Abstract
Purpose
Commercial production and the use of liquid vermicompost extract (LVE) is gaining attention as a technique that supports integrated soil-microbial-crop management for sustainable agriculture. However, the interaction effects of LVE, arbuscular mycorrhizal fungi (AMF), and host plants on the delivery of agroecosystem services in alkaline soil have been less studied.
Methods
We carried out a 3-year field experiment in Central Italy, to investigate the short-term effect of LVE on soil mycorrhizal inoculum potential (MIP), AMF root colonization, and productivity of berseem clover, lentil, and sunflower. LVE produced in different years were screened for microbial properties using Illumina Miseq sequencing. LVE was applied at seeding, crop stem elongation and flowering stages. Control crops received water as a placebo.
Results
LVE bacterial communities were more diverse and showed a higher turnover between 2019 and 2020 than fungal communities. Diverse microbial groups, the majority of which belonged to phyla Proteobacteria, Bacteroidetes, Firmicutes, and Mucoromycota, were detected, including N-fixers (Flavobacterium, Malikia, and Citrobacter), P-solubilizers (Pseudomonas), and C-degraders (Tolumonas, Arcobacter, and Mucor). Notably, LVE treatment enhanced soil MIP and AMF root colonization in most crops, but selectively improved shoot biomass of berseem clover (+ 32%) and sunflower (+ 34%), and grain yield (+ 37%) and oil concentration (+ 5%) in sunflower, compared to the corresponding non-treated controls.
Conclusions
LVE had diverse groups of bacteria and a few fungal taxa, and its application enhanced mycorrhizal properties and selected growth- and yield-related variables in lentil, berseem clover, and sunflower. This could be due to LVE’s biostimulating effect arising from the vermicompost-associated microbiome and biomolecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liquid vermicompost extracts (LVE), also commonly known as vermicompost leachates or teas, are by-products obtained during vermicomposting, a non-thermophilic process mediated by earthworms and microorganisms that bio-oxidize and stabilize organic materials into usable bio-stimulants (Lazcano and Domínguez 2011). For that reason, LVE is a microbiologically active product that contains a wide range of macro-and micro-nutrients, extracellular enzymes, chemical attractants, and hormone-like molecules that confer various benefits to soil and plants (Gudeta et al. 2021). Some of these substances may affect soil microbes including arbuscular mycorrhizal fungi (AMF) that stimulate solubilization of nutrients such as phosphorus (P) bound to organic compounds, particularly in alkaline soils (Uz and Tavali 2014) and enhance biocontrol of pests and pathogens (Rostami et al. 2021). The biochemical quality and microbiological profile of LVE are dictated by the vermicompost nature, which in turn is affected by the type of initial feeding substrate, environmental conditions, and the species of earthworm used (Yasir et al. 2009; Huang et al. 2013; Domínguez et al. 2019; Yatoo et al. 2021). This variation can likely affect the delivery of microbial-associated agroecosystem services such as nutrient acquisition and facilitation, particularly in low input systems that rely on limited or no use of external fertilizers.
Considerable input has been put into understanding the microbiology and effect of solid vermicompost casts on various crops (Zhao et al. 2017; Cai et al. 2018; Kolbe et al. 2019; Zhang et al. 2020; Khodabin et al. 2022). Still, much less has been done to assess the microbial composition of LVE (Fritz et al. 2012; del Pilar et al. 2021) and its effect on soil microbiota, particularly the local AMF communities. Previous research has demonstrated that humic acid rich vermicompost act synergistically with Rhizobium and AMF in improving the growth of Capsicum assamicum, Lactuca sativa L., and Pisum sativum L. crops, and soil health under microcosm conditions (Khan et al. 2014; Maji et al. 2017; Liu et al. 2020). Host specificity and abiotic factors i.e., edaphic, climatic, and anthropogenic farm practices, can differentially affect the local diversity and functioning of the AMF symbionts, thus, interrupting the outcome of plant-microbial symbioses (Turrini et al. 2018), bringing forward the need for biostimulation (Fig. 1). To our knowledge, reports on field studies elucidating the tripartite LVE-plant-soil AMF synergistic interactions are scarce.
Biostimulatory effect of liquid vermicompost extract (LVE) on (A) plant microbial symbionts such as plant growth promoting rhizobacteria (PGPRs) and arbuscular mycorrhizal fungi (AMF) involved in soil biogeochemical cycles such as N-fixation, P, and K solubilization, Fe, Zn, and Mn bioavailability. Photos showing root system of sunflower that received (B) No LVE treatment and (C) LVE treatment. Falcon tubes were used for storing roots prior staining for AMF colonization assessment. Roots were collected 76 days after sowing (1st June 2021)
Previous studies of vermicompost prokaryotic communities, based on 16S rRNA molecular analyses, identified Proteobacteria, Bacteroidetes, and Firmicutes (Yasir et al. 2009; Munoz-Ucros et al. 2020), exemplified by P-solubilizers belonging to Pseudomonas and Bacillus spp. (Rostami et al. 2021), Terrimonas and Trichococcus spp. (Zhang et al. 2020), N-fixers and nitrifiers belonging to Flavobacterium, Azotobacter, Nitrobacter, Azospirillum, and Rhizobium spp. (Pathma and Sakthivel 2012), and biocontrol agents such as Pseudomonas resinovorans, and Bacillus megaterium (Rostami et al. 2021), as the most dominant functional groups. Few studies on fungal communities, using molecular tools, identified representatives of all major phyla (Cai et al. 2018; Domínguez et al. 2021), and at the class level, Mucoromycetes, Sordariomycetes, Agaricomycetes, Eurotiomycetes, Saccharomycetes, Orbiliomycetes, Chytridiomycetes, and Pezizomycetes (Huang et al. 2013; Silawat et al. 2013; Cai et al. 2018).
Substantial differences in bacterial and fungal communities have also been observed in LVE derived from different vermicomposting materials (Fritz et al. 2012; Munoz-Ucros et al. 2020; del Pilar et al. 2021). Therefore, it is crucial to thoroughly characterize the microbial content of LVE derived from the commonly available farm crop and livestock residues. Though, there is a poor knowledge on the microbiota occurring in LVE derived from wheat straw and horse dung manure, widely used in compost preparation, and studies assessing the effect of LVE application on AMF activity, colonization, and crop yield under alkaline field conditions of the Mediterranean soils are scarce. Three spring–summer crop species: a forage legume represented by berseem clover (Trifolium alexandrinum L. cv. Akenaton), a pulse grain legume represented by lentil (Lens culinaris L. cv. Elsa), and a non-legume control represented by sunflower (Helianthus annuus L. cv. Sangria), were used as test plants to study the impact of LVE application on plant-mycorrhizal interactions. The three crop species are widely grown by farmers in the Mediterranean region as sole crops or in rotations for human food, animal feed, and oil production, and their growth cycles synchronize well with the Mediterranean’s spring–summer conditions. Besides, the crops are known to be mycorrhizal dependent especially in soils with low P, typical of the Mediterranean region (Molla et al. 2010; Saia et al. 2014; Vangelisti et al. 2018).
