Abstract
Aims
Root exudation may have a large impact on soil biological activity and nutrient cycling. Recent advances in in situ-measurement techniques have enabled deeper insights into the impact of tree root exudation on rhizosphere processes, but the abiotic and biotic controls of exudation rate remain poorly understood. We explored the temperature dependence of root exudation in mature beech (Fagus sylvatica L.) trees.
Methods
We measured fine root exudation in seven beech forests along an elevational gradient (310–800 m a.s.l.) and related carbon (C)-flux rates to mean daily temperature, actual precipitation, mean summer temperature (MST) and precipitation (MAP), soil moisture (SWC), and stand structure.
Results
Average mass-specific exudation (averaged over all sampling dates) ranged from 12.2 µg C g−1 h−1 to 21.6 µg C g−1 h−1 with lowest rates measured at highest elevations and peak rates at mid-elevation (490 m). Regression analyses showed a highly significant positive effect of site-specific daily air and soil temperature on exudation rates (p < 0.01) with an average increase by 2 µg C g−1 h−1 per 1 °C-temperature increase, while the relation to mean summer or annual temperature and mean temperature of the measuring year was less tight. Exudation decreased with increases in mean annual precipitation and soil moisture, but increased with increasing stem density.
Conclusions
The root exudation rate of beech trees roughly triples between 10 °C and 20 °C mean daily temperature, evidencing a large temperature influence on root-borne C flux to the soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate warming affects biogeochemical processes and the carbon (C) cycle of forests through various direct and indirect interactions, notably temperature effects on photosynthesis, plant respiration, soil organic matter (SOM) decomposition, nutrient diffusion in soil, and root nutrient uptake kinetics, and the abundance and activity of mycorrhizal partners (Norby et al. 2007; Yin et al. 2013b). While much experimental research with trees has addressed warming effects on photosynthesis, respiration, belowground C allocation and fine root dynamics (Bai et al. 2010; Graham et al. 2014; Liang et al. 2013; Majdi and Ohrvik 2004), and soil microbial activity and SOM decomposition (von Lützow and Kögel-Knabner 2009), much less is known about the temperature dependence of rhizodeposition, i.e. the loss of carbon from roots (Yin et al. 2013b). Rhizodeposition, which includes exudation (the leakage of soluble organic compounds from living root cells), the secretion of mucilage and other organic substances, and the shedding of dead cells and tissues from roots, represents a net flow of C from roots to the soil which can have profound effects on C and nutrient cycling in forest ecosystems (Jones et al. 2009). Root exudation is thought to be a key mediator in plant-soil interactions that influences the composition and activity of soil microbial communities and thus soil enzyme activity, SOM decomposition and nutrient cycling (Cheng et al. 2014; Fransson and Johansson 2010; Gougherty et al. 2018; Jones et al. 2004; Nguyen 2003; Pausch and Kuzyakov 2017). Estimates of the size of the C flux with root exudation in forest ecosystems vary widely from a few percent up to 21% of net primary productivity (Badri and Vivanco 2009; Jones et al. 2004; Kannenberg and Phillips 2017; Meier et al. 2020; Pinton et al. 2007), or up to a third of the photosynthetic carbon gain of tree saplings (Liese et al. 2018). It is not well understood how an increase in temperature influences exudation rate, as this C flux is dependent on a multitude of biotic and abiotic factors and is rarely quantified under field conditions (Yin et al. 2013b). In fact, it may represent the most uncertain part of the C cycle in ecosystems, especially in mature forests (Price et al. 2012).
