delgado MJ (2021) Bacterial nitric oxide metabolism: recent insights in rhizobia. Adv Microb Physiol 78:259–315. https://doi.org/10.1016/bs.ampbs.2021.05.001
Sánchez C, Minamisawa K (2019) Nitrogen Cycling in soybean Rhizosphere: sources and sinks of Nitrous Oxide (N2O). Front Microbiol 10:1943. https://doi.org/10.3389/fmicb.2019.01943
Simon R, Priefer U, Pühler A (1983) Vector plasmids for in vivo and in vitro manipulation of gram-negative bacteria. In: Pühler A (ed) Molecular genetics of the bacteria-plant interaction. Springer Science, Heidelberg, pp 98–106
Steffen W, Richardson K, Rockström J, Cornell SE, Fetzer I, Bennett EM et al (2015) Planetary boundaries: guiding human development on a changing planet. Science 347:1259855. https://doi.org/10.1126/science.1259855
Sullivan MJ, Gates AJ, Appia-Ayme C, Rowley G, Richardson DJ (2013) Copper control of bacterial nitrous oxide emission and its impact on vitamin B12-dependent metabolism. Proc Natl Acad Sci USA 110(49):19926–19931. https://doi.org/10.1073/pnas.1314529110
Torres MJ, Ávila S, Bedmar EJ, Delgado MJ (2018) Overexpression of the periplasmic nitrate reductase supports anaerobic growth by Ensifer meliloti FEMS Microbiol Lett 365(7):fny041. https://doi.org/10.1093/femsle/fny041
Torres MJ, Hidalgo-García A, Bedmar EJ, Delgado MJ (2013) Functional analysis of the copy 1 of the fixNOQP operon of Ensifer meliloti under free-living microoxic and symbiotic conditions. J Appl Microbiol 114(6):1772–1781. https://doi.org/10.1111/jam.12168
Torres MJ, Rubia MI, Bedmar EJ, Delgado MJ (2011) Denitrification in Sinorhizobium meliloti Biochem Soc Trans 39(6):1886–1889. https://doi.org/10.1042/BST20110733
Torres MJ, Rubia MI, de la Peña TC, Pueyo JJ, Bedmar EJ, Delgado MJ (2014) Genetic basis for denitrification in Ensifer meliloti BMC Microbiol 14:142. https://doi.org/10.1186/1471-2180-14-142
Torres MJ, Simon J, Rowley G, Bedmar EJ, Richardson DJ, Gates AJ, Delgado MJ (2016) Nitrous oxide metabolism in nitrate-reducing bacteria: physiology and regulatory mechanisms. Adv Microb Physiol 68:353–432. https://doi.org/10.1016/bs.ampbs.2016.02.007
Tortosa G, Hidalgo-García A, Salas A, Bedmar EJ, Mesa S, Delgado MJ (2015) Nitrate and flooding induce N2O emissions from soybean nodules. Symbiosis 67:125–133. https://doi.org/10.1007/s13199-015-0341-3
Tortosa G, Pacheco PJ, Hidalgo-García A, Granados A, Delgado A, Mesa S, Bedmar EJ, Delgado MJ (2020) Copper modulates nitrous oxide emissions from soybean root nodules. Environ Exp Bot 180:104262. https://doi.org/10.1016/j.envexpbot.2020.104262
van Spanning RJM, Delgado MJ, Richardson DJ (2005) The nitrogen cycle: denitrification and its relationship to N2 fixation. In: Werner D, Newton WE (eds) Nitrogen fixation in agriculture, forestry, ecology and the environment. Springer Science, Dordrecht, pp 277–342
van Spanning RJM, Richardson DJ, Ferguson SJ (2007) Introduction to the biochemistry and molecular biology of denitrification. In: Bothe H, Ferguson SJ, Newton WE (eds) Biology of the nitrogen cycle. Elsevier Science, Amsterdam, pp 3–20
Woliy K, Degefu T, Frostegård à (2019) Host range and symbiotic effectiveness of N2O reducing Bradyrhizobium strains. Front Microbiol 10:2746. https://doi.org/10.3389/fmicb.2019.02746
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36(5):409–430. https://doi.org/10.1071/FP08288
Acknowledgements
The authors are grateful to Socorro Muñoz and Alba Hidalgo García for their technical support, the Instrumental Technical Service (EEZ-CSIC) for the ICP-OES determinations and N/C analyses, and the Greenhouse and Growth Chamber Service (GGCS) (EEZ-CSIC). Authors also thank Dr. Juan Sanjuán for supplying pGUS3 plasmid.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by MCIN/AEI/ https://doi.org/10.13039/501100011033 and by “European Regional Development Fund (ERDF) A way of making Europe”, grants AGL2017-85676-R, and PID2020-114330GB-100 and also by PAIDI2020 from Junta de Andalucía, grant P18-RT-1401. P.J.P. was supported by a FPU fellowship from Ministerio de Universidades (MIU) (formerly MECD).
Author information
Authors and Affiliations
Contributions
Conceptualization: Pacheco PJ, Tortosa G, Delgado MJ; Methodology: Pacheco PJ, Tortosa G; Formal analysis and investigation: Pacheco PJ, Tortosa G; Writing – original draft preparation: Pacheco PJ, Tortosa G, Delgado MJ; Writing, review and editing: Pacheco PJ, Bedmar EJ, Mesa S, Tortosa G, Delgado MJ; Funding acquisition: Mesa S, Delgado MJ; Resources: Delgado MJ; Supervision: Tortosa G, Delgado MJ.
Corresponding authors
Ethics declarations
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Euan K. James.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pacheco, P.J., Bedmar, E.J., Mesa, S. et al. Ensifer meliloti denitrification is involved in infection effectiveness and N2O emissions from alfalfa root nodules. Plant Soil 486, 519–534 (2023). https://doi.org/10.1007/s11104-023-05946-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-05946-3