The area where this experiment was carried out is located in a ‘mycorrhizal diversity hotspot’ (Turrini and Giovannetti 2012; Njeru et al. 2015; Turrini et al. 2018), so incorporating farm practices that could protect and augment AMF activities would be fundamental in maintaining soil-AMF productivity amidst the changing climatic and edaphic conditions occurring in the Mediterranean region. Besides, it is necessary to make better use of the readily available organic residues, identify functional groups of microorganisms in the LVE, and understand their interaction with host plants and rhizosphere microbiomes.
In this study, we investigated (i) the microbial composition and diversity in a commercial LVE through Illumina Miseq sequencing, (ii) the biostimulatory effects of LVE on local AMF abundance and plant root colonization, (iii) the effect of LVE in enhancing growth- and production-related traits of lentil (pulse legume), berseem clover (forage legume), and sunflower (oil-seed crop) grown under low-input field conditions. We hypothesized that LVE might contain a diverse array of bacteria and fungi of different genera and species with linkable plant growth-promoting traits and biostimulatory effects on the activity of the local AMF. Lastly, we expected that the application of LVE would increase biomass, grain yield, and nutrition of crops managed under a rain-fed low input system.
Materials and methods
Study site
The experiment was carried out and replicated in three years (2019, 2020, and 2021) on three separate but adjacent fields with no history of vermicompost application at the Centre for Agri-Environmental Research ‘Enrico Avanzi’ of the University of Pisa (43° 40′ 5.3'' N, 10° 18′ 34.2'' E), Pisa, Central Italy. The soils are classified as Xerofluvent by USDA (Soil Survey Stuff 1999) and as Fluvisol by FAO (IUSS Working Group WRB 2015), and have a typically alkaline sandy loam texture, pH (7.6 – 8.1), low in total Kjeldahl N (0.52 – 0.92 g kg−1), available Olsen P (8 – 10 mg kg−1), and Walkley–Black organic carbon (0.66 – 0.84%). The three fields had a previous rotational cultivation history of durum wheat (Triticum durum Desf.), sorghum (Sorghum bicolor L.), and alfalfa (Medicago sativa L.). All fields were prepared by shallow plowing at 25 cm depth and subsequent harrowing at 10 cm depth. The crops were mechanically sown in 13 × 3 m plots and managed as a rain-fed low-input system with no pesticides, herbicides, or inorganic fertilizers. Hand hoeing was constantly done to reduce weed infestation until the flowering stage of each crop. The total mean monthly rainfall during the first three months (April to June) critical for seed germination, flowering, and grain formation was 198 mm, 126 mm, and 103 mm in 2019, 2020, and 2021, respectively (Fig. S1).
Experimental materials and design
A commercial LVE, produced from wheat straw (20%) amended with horse manure (80%) in the presence of Eisenia fetida and Eisenia andrei earthworms, was sourced from a local company. Briefly, the LVE were made from a mixture of mature compost manure (8 – 12 weeks old, kept at < 25 °C) and wheat straws, periodically mixed and moisturized with water, and covered in piles. Worm density of 9 kg living biomass m−2 (about 15,000 individuals per m−2) was used and the worm-bedding was maintained for a 4 to 5-month cycle to allow the LVE to accumulate, mature, and stabilize. LVE biochemical properties for 2019 and 2020 batches were pH 5.0 and 6.0, electrical conductivity 2.55 and 3.10 mS cm−1, total N 89 and 102 mg kg−1, organic C 0.10 and 0.14%, total bacteria load 7.75 × 107 and 1.62 × 107 CFU g−1, Escherichia coli < 5 and < 4 CFU g−1, and humidity 99.6 and 99.5%, respectively. Three spring–summer crop species were used as test plants and were sourced locally: berseem clover cv. Akenaton, lentil cv. Elsa, and sunflower cv. Sangria. Experimental treatments included LVE application on the test crops laid under a split-plot design with crop species as the main plot factor and LVE treatment (with or without LVE) as a sub-plot factor, replicated in five blocks each year. An additional plot with no crop but with spontaneous weed vegetation was used as a control. A total of 40 plots were laid out each year.
Vermicompost microbiome
Bacterial and fungal DNA extraction, amplification, and sequencing
Three aliquots (2 ml each) of LVE batches per year (2019 and 2020) were collected to analyze the composition of the bacterial and fungal communities using the Next-generation high throughput DNA sequencing (Ansorge 2009). Total community DNA was extracted from each sample using the DNeasy 96 PowerSoil Pro QIAcube HT Kit (Milan, Italy). Three 16S rRNA and three ITS gene amplicon libraries were prepared by PCR amplification of the hypervariable V3-V4 regions of the 16S rRNA gene and the ITS2 region, respectively, according to the Illumina 16S and ITS metagenomic sequencing library protocol. PCR amplification of the bacterial communities was performed with primers Pro341F and Pro805R following the standard procedures described by Takahashi et al. (2014). The universal fungal primers ITS3 and ITS4 designed by White et al. (1990) were used to amplify the fungal ITS2 region. Amplicons were obtained using the Platinum Taq DNA Polymerase High Fidelity (Thermofisher, Italy). Cycle conditions were an initial step at 94 °C for 1 min; 25 cycles of 94 °C (30 s), 55 °C (30 s), 68 °C (45 s); a final extension of 7 min at 62 °C. Libraries were purified using Agencourt AMPure XP (LABPLAN; Naas, Ireland) according to the Illumina metagenomic sequencing library protocol. Dual indices and Illumina sequencing adapters from the Illumina Nextera XT index kits v2 B and C (Illumina, San Diego, USA) were added to the target amplicons in a second Index PCR step according to the Illumina metagenomic sequencing library protocols to generate sequencing index libraries. Sequencing was done as a 2 × 300 bp paired-end run on the Illumina MiSeq platform. The NGS sequencing procedure was performed by BMR genomics (Padua, Italy).
Microbial diversity and sequence analyses
Fungal and bacterial sequence processing and analyses were carried out using the Microbial Ecological tool QIIME2 (Bolyen et al. 2019) version 2020.2 pipeline. The high throughput fungal and bacterial sequence reads were pre-processed using Cutadapt v.10 (Martin 2011) included in the QIIME2 to eliminate adapter, and unwanted primer, followed by denoising, chimeras’ removal, dereplication, and OTUs construction using DADA2 (Callahan et al. 2016) at 99% accuracy level. Taxonomy assignment and classification of fungi were done against the UNITE (Kõljalg et al. 2005) fungal database (version 8.2 2020). Alignment and taxonomic assignment of bacterial OTUs were done against the GreenGenes (Mcdonald et al. 2012) database version 13.8. Alpha-diversity was presented as Hill numbers (Hill 1973) while Bray–Curtis dissimilarity index (Bray and Curtis 1957) was used to estimate the microbial dissimilarity between the two different LVE batches. The sequence data were submitted to the NCBI GenBank database under the accession numbers: ON819887 – ON820109 and ON833101 – ON833262 for bacteria in 2019 and 2020, respectively, and ON782302 – 782,356 and ON758673 – ON758680 for fungi in 2019 and 2020, respectively.