Biotic factors influencing root exudation are carbohydrate availability and allocation to roots, plant phenology and development stage, root morphology (notably specific root length, SRL), the type of mycorrhizal fungi, and the presence of pathogenic soil microbes (Kuzyakov 2002; Meier et al. 2013; Neumann and Römheld 2007; Tückmantel et al. 2017) as well as root membrane conductivity. Abiotic factors that have been found to influence root exudation rate include radiation through its influence on photosynthesis (Nakayama and Tateno 2018), deficiency of nutrient elements such as phosphorus (P) and nitrogen (N) (Meier et al. 2020; Yin et al. 2014), soil moisture (Brunn et al. 2022; Jakoby et al. 2020; Preece et al. 2018), and temperature (Yin et al. 2013a). The majority of root exudates are believed to be passively lost from the root, thus being proportional to the root – soil solute concentration gradient (Jones et al. 2004). This suggests a possible influence of microorganism absorption on exudation rate (Canarini et al. 2019).
The few studies that have addressed temperature effects on tree root exudation employed either experimental soil warming of seedling cultures (Karst et al. 2017; Uselman et al. 2000; Yin et al. 2013a, b) or compared trees of the same species at sites differing in mean temperature (Yang et al. 2020). While most studies with trees indicate higher exudation under elevated temperatures, higher exudation at lower temperatures was also reported (Karst et al. 2017; Yang et al. 2020). Due to the multitude of possible influential factors and the difficulties associated with measuring tree root exudation under field conditions, our understanding of climate warming effects on exudation is very limited (Wang et al. 2021). This weakens our capacity to predict changes in soil biological activity and C and nutrient cycling in forest soils in a future warmer world.
In this study, we investigated changes in root exudation in seven mature forests of European beech (Fagus sylvatica L.) along an elevation transect (310 – 800 m a.s.l.) on acidic soil with the aim to explore the responses of root exudation of this tree species to both prevailing thermal conditions and varying mean annual temperature (8.4 – 6.0 °C) at the site. Earlier research in Central European beech forests with an in-situ cuvette-based method showed that mass-specific exudation rates decrease greatly from the topsoil to the subsoil (Tückmantel et al. 2017) and increase with soil acidity and N deficiency of the soil (Meier et al. 2020). To capture the assumed temperature effects, we here extend the sample of studied beech forests to an elevational transect on base-poor bedrock from the colline to the montane zone with a marked temperature decrease and precipitation increase, while soil pH (pH(CaCl2 in 0–10 cm mineral soil: 3.1 – 3.7) and soil N availability (15.2 – 19.9 g g−1) varied only little. We hypothesized that the root exudation rate increases with increasing mean daily temperature due to an overall increase in the tree’s metabolic activity, while the cumulative annual C flux with exudation is primarily determined by growing season length, which increases from the colder to the warmer stands.
Material and methods
Study sites, climate, and geology
The study was conducted in seven European beech forests of mature age along an elevational gradient between 310 and 800 m a.s.l. on the eastern slopes of the Rothaar Mountains in the state of Hesse, central Germany. The study region has a cool-temperate humid climate with MAT decreasing from 8.4 to 6.0 °C and mean annual precipitation (MAP) increasing from 600 to 1200 mm yr−1 from the colline to the montane zone due to orographic lift of air masses that mostly arrive from western directions (German Weather Service, period 1981–2010; Table 1).The elevation transect had a length of approx. 30 km in east–west direction and ranged from the colline/submontane to the montane belt, covering a gradient in mean annual temperature (MAT) of about 2.4 °C and a MAP gradient of 600 mm yr−1. The studied beech forests are thus exposed in downslope direction to increasingly warmer and drier summers, as is predicted to happen in the course of climate warming in the twenty-first century in Central Europe and elsewhere (Kaspar et al. 2017). By employing a space-for-time substitution, this setting may allow rough estimates of anticipated future changes in tree root exudation with climate warming. Besides temperature, we measured soil moisture and several soil chemical parameters as well as fine root biomass (FRB) and fine root morphological traits in order to relate exudation to possible controlling abiotic and biotic factors. All stands belonged to the Luzulo-Fagetum forest community (beech forests on acidic soils) and stocked on acidic bedrock (Triassic sandstone or Paleozoic clay shale) in level to slightly inclined terrain (Table 1). In the forests, study plots of 30 m × 30 m size were selected in sections with closed canopy. While mean diameter at breast height (DBH) varied only between 32 and 45 cm in the seven stands and tree ages ranged mostly between 100 and 180 years according to information from the forest offices, stem density was more variable (150–578 stems ha−1). Mean tree height as measured in 15 trees per plot with a Vertex III height meter (Haglöf, Längsele, Sweden) with at least three measurements taken per tree from different directions decreased with elevation from 33 to 20 m.