Vermicompost application and crop management
All test crops were sown in the last week of March of each year using a mechanical plot seeder and harvested in July–August, depending on the maturity period of the crop. The crop seeding rate was adjusted to 207 seeds m−2 for lentils, 2.5 g seeds m−2 for berseem clover, and 9 seeds m−2 for sunflower. The row spacing for legumes was 15 cm, while sunflowers were spaced at 45 × 30 cm. Rhizobia inoculations (> 1 × 109 CFU g˗1) supplied by Alosca Technologies Pty Ltd (Australia) were done at a constant rate of 3 kg ha−1, equivalent to one-third of the recommended amount for each legume. LVE application was done three times in two ways; as a seed dresser (applied 3 h before sowing in an optimally recommended 1:40 LVE/water dilution i.e., 1 ml LVE + 40 ml water, total volume of the mixture 100 ml plot−1 seeds) and as a root-base spray (25.6 L ha−1, 1:40 LVE/water dilution) delivered manually in the field at crop stem elongation and flowering stages. Control crops received water as a placebo instead of LVE. Different batches of LVE were used in the 2019, 2020, and 2021 field trials.
Soil mycorrhizal inoculation potential (MIP) bioassay
Soil samples were collected from a depth of 0 – 20 cm in three randomly selected points in each plot using a 5-cm diameter soil probe; the three sub-samples were mixed to form one homogenous representative sample plot−1. The first sampling was done before sowing (TSow) and the second after crop harvesting (THarv). The pooled soil samples were used to assess mycorrhizal activity in the soil by a soil MIP bioassay. Soil MIP is commonly used as a biological indicator of mycorrhizal propagules to colonize plant roots within a given period (Bedini et al. 2013; Njeru et al. 2014). Briefly, Cichorium intybus (L.) seeds were sown in three replicates of 50 ml sterile Falcon tubes filled with 45 g of each plot soil sample. Tubes were watered, wrapped in sterile polythene bags, and placed in a growth chamber with a temperature regime of 25/21 °C (day/night) and 16 h of daylight. Five days after emergence (DAE), C. intybus plants were thinned to three plants tube−1. The whole root system of each plant was harvested 35 DAE by carefully washing away the soil to minimize root disturbance. The roots were separated from the shoots and prepared for staining with acidified trypan blue dye following the procedure described by Phillips and Hayman (1970). The percentage of colonized root length was determined under a dissecting microscope at × 40 magnification using the gridline intersect method described by Giovanetti and Mosse (1980).
Plant sampling and harvesting
Plant sampling in the field was done at two plant growth stages according to the BBCH scale (Meier et al. 2009). At flowering (BBCH 60/62), three plants near the soil sampling points in each plot were uprooted gently using a hand spade, at a depth of about 20 cm and 15 cm radius; roots washed and assessed for nodulation and AMF root colonization. The total nodule number was counted from the whole root system of the three plant samples and averaged to obtain the mean nodule number plant−1 (Menge et al. 2018). Ten sections of 3-cm long pieces of lateral roots from each plant were randomly chosen, cut, and pooled for mycorrhizal staining following the procedure described by Phillips and Hayman (1970). At the same time, shoot biomass was cut from two quadrats in each plot. At harvesting (BBCH 90), crops were manually cut from three quadrats in each plot, packaged independently, shelled, sorted, and cleaned to obtain pure grains. The quadrat size for lentil and berseem clover was 50 × 50 cm, while that of sunflower measured 90 × 60 cm. The biomass cut was used to determine the biomass dry weight (t ha−1) and shoot nutrition quality (N and P) at flowering stage, and grain yield (t ha−1) at harvesting stage. Dried tissues were ground to fine powder and N concentration was measured on Kjeldhal digests and determined with an elemental auto-analyzer (Flowsys Analyzer, Systea, Italy) while P concentration was assessed using a sulfuric acid-perchloric acid digestion method and measured on an Agilent 8453 UV–Visible spectrophotometer (Labstuff, Ireland) (Cresser and Parsons 1979). Whole grain protein concentration in lentil seeds and oil concentration in sunflower flour were analyzed using a Foss Infratec™ 1241 Grain Analyzer (Milan, Italy) that uses a near-infrared transmittance (NIT) spectrophotometric technique.
Data analyses
Although the experiments were carried out in 2019, 2020, and 2021 not all the tests reported were done in all the three years. The harvest data on grain yield and dry biomass and AMF root colonization were collected in 2019, 2020, and 2021. The samples for determining legume whole grain protein concentration and sunflower oil concentration, the nodule number, shoot nutrition quality (N and P), and shoot biomass at flowering were collected in 2020 and 2021. The samples for LVE microbial analyses and soil mycorrhizal inoculation potential (MIP) were collected in 2019 and 2020.
All statistical analyses were carried out in R environment version 4.1.0 (R Core Team 2021). Depending on the data type and error distribution, linear and generalized linear mixed-effects models in ‘Lme4’ R package (Bates et al. 2015) were fitted to determine the effect of LVE treatment. Soil mycorrhizal activity (% MIP THarv vs. % MIP TSow), AMF root colonization percentage, shoot nutrition (P and N concentration and content), biomass, grain yield, grain protein, and oil concentration were considered dependent variables, while crop species and LVE application were set as fixed factors. Year was treated as a fixed factor only if there was a significant year × treatment interaction. Blocks and plot pseudo-replicates were used as random factors. The Akaike Information Criteria (Akaike 1974) was used for refining the model comparison. For each model, the Kolmogorov–Smirnov test of normality in the ‘DHARMa’ R package (Hartig and Lohse 2021) complemented by the Shapiro–Wilk test and graphical representations for normality (Zuur et al. 2009) were used to assess the goodness of fit on the scaled residuals of the models. Tukey’s post hoc test using R/emmeans (Lenth 2019) was run in each model to check for the differences of significant explanatory variables at p ≤ 0.05. Principal component analyses (PCA) were performed to describe the relevant associations between agronomic and mycorrhizal variables recorded in 2020 and 2021.
Results
Alpha diversity of bacteria and fungi in the LVE
NGS analysis produced 256,299 and 184,605 total bacterial reads and 313,550 and 248,459 fungal reads in 2019 and 2020, respectively (Table S1). Approximately 53% and 57% of the bacterial and 88% and 42% of the fungal raw reads passed merging, trimming, and chimera filtering steps in 2019 and 2020, respectively, and were analyzed for operational taxonomic unit (OTU) search. In total, 223 and 162 bacterial OTUs, and 55 and 8 fungal OTUs were generated from the final sequence reads in 2019 and 2020, respectively (Table S1).
Bacterial communities extracted from LVE were more diverse than fungal communities, though, both showed a strong dominance of a few taxa, as attested by Hill numbers (Table 1). In 2020, bacterial diversity was significantly less than in 2019 but without a change in evenness (p = 0.53) as attested by the Pielou’s index. In contrast, the evenness of the fungal community was substantially (p < 0.0001) higher in 2020 than in 2019 (Table 1).
Bacterial and fungal composition in the LVE
Microbial community turnover between 2019 and 2020 batches was higher in bacteria than in fungi (Bray-Curti’s dissimilarity index 0.88 and 0.04, respectively). The fungal community in the LVE was dominated by a species of Mucor circinelloides in both 2019 and 2020 batches (Fig. 2). Other taxa identified, although with low frequency (OTUs < 1% of the total fungal reads), included Saccharomycetes (Cyberlindnera, Ogataea, and Geotrichum), Chytridiomycetes (Triparticalcar), Microbotryomycetes (Rhodotorula), Blastocladiomycetes (Allomyces), Mortierellomycetes (Mortierella) and Agaricomycetes (Fig. 2, Table S2). On the other hand, bacterial communities were dominated by Proteobacteria (86%), Bacteroidetes (11%), and Firmicutes (2%) in both years (Fig. 2). Proteobacteria were represented by Gammaproteobacteria (61%), Epsilonproteobacteria (18%), and Betaproteobacteria (6%). Other phyla with OTUs < 1% included Verrucomicrobia, Actinobacteria, Acidobacteria, Chloroflexi, and Fusobacteria. The most abundant identified bacterial genera in 2019 were Citrobacter, Arcobacter, and Pseudomonas while in 2020 were Tolumonas and Flavobacterium.
Effect of LVE application on soil mycorrhizal activity
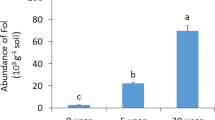
Soil mycorrhizal inoculum potential (MIP) bioassay at the start of the experiment gave similar results within each year, ranging from 25 – 35% in 2019 and 37 – 42% in 2020 (Fig. 3) but showing a significant difference between 2019 and 2020. At the end of the crop cycle, MIP values were generally higher than at the start of the cycle with variation depending on crop species, LVE treatment, and cropping year since significant crop species × LVE treatment (p < 0.0001) and crop × year (p < 0.0001) interactions were observed (Fig. 3, Table S3). Actually, LVE treated crops showed higher increase of MIP values (44 – 62%) than that of non-treated crops (43 – 52%), except in lentil in 2019, and, than non-cropped (43 – 45%) controls in both years (Fig. 3, Table S4). Interestingly, the increase in MIP values was larger in 2019 than in 2020.
Soil mycorrhizal inoculum potential (MIP) activity as influenced by liquid vermicompost extract (LVE) application and crop species. Asterisks indicate statistically significant differences between LVE treatments in each crop species (Tukey’s post hoc test); *** (p < 0.001) and n.s (p > 0.05). Error bars represent the standard errors of the means. The bars indicate the mean MIP values after harvesting (THarv). The blue triangles indicate the mean MIP values of each treatment before sowing (TSow). The dotted horizontal blue line indicates the baseline MIP averaged among the treatments before sowing. The control treatment labelled ‘No crop’ had spontaneous weed vegetation
Effect of LVE application on AMF root colonization
At the flowering stage, significant LVE treatment × year (p = 0.001) and crop species × year (p < 0.0001) interactions were observed, since AMF root colonization was differentially affected in 2019 compared to 2020 and 2021. In 2019, LVE application had no or small (as in lentil) effect on different crop species while in 2020 and 2021, all species showed a larger colonization when treated with LVE, with an average increase of 20%, 17%, and 13% colonized root length in sunflower, lentil, and berseem clover, respectively (Fig. 4, Table S5). On average, legumes had a higher (74 ± 8%) percentage of AMF-colonized root length than treated sunflower (57 ± 7%). In addition, a trend in reducing colonization was observed, especially from 2020 to 2021 (Fig. 4, Table S6).
Arbuscular mycorrhizal (AMF) colonization on plant roots as influenced by liquid vermicompost extract (LVE) application and crop species. Asterisks indicate statistically significant differences between LVE treatments in each crop species (Tukey’s post hoc test); *** (p < 0.001), * (p ≤ 0.05), and n.s (p > 0.05). Error bars represent standard errors of the means. The bars indicate the mean AMF (%) colonization values
Effect of LVE application on nodule number, shoot biomass, P and N concentration and content at flowering
At flowering stage, the number of root nodules was higher in berseem clover than in lentil, and LVE application enhanced nodulation in berseem clover (p < 0.0001), but not in lentil (p = 0.142) (Table 2, Table S7, S8). LVE application enhanced shoot biomass depending on crop species, that is, in berseem clover (+ 32%) and sunflower (+ 34%) compared to non-treated controls (Table 2, Table S9, S10). No significant differences were observed in shoot P (p = 0.082) and N (p = 0.105) concentrations of all the LVE treated and non-treated crop species (Table S11). However, a significant LVE treatment × crop (p = 0.023) interaction was observed in shoot N accumulation. LVE treated berseem clover accumulated a higher N content (124.96 ± 6.26 kg N ha−1) than the non-treated control (90.69 ± 4.7 kg N ha−1) while no significant differences were observed in lentil and sunflower shoot N contents (Table S11). LVE application significantly (p = 0.0003) enhanced shoot P accumulation in all the three test crops compared to the non-treated controls (Table S11).
Effect of LVE application on grain yield, protein, and oil concentration
Grain yield was affected by LVE treatment but varied depending on year of application and crop species. Actually, sunflower was strongly affected by LVE treatment in 2021 (+ 54%) and 2020 (+ 45%), while in 2019, there was no effect in sunflower (Fig. 5). LVE treatment did not affect legume grain production (Table S12, S13). While LVE treatment did not affect lentil grain protein concentration (p = 0.439; 25.5% vs 25.3%, (Table S14, S15), it enhanced oil concentration in sunflower (p < 0.0001) whose value increased by 5% in LVE treated (58%) compared to the non-treated (55%) plants (Table S16, S17).
Effect of liquid vermicompost extract (LVE) application on crop grain yield at harvesting stage. Asterisks indicate statistically significant differences between LVE treatments in each crop species at p ≤ 0.05 level (Tukey’s post hoc test); *** (p < 0.001), and n.s (p > 0.05). Error bars represent the standard errors of the means. The bars indicate the mean grain yield (t ha−1) values
Relationships between agronomic and mycorrhizal variables as influenced by LVE application
PCA was used to correlate production data such as grain yield and shoot biomass for the three crops, and oil seed or grain protein concentration in sunflower and lentil, respectively, with microbiological features (percent mycorrhizal length and rhizobia nodule density) of crops as influenced by LVE application. Variance explained by the two principal components is larger than 60% in the three crops. Along the PCA1 axis, a clear separation is identified between LVE treated and non-treated sunflower and berseem clover (Fig. 6). Evidently, in both crops, LVE treatments increased mycorrhizal colonization, and nodule density in berseem clover and oil concentration in sunflower (Fig. 6). Similarly, LVE application was associated with the shoot biomass at harvest in lentil (Fig. 6).