During the study period from spring 2014 to winter 2015, air and soil temperature were continuously measured in 2015 with iButton sensors (Maxim, Dallas, USA) installed at 1.5 m height above the forest floor and in the topsoil (3 cm depth) in the seven stands. The sensors were read every 60 min. Dendrometer tapes (type D1, UMS, Munich, Germany; precision of 0.1 mm) were permanently installed at 1.5 m height on 15 trees per plot to determine DBH and annual stem diameter increment through annual DBH recording.
Soil chemical and physical analyses
To characterize soil chemical factors, each five samples were collected in summer 2015 from the uppermost 15 cm of the soil with a 6.6 cm-diameter corer at random position in the study plots. Subsequently, the thickness of the organic layer was measured in the cored hole. All soil samples were separated into organic layer and mineral topsoil material (0–10 cm), transferred to the laboratory in a cooling box, sieved (< 5 mm for organic layer material; < 2 mm for mineral soil), and stored in polyethylene bags at 4 °C for further processing. Subsamples were analyzed in field-moist condition for pH (measured in H2O: 10 g fresh soil in 25 ml deionized water, or in CaCl2: 10 g soil suspended in 0.01 M CaCl2) after 1 h of equilibration. Additional subsamples were dried (60 °C, 48 h), ground, and analyzed for total carbon and nitrogen concentrations through gas chromatography with an elemental analyzer (vario EL III, Elementar Analysensysteme GmbH, Hanau, Germany). Since all soils were highly acidic, total C content was assumed to be organic C (SOC). The total P content was determined by ICP-OES analysis (Perkin Elmer Optima 5300 DV) after acid-pressure digestion (65% HNO3 at 195 °C for 6 h) of the ground soil samples. Plant-available phosphorus was estimated with the resin bag method according to Bowman and Cole (1978) using Dowex 1 × 8–50 anion exchange gel (Dow Water & Process Solutions, USA) that was placed for 16 h in a solution of 1 g of soil material suspended in 30 ml water (Sibbesen 1977). Extracted P was re-exchanged with NaCl and NaOH solutions and the P concentration measured in a spectrophotometer (Libra S 21, Biochrom, UK) at 712 nm after adding 5 mM hexaammonium-heptamolybdate solution (Murphy and Riley 1962).
The water content of the topsoil was determined gravimetrically in each five soil samples collected at random position in the plots every month from March to December 2015. The sampling in August and September was conducted synchronously with the collection of root exudates.
Root exudate collection
Root exudates were collected in three sampling campaigns in July of 2014, August 2015, and September 2015 in nine soil pits excavated at each site in at least 3 m distance to the nearest mature beech tree, employing the cuvette-based in situ-collection approach (after Phillips et al. 2008; Freschet et al. 2021). Beech fine root strands were carefully extracted from the uppermost 10 cm of the soil profile and cleaned with fine forceps and deionized water. Since organic layer thickness increases with elevation (Table 2), the sampled roots were at the uppermost sites 6 and 7 entirely located in the thick organic OF and OH layers, while a greater part of the studied root strands grew in the uppermost humus-rich mineral soil Ah-horizon in the lower plots (1–5) with somewhat thinner organic layers. After cleansing, roots were placed overnight in moist, sandy soil to allow recovery from the excavation process. On the next day, the living terminal root systems (average cumulative length of all parts of the strand c. 16.5 cm and mean diameter c. 0.45 mm) were placed into root cuvettes filled with sterile 2 mm-diameter glass beads to simulate the porosity of the soil and mechanical impedance in a matrix free of carbon. The beads covering the root were moistened with a sterile carbon-free dilute nutrient solution (0.5 mM NH4NO3, 0.1 mM KH2PO4, 0.2 mM K2SO4, 0.15 mM MgSO4, 0.3 mM CaCl2) used as a culture medium.