Biplots from the principal component analysis (PCA) showing relationships between agronomic and mycorrhizal variables as influenced by liquid vermicompost extract (LVE) application on (A) sunflower, (B) berseem clover, and (C) lentil. Variance explained by each principal component is shown in parentheses. Data are pooled from 2020 and 2021 observations. AMF_flr, arbuscular mycorrhizal fungi root colonization at flowering stage; Nodno, number of nodules plant−1 at flowering; BM_flr, aboveground biomass at flowering; BM_hav, aboveground biomass at harvest; GrainYld, grain yield at harvest; Protncont, protein concentration; Oilcont, oil concentration; LVE, liquid vermicompost extract
Discussion
Diversity and composition of bacteria and fungi in the LVE
In this study, LVE produced by a local company was characterized for its microbial diversity and its effect on crop production during the three years of field experimentation. LVE was obtained from the same raw materials (horse dung + wheat straw), but these materials were collected at different times, therefore, producing different LVE batches. The batches supplied in 2019 and 2020 showed a high dominance of Mucoromycota and Proteobacteria, although differences in abundance occurred between the batches. Notably, the microbial community turnover between 2019 and 2020 batches was higher in bacteria than in fungi. The bacteria turnover has been driven by the gain or loss of specific taxa and changes in the abundance of individual taxa. For instance, Citrobacter and Arcobacter were the most abundant species in the 2019 LVE batch, while Tolumonas and Flavobacterium were dominant in 2020. Unlike bacteria, fungal abundance was higher and evenness lower in 2019 batches than in 2020. The low turnover in fungal communities could be explained by the presence of a dominant genus, Mucor, represented by a high relative abundance of the species Mucor circinelloides in both years.
Horse dung + wheat straw raw materials were used as the feeding substrates for Eisenia fetida and Eisenia andrei earthworms, which are highly effective in vermicomposting. Domínguez et al. (2021) have shown that earthworm’s gut can induce drastic changes in microbial composition and can eliminate up to 91% of the ingested fungal taxa and 96% of the bacterial taxa. Under stressful conditions, earthworms may utilize fungi as their feed more preferably than bacteria (Yasir et al. 2009; Shan et al. 2013), which can decrease the fungal community profile in the vermicompost. Since LVE was collected at the end of vermicomposting, there is a likelihood of obtaining simple microbial communities but with varying abundances. At the same time, the microbial changes may have also been induced by the effect of other parameters that cannot be easily controlled at the horse farm level, such as dung maturation time and horse feeding strategy, and at the wheat farm level, such as field conditions and management practices. In addition, the season during the transformation process may partly account for the microbial differences. These observations are consistent with the previous findings (Huang et al. 2013) reporting a comparatively smaller fungal population than that of bacteria in vegetable wastes vermicomposted by E. fetida species. Moreover, other fungal groups such as the members of Glomeromycota were not detected in the LVE, probably due to ecological related issues considering that the LVE product comes from the straw-dung vermicompost that has been processed for 4 – 5 months which can considerably reduce the viability or the population of the AMF communities. The absence of the AMF communities has also been reported in a vermicomposting study by Domínguez et al. (2021).
The occurrence of members of the phyla Mucoromycota and Proteobacteria in the LVE is in line with the findings of other studies (del Pilar et al. 2021; Grantina-Ievina et al. 2013; Yasir et al. 2009). Mucoromycetes play a key role in the biodegradation of complex carbon materials and have been found in vermicomposting materials (Grantina-Ievina et al. 2013; Silawat et al. 2013), although, not as dominant as it is reported for the first time in this study. Their remarkable versatility to utilize different carbon sources as food and to withstand the highly variable vermicomposting environment distinguish them from other fungi. Hence, finding them a dominant fungal group in our LVE batches is unsurprising. Proteobacteria is considered the most taxonomically diverse bacterial group whose members can utilize different physiological and metabolic pathways, including degradation of complex lignocellulose and chitin, for survival (Ho et al. 2017; Chen et al. 2018; Gómez-Brandón et al. 2020). The abundance of diverse functional microbial groups, including N-fixers (Flavobacterium, Malikia, and Citrobacter), P-solubilizers (Pseudomonas), and C-degraders (Tolumonas, Arcobacter, and Mucor) suggests that there is a high microbial functional diversity and redundancy in the LVE that could potentially be beneficial in promoting plant growth, nutrient recycling, and soil health. The functional redundancy of the microbial groups in the two LVE batches may be beneficial in case of absence of one functional group.
The microbial functional groups reported in this study are also reflected in the recent vermicomposting studies of Gómez-Brandón et al. (2020) and Kolbe et al. (2019), which reported not only increased bacterial composition and diversity, but also enhanced abundance of functional genes important in cellulose-lignin degradation, antibiotic synthesis, and plant hormone synthesis such as salicylic acid, useful in mediating defense responses against phytopathogens. The authors identified Proteobacteria (Pseudomonas, Citrobacter, Comamonas, and Arcobacter), Bacteroidetes (Flavobacterium), and Firmicutes (Bacillus) groups as the main taxa in the vermicompost contributing to the metabolic, antibiotic, and plant hormone gene synthesis. Our results are further supported by the findings of Ravindran et al. (2016) who linked the production of indole 3-acetic acid, cytokinin, and gibberellin to specific microbial functional groups present in the vermicompost. The high abundance of Tolumonas in the LVE is in line with the findings of the previous studies that isolated the bacterium from environmental samples (Caldwell et al. 2011; Billings et al. 2015). The authors linked Tolumonas abundance to ferulic acid metabolism and lignin degradation under anaerobic conditions (like vermicomposting conditions).
LVE enhances soil mycorrhizal activity, root nodulation, and AMF colonization
Soil MIP bioassay showed a uniform mycorrhizal activity at the start of the experiment but differed between 2019 and 2020. This allowed us to consider mycorrhizal colonization data as only dependent on crop species and LVE treatments and not biased by a possible heterogeneous distribution of AMF propagules in the field in each year. The differences in mycorrhizal activity observed at the start of the experiment in the two years could be attributed to use of different fields that had different rotational crops. In 2019 and 2020 fields, the preceding crops were durum wheat and sorghum, respectively. Sorghum is known to be more mycotrophic than durum wheat (Rodriguez-Heredia et al. 2020). As expected, MIP increased during the growth season and LVE application induced a higher mycorrhizal activity in fields sown with sunflower and berseem clover in both years and in lentil in 2020, compared to non-treated controls. The LVE effect was further supported by the AMF root colonization data at the flowering stage, and the findings of Cruz-Koizumi et al. (2018), who reported an increased AMF colonization in tomatillo (Physalis ixocarpa Brot.) inoculated with LVE. In contrast, molecular analyses of soil microbial community showed that Glomeromycota population was reduced after the application of vermicompost (Zhao et al. 2017), although this may not be linked to root colonization, as the colonization factor was not assessed by the authors. The mechanistic effects of the LVE use on mycorrhiza could be related to that of Coelho et al. (2014) who found that adding vermicompost onto sand + vermiculite substrate induced spore germination, hyphal growth, and mycelium development of the AMF species Claroideglomus etunicatum, Gigaspora albida, and Acaulospora longula. Similarly, Khan et al. (2014) also observed an increased AMF root colonization in vermicompost treated Capsicum assamicum L. plants compared to the non-treated controls, which they attributed to the priming effect of soil microbiota mediated by humic acid in the vermicompost, leading to an improved soil quality, and microbial biomass.
Besides the mycorrhization effect, positive effects of LVE treatment were also observed on nodulation of legumes, therefore, corroborating the results obtained with solid vermicompost derived from rice straw + cow dung (Maji et al. 2017). A strong correlation between nodulation and AMF colonization at flowering in berseem clover may suggest the synergistic action of LVE and soil rhizobia in enhancing belowground plant–microbe interactions, possibly by favoring the recruitment, early establishment, and proliferation of host-specific microbial symbionts. In fact, recent studies have highlighted that AMF, through their extensive extraradical mycelium network, may facilitate rhizobia translocation to the legume host plant leading to the formation of root nodules (de Novais et al. 2020; Ujvári et al. 2021). However, neither the increased AMF colonization in LVE-treated sunflower nor the enhanced nodulation in LVE-treated legumes significantly influenced P and N concentration in the shoots. Possibly, this was due to the strong influence of the environmental conditions such as water stress during the plant growth cycle. In fact, the amount of rainfall at crop flowering stage (May and June) was on average < 50 ± 5 mm (Fig. S1). Water stress reduces the mobility of nutrients in the soil, therefore, limiting the plant–microbe associated translocation of nutrients. Consistent to our findings, Chareesri et al. (2020) did not find any significant change in the P concentration of AMF colonized rice (Oryza sativa L.) grown under water stress regime. Overall, the N and P content accumulated by the three crops was relatively higher despite the low N and P content of the field soil and considering that a fertilizer-free low-input management system was used in this study. The significant effect of LVE application on shoot P accumulation in sunflower and berseem clover could be due to the differences in shoot biomass, which was evidently enhanced by LVE application.
Selective effect of LVE on agronomic variables and their associations with mycorrhiza
PCA analyses suggest that the overall effect of LVE application could be stronger when applied to sunflower than to legumes. Actually, sunflower oil concentration was evidently influenced by LVE application unlike lentil grain protein concentration. In support of our findings, Sharma et al. (2021) found that the presence of Glomus mosseae and Pseudomonas fluorescens increased sunflower oil concentration under drought stress conditions. The authors associated greater adaptability of the mentioned microorganisms to the local ecological soil conditions and efficacy in enhancing sunflower seed nutrition. However, it is evident from the PCA that grain yield and crop biomass at harvest are unrelated to AMF root colonization at flowering (the angle between their vectors is close to 90°), indicating a lack of correlation; therefore, the biostimulants effect of LVE on biomass/yield might be attributed to other components other than the AMF. It has also been reported that some indigenous AMF species may colonize the plant, but are inefficient in improving plant growth under certain field conditions, hence the need for priming with more effective stimulants suitable for that specific agroecological condition (Njeru and Koskey 2021). It should be noted that the LVE was diluted to minimal concentrations, and therefore its ‘fertilizer effect’ could be minimal compared to its biostimulant role in enhancing plant growth.
Farm management practices (e.g., frequent tillage), soil and climatic variability may have masked the LVE effects on different crops or across the years. In addition, it is important to note that the variable composition of LVE microbiota in the 2019 and 2020 batches may affect differently the soil microbial communities, including AMF and PGPRs, and cannot be underestimated, opening a road for further investigation to understand the interactive effects of LVE with other functional groups of soil bacteria and fungi. To date, there are few studies focusing on the use of LVE in open field cultivation of spring–summer crops under typically alkaline soils (Ayyobi et al. 2014). To our knowledge, the results of this study, which demonstrate for the first time the enhanced stimulation of LVE on AMF activity at the field scale, and its selective effect on plant growth, and grain yield of spring–summer crops in alkaline soil under low input conditions, are encouraging in promoting soil health and agricultural sustainability. More studies aimed at targeting different biological aspects of LVE microbiota using both non- and culture-based methods, e.g., isolation and metabolic characterization of culturable strains, community-level physiological profiles, metagenomics, and meta-transcriptomics, may confirm, and provide a better understanding of the functional equivalence occurring among different communities hosted by the LVE batches.
Conclusions
The LVE derived from vermicomposting wheat straw and horse dung in the presence of selected earthworm species had diverse functional groups of bacteria and fungi dominated by N-fixers, P-solubilizers, and C-degraders. The multiple application of LVE generally enhanced soil mycorrhizal activity and AMF root colonization in lentil, berseem clover, and sunflower but selectively improved shoot biomass of sunflower and berseem clover, sunflower grain yield, and oil concentration. The microbial and agronomic effects exerted by the LVE may partly be due to its nutritional content and, to a greater extent to the biostimulating processes arising from the vermicompost associated microbiome and biomolecules. Future studies should focus on exploring mechanisms behind the differential effects of LVE on different crop species emphasizing on belowground and aboveground interactions in alkaline field soil.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716–723. https://doi.org/10.1109/TAC.1974.1100705
Ansorge WJ (2009) Next-generation DNA sequencing techniques. N Biotechnol 25:195–203. https://doi.org/10.1016/j.nbt.2008.12.009
Ayyobi H, Hassanpour E, Alaqemand S et al (2014) Vermicompost leachate and vermiwash enhance French dwarf bean yield. Int J Veg Sci 20:21–27. https://doi.org/10.1080/19315260.2012.753496
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bedini S, Avio L, Sbrana C et al (2013) Mycorrhizal activity and diversity in a long-term organic Mediterranean agroecosystem. Biol Fertil Soils 49:781–790. https://doi.org/10.1007/s00374-012-0770-6
Billings AF, Fortney JL, Hazen TC et al (2015) Genome sequence and description of the anaerobic lignin-degrading bacterium Tolumonas lignolytica sp. nov. Stand Genomic Sci 10:1–11. https://doi.org/10.1186/S40793-015-0100-3/FIGURES/5
Bolyen E, Rideout JR, Dillon MR et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Cai L, Gong X, Sun X et al (2018) Comparison of chemical and microbiological changes during the aerobic composting and vermicomposting of green waste. PLoS One 13:1–16. https://doi.org/10.1371/journal.pone.0207494