The roots in the cuvettes were allowed to recover for 48 h before flushing and cleaning the cuvettes 3 to 5 times with culture medium using gentle, low-pressure vacuum (≤ -0.2 bar) induced by a syringe. New sterile culture medium (c. 40 mL) was added and after a full diurnal (photosynthetic) cycle of approx. 24 h, these trap solutions containing exudates were collected for analysis. These solutions were subsequently filtered through sterile glass fiber filters (GE Healthcare Life Sciences Whatman, Glass Microfibre Filters, Grade GF/F) and stored at -20 °C until further analysis. Control samples were taken from rootless cuvettes treated similarly. The samples were analyzed for their dissolved organic carbon using a total carbon analyzer (Shimadzu TOC-L CPH/CPN; Shimadzu Scientific Instruments, Duisburg, Germany). Taking fresh root biomass as a calculation basis, net mass-specific exudation rates (µg C g−1 h−1) and annual C fluxes with exudation per root mass or ground area (mg g−1 yr−1, g C m−2 g−1) were calculated, the latter from site-specific exudation-temperature relationships and temperature variation across the growing season, taking all days with average temperatures > 10 °C into account. This is a rough estimate, as it assumes a constant temperature dependence of exudation across the growing season. To estimate growing season length for the seven sites, we used gridded temperature data provided by the German Weather Service (DWD). Growing season length decreased with decreasing MAT from 170 to 125 d between 310 and 800 m a.s.l. (Table 1).
Fine root biomass and root morphology
In November 2018, 12 root samples were taken at random locations in each of the 30 m × 30 m plots using a soil corer (6.6 cm in diameter) and the material separated into organic layer and mineral topsoil (0–10 cm) material. Samples were transported in a cooling box to the laboratory where they were kept at 4 °C and processed within four weeks. Only fine roots (diameter < 2 mm) of beech were considered in the analysis. All fine root segments were picked out by hand and sorted into live and dead fine root mass under a stereomicroscope (40 × magnification). Root vitality was assessed by means of root color and structure of the root surface, root elasticity and turgescence, branching structure, and the degree of cohesion of cortex, periderm, and stele (for criteria, see Persson, 1978; Meier and Leuschner 2008). Standing FRB was expressed as profile total (organic layer and uppermost 10 cm of mineral soil; in g m−2). Specific root length (SRL, m g−1), specific root surface area (SRA, cm2 g−1), root tissue density (TD, mg cm−3), and root tip frequency (RTF, number of root tips per fine root mass; n g−1) were determined for the root material using a flatbed scanner and the software WinRhizo (Régents Instruments, Quebec, Canada). Fine roots used for exudate collection were clipped off and the biomass of that root segment determined by drying (48 h, 78 °C) and weighing the sample.
Statistical analyses
All statistical analyses were conducted with SPSS software. The data was tested for fit to normal distribution using a Shapiro–Wilk test. Normally distributed data were tested for homogeneity of variances with a Levene test. Site differences between means of edaphic (total C and N content, C/N-ratio, pH (H2O), pH (CaCl2), total and plant-available P content) and root morphological variables (TD, SRA, SRL, and RTF), and exudation rates (net mass-specific exudation rate and annual C flux with exudation) were examined with one-way analysis of variance for parametric data and a Kruskal–Wallis test for non-parametric data. ANOVAs were followed by a Scheffé or a Dunnett T3 test, if homogeneity of variances was not fulfilled. Kruskal–Wallis tests were followed by pairwise comparisons to locate differences.