Caldwell ME, Allen TD, Lawson PA, Tanner RS (2011) Tolumonas osonensis sp. nov., isolated from anoxic freshwater sediment, and emended description of the genus Tolumonas. Int J Syst Evol Microbiol 61:2659–2663. https://doi.org/10.1099/IJS.0.023853-0/CITE/REFWORKS
Callahan BJ, McMurdie PJ, Rosen MJ et al (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Chareesri A, De Deyn GB, Sergeeva L et al (2020) Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 30:315–328. https://doi.org/10.1007/S00572-020-00953-Z/FIGURES/5
Chen Y, Chang SKC, Chen J et al (2018) Characterization of microbial community succession during vermicomposting of medicinal herbal residues. Bioresour Technol 249:542–549. https://doi.org/10.1016/j.biortech.2017.10.021
Coelho IR, Pedone-Bonfim MVL, Silva FSB, Maia LC (2014) Optimization of the production of mycorrhizal inoculum on substrate with organic fertilizer. Braz J Microbiol 45:1173–1178. https://doi.org/10.1590/s1517-83822014000400007
Cresser MS, Parsons JW (1979) Sulphuric—Perchloric acid digestion of plant material for the determination of nitrogen, phosphorus, potassium, calcium and magnesium. Anal Chim Acta 109:431–436
Cruz-Koizumi YP, Alayón-Gamboa JA, Morón-Ríos A et al (2018) Effects of organic and chemical agriculture systems on arbuscular mycorrhizal fungi and green tomato production in Calakmul, Mexico. Agric Sci 09:1145–1167. https://doi.org/10.4236/as.2018.99080
de Novais CB, Sbrana C, da Conceição JE et al (2020) Mycorrhizal networks facilitate the colonization of legume roots by a symbiotic nitrogen-fixing bacterium. Mycorrhiza 30:389–396. https://doi.org/10.1007/s00572-020-00948-w
del Pilar SRR, Mario HG, Manuel RVV et al (2021) Study of bacterial communities in different types of leachates and their impact on solanum lycopersicum production in greenhouses. J Soil Sci Plant Nutr 21:1–12. https://doi.org/10.1007/s42729-021-00430-2
Domínguez J, Aira M, Crandall KA, Pérez-Losada M (2021) Earthworms drastically change fungal and bacterial communities during vermicomposting of sewage sludge. Sci Rep 11:1–10. https://doi.org/10.1038/s41598-021-95099-z
Domínguez J, Aira M, Kolbe AR et al (2019) Changes in the composition and function of bacterial communities during vermicomposting may explain beneficial properties of vermicompost. Sci Rep 9:. https://doi.org/10.1038/s41598-019-46018-w
Fritz JI, Franke-Whittle IH, Haindl S et al (2012) Microbiological community analysis of vermicompost tea and its influence on the growth of vegetables and cereals. Can J Microbiol 58:836–847. https://doi.org/10.1139/W2012-061
Giovanetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. https://doi.org/10.1111/j.1469-8137.1980.tb04556.x
Gómez-Brandón M, Aira M, Santana N et al (2020) Temporal dynamics of bacterial communities in a pilot-scale vermireactor fed with distilled grape marc. Microorganisms 8:642. https://doi.org/10.3390/MICROORGANISMS8050642
Grantina-Ievina L, Andersone U, Berkolde-Pīre D et al (2013) Critical tests for determination of microbiological quality and biological activity in commercial vermicompost samples of different origins. Appl Microbiol Biotechnol 97:10541–10554. https://doi.org/10.1007/s00253-013-4825-x
Gudeta K, Julka JM, Kumar A et al (2021) Vermiwash: an agent of disease and pest control in soil, a review. Heliyon 7:. https://doi.org/10.1016/J.HELIYON.2021.E06434
Hartig F, Lohse L (2021) residual diagnostics for hierarchical (Multi-Level / Mixed) regression models. In: R Packag. version 0.4.3. https://CRAN.R-project.org/package=DHARMa. Accessed 25 Jul 2021
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432. https://doi.org/10.2307/1934352
Ho A, Di Lonardo DP, Bodelier PLE (2017) Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol Ecol 93:6. https://doi.org/10.1093/FEMSEC/FIX006
Huang K, Li F, Wei Y et al (2013) Changes of bacterial and fungal community compositions during vermicomposting of vegetable wastes by Eisenia foetida. Bioresour Technol 150:235–241. https://doi.org/10.1016/j.biortech.2013.10.006
IUSS Working Group WRB (2015) World reference base for soil resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. Rome
Khan MH, Meghvansi MK, Gupta R et al (2014) Foliar spray with vermiwash modifies the arbuscular mycorrhizal dependency and nutrient stoichiometry of bhut jolokia (Capsicum assamicum). PLoS One 9:1–8. https://doi.org/10.1371/journal.pone.0092318
Khodabin G, Lightburn K, Hashemi SM et al (2022) Evaluation of nitrate leaching, fatty acids, physiological traits and yield of rapeseed (Brassica napus) in response to tillage, irrigation and fertilizer management. Plant Soil 473:423–440. https://doi.org/10.1007/s11104-021-05294-0
Kolbe AR, Aira M, Gómez-Brandón M et al (2019) Bacterial succession and functional diversity during vermicomposting of the white grape marc Vitis vinifera v. Albariño. Albariño Sci Rep 9:1–9. https://doi.org/10.1038/s41598-019-43907-y
Kõljalg U, Larsson KH, Abarenkov K et al (2005) UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol 166:1063–1068. https://doi.org/10.1111/j.1469-8137.2005.01376.x
Lazcano C, Domínguez J (2011) The use of vermicompost in sustainable agriculture: impact on plant growth and soil fertility. In: Mohammad M (ed) Soil Nutrients. Nova Science Publishers Inc, New York, pp 211–234
Lenth R (2019) Emmeans: estimated marginal means. In: R Packag. version 1.4.2. https://github.com/rvlenth/emmeans. Accessed 25 Jul 2021
Liu M, Zhu C, Wang C (2020) Vermicompost assisted arbuscular mycorrhizal fungi to transfer 15N from crop residues to lettuce. Plant Soil 456:175–187. https://doi.org/10.1007/s11104-020-04711-0
Maji D, Misra P, Singh S, Kalra A (2017) Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl Soil Ecol 110:97–108. https://doi.org/10.1016/J.APSOIL.2016.10.008
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J 17:10–12
Mcdonald D, Price MN, Goodrich J et al (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6:610–618. https://doi.org/10.1038/ismej.2011.139
Meier U, Buhr L, Feller C et al (2009) The BBCH system to coding the phenological growth stages of plants—History and publications, 2nd edn. Federal Biological Research Centre for Agriculture and Forestry, Berlin
Menge EM, Njeru EM, Koskey G, Maingi J (2018) Rhizobial inoculation methods affect the nodulation and plant growth traits of host plant genotypes: a case study of Common bean Phaseolus vulgaris L. germplasms cultivated by smallholder farmers in Eastern Kenya. Adv Agric Sci 6:77–94
Molla MN, Solaiman ARM, Mridha M, Khanam D (2010) The dependence of some pulses and oil seeds on arbuscular mycorrhizal fungi. Bull Inst Trop Agric Kyushu Univ 33:27–36. https://doi.org/10.11189/BITA.33.27
Munoz-Ucros J, Panke-Buisse K, Robe J (2020) Bacterial community composition of vermicompost-treated tomato rhizospheres. PLoS One 15:1–12. https://doi.org/10.1371/journal.pone.0230577
Njeru EM, Avio L, Sbrana C et al (2014) First evidence for a major cover crop effect on arbuscular mycorrhizal fungi and organic maize growth. Agron Sustain Dev 34:841–848. https://doi.org/10.1007/s13593-013-0197-y
Njeru EM, Bocci G, Bàrberi P et al (2015) Contrasting effects of cover crops on ‘hot spot’ arbuscular mycorrhizal fungal communities in organic tomato. Biol Fertil Soils 51:151–166. https://doi.org/10.1007/s00374-014-0958-z
Njeru EM, Koskey G (2021) Using beneficial microorganisms to promote sustainable crop production and resilience of smallholder agroecosystems to changing climate. In: Mallappa VKH, Shirur M (eds) Climate change and resilient food systems. Springer Nature, Singapore, pp 287–314
Pathma J, Sakthivel N (2012) Microbial diversity of vermicompost bacteria that exhibit useful agricultural traits and waste management potential. Springerplus 1:1–19. https://doi.org/10.1186/2193-1801-1-26
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158-IN18. https://doi.org/10.1016/s0007-1536(70)80110-3
R Core Team (2021) R: A language and environment for statistical computing. In: R Found. Stat. Comput. https://www.r-project.org/. Accessed 25 Jul 2021
Ravindran B, Wong JWC, Selvam A, Sekaran G (2016) Influence of microbial diversity and plant growth hormones in compost and vermicompost from fermented tannery waste. Bioresour Technol 217:200–204. https://doi.org/10.1016/j.biortech.2016.03.032
Rodriguez-Heredia M, Djian-Caporalino C, Ponchet M, et al (2020) Protective effects of mycorrhizal association in tomato and pepper against Meloidogyne incognita infection, and mycorrhizal networks for early mycorrhization of low mycotrophic plants. Phytopathol Mediterr 59:377–384. https://doi.org/10.14601/Phyto-11637
Rostami M, Karegar A, Taghavi SM (2021) Biocontrol potential of bacterial isolates from vermicompost and earthworm against the root-knot nematode Meloidogyne javanica infecting tomato plants. Egypt J Biol Pest Control 31:. https://doi.org/10.1186/s41938-021-00383-9
Saia S, Amato G, Frenda AS et al (2014) Influence of arbuscular mycorrhizae on biomass production and nitrogen fixation of berseem clover plants subjected to water stress. PLoS One 9:1–7. https://doi.org/10.1371/JOURNAL.PONE.0090738
Shan J, Liu J, Wang Y et al (2013) Digestion and residue stabilization of bacterial and fungal cells, protein, peptidoglycan, and chitin by the geophagous earthworm Metaphire guillelmi. Soil Biol Biochem 64:9–17. https://doi.org/10.1016/j.soilbio.2013.03.009
Sharma M, Delta AK, Kaushik P (2021) Glomus mosseae and pseudomonas fluorescens application sustains yield and promote tolerance to water stress in Helianthus annuus L. Stresses 1:305–316. https://doi.org/10.3390/stresses1040022
Silawat N, Batav N, Chouhan S et al (2013) Fungal flora of vermicompost and organic manure: a case study of molecular diversity of mucor racemosus using RAPD analysis. Biosci Biotechnol Res Asia 10:509–514. https://doi.org/10.13005/bbra/1224
Soil Survey Stuff (1999) Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd edn. United States Department of Agriculture (USDA), Washinghton D.C.
Takahashi S, Tomita J, Nishioka K et al (2014) Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS One 9:1–9. https://doi.org/10.1371/journal.pone.0105592
Turrini A, Giovannetti M (2012) Arbuscular mycorrhizal fungi in national parks, nature reserves and protected areas worldwide: a strategic perspective for their in situ conservation. Mycorrhiza 22:81–97. https://doi.org/10.1007/s00572-011-0419-6
Turrini A, Bedini A, Loor MB et al (2018) Local diversity of native arbuscular mycorrhizal symbionts differentially affects growth and nutrition of three crop plant species. Biol Fertil Soils 54:203–217. https://doi.org/10.1007/s00374-017-1254-5
Ujvári G, Turrini A, Avio L, Agnolucci M (2021) Possible role of arbuscular mycorrhizal fungi and associated bacteria in the recruitment of endophytic bacterial communities by plant roots. Mycorrhiza 31:527–544. https://doi.org/10.1007/s00572-021-01040-7
Uz I, Tavali IE (2014) Short-term effect of vermicompost application on biological properties of an alkaline soil with high lime content from mediterranean region of Turkey. Sci World J 2014:1–12. https://doi.org/10.1155/2014/395282
Vangelisti A, Natali L, Bernardi R, et al (2018) Transcriptome changes induced by arbuscular mycorrhizal fungi in sunflower (Helianthus annuus L.) roots. Sci Reports 2017 81 8:1–14. https://doi.org/10.1038/s41598-017-18445-0
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: A Guide to Methods and Applications. Academic Press Inc., New York, pp 315–322
Yasir M, Aslam Z, Kim SW et al (2009) Bacterial community composition and chitinase gene diversity of vermicompost with antifungal activity. Bioresour Technol 100:4396–4403. https://doi.org/10.1016/j.biortech.2009.04.015
Yatoo AM, Ali MN, Baba ZA, Hassan B (2021) Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea. A review. Agron Sustain Dev 41:1–26. https://doi.org/10.1007/s13593-020-00657-w
Zhang H, Li J, Zhang Y, Huang K (2020) Quality of vermicompost and microbial community diversity affected by the contrasting temperature during vermicomposting of dewatered sludge. Int J Environ Res Public Health 17:1–12. https://doi.org/10.3390/ijerph17051748
Zhao HT, Li TP, Zhang Y et al (2017) Effects of vermicompost amendment as a basal fertilizer on soil properties and cucumber yield and quality under continuous cropping conditions in a greenhouse. J Soils Sediments 17:2718–2730. https://doi.org/10.1007/s11368-017-1744-y
Zuur AF, Ieno EN, Walker NJ et al (2009) Mixed Effects Models and Extensions in Ecology with R, 1st edn. Springer, New York
Acknowledgements
The authors would like to thank Mariateresa Lazzaro and Fernando Pellegrini for their input in conceptualizing the first part of this study. In addition, special appreciation goes to Giacomo Nardi, Federico Leoni, Stefano Carlesi, and all the technicians of the Centre for Agri-Environmental Research “Enrico Avanzi” of the University of Pisa for their technical support and efforts in managing the field trials. The authors are also grateful to Giulia Carpi and the Centro Lombricoltura Toscano Soc. Semp. Agr. (Pisa, Italy) for their support in supplying the vermicompost material.
Funding
Open access funding provided by Scuola Superiore Sant'Anna within the CRUI-CARE Agreement. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 727672 (LegValue). Gilbert Koskey has a study grant from the Ph.D. programme in Agrobiodiversity at Scuola Superiore Sant’Anna, Pisa, Italy.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Gilbert Koskey, Luciano Avio, Alessandra Turrini and Cristiana Sbrana. The first draft of the manuscript was written by Gilbert Koskey and all authors, reviewed, and commented on previous versions of the manuscript. Project fund acquisition was done by Paolo Bàrberi. Supervision was done by Paolo Bàrberi and Luciano Avio. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Janusz J. Zwiazek.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koskey, G., Avio, L., Turrini, A. et al. Biostimulatory effect of vermicompost extract enhances soil mycorrhizal activity and selectively improves crop productivity. Plant Soil 484, 183–199 (2023). https://doi.org/10.1007/s11104-022-05783-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05783-w