Pearson correlations were used for investigating the relation between root exudation rate and elevation, climatic and edaphic variables, and root morphological traits. If data were non-normally distributed, Spearman rank correlation analysis was employed. Correlations were tested for the variables long-term mean temperature and precipitation, average summer temperatures of 2014 and 2015, soil water content at the date of exudate sampling, and air and soil temperatures averaged over the seven days prior to sampling. The p-values were adjusted by the Benjamini–Hochberg procedure for multiple testing.
Multiple regression analyses with backward variable elimination were conducted to test for significant independent predictors of root exudation rates and the estimated annual C flux with exudation per m2 ground area. As variation in soil chemical factors across sites was moderate to low due to the sampling design, and root morphology varied little, we ran the initial model with the abiotic factors site-specific daily temperature, soil moisture and elevation, and diameter at breast height and stem density as key characteristics of stand structure. At each elimination step, the variable showing the smallest contribution to the model was deleted until all variables remaining in the model produced significant F statistics. The p-values were calculated via the bootstrapping method because most of the data showed no fit to normal distribution. Variables were tested for multi-collinearity and were excluded when they were highly correlated and collinearity diagnostics (variance inflation factor and tolerance) were critical.
Results
Elevational change in climatic and edaphic conditions
During the two years of measurement, annual mean air temperature (weather station data) decreased at a lapse rate of c. 0.57 °C per 100 m from 9.9 to 7.5 °C in 2014 (and from 9.3 to 6.9 °C in 2015) along the elevational gradient. Both years were on average by about 1.5 °C (2014) and 1.0 °C (2015) warmer than the long-term average of the 1981–2010 period (Tab. 1). Annual precipitation increased from 706 mm at 310 m to 1115 mm at 800 m in 2014, and from 571 to 1054 mm in 2015, corresponding to average increases by 83 and 99 mm per 100-m increase in elevation during the two study years.
The recording of climatic conditions in the stands directly before exudate sampling in summer 2015 gave smaller air temperature (Ta) and soil temperature (Ts) decreases with elevation during the sampling campaigns (Ta decrease by 0.37 and 0.53 °C 100 m−1 and Ts decrease by 0.41 and 0.45 °C 100 m−1 in August and September 2015, respectively; Fig. 1a). The amount of precipitation recorded one week prior to each sampling campaign showed a very slight decrease with elevation in 2014, with the lowest amount measured at the highest sites at 800 m (244 mm) and 690 m (260 mm) and the highest amount at the intermediate sites at 490 m (291 mm) and 560 m (281 mm). Precipitation increased with elevation during the August and September 2015 sampling campaigns (Fig. 1b). Gravimetric soil water content (SWC) of the organic layer plus uppermost mineral soil (0–10 cm) determined for the sampling in August and September 2015 roughly doubled between the lowest and highest sites from 15–25 wt. % at the low-elevation sites to 38–47 vol. % at the high-elevation sites (Fig. 1c). In summer 2015, SWC increased along the gradient from 46.9% at 310 m to 67.0% at 800 m in the organic layer, and from 22.8% to 43.6% in the mineral topsoil (Table 2).
Soil and air temperature (a), precipitation (b) and soil water content (SWC; wt.%) (c) measured in seven forests at two sampling dates in summer/autumn 2015 and one sampling date in summer 2014 (precipitation only) (means ± SE). Temperatures are means of seven-day periods prior to exudate sampling of each 5 sensors per stand, precipitation data are interpolated from gridded weather station data of the German Weather Service (DWD) corrected for elevation, and SWC data are gravimetric samples taken in the organic layer and 0–10 cm mineral soil at the date of sampling (n = 5)
Among the most conspicuous changes observed in soil chemical and physical factors was the doubling in organic layer thickness from about 5.5 cm at 310 m to 10.5 cm at 800 m a.s.l., which was associated with an increase in organic layer mass from 8.0 kg m−2 at 310 m to 15.0 kg m−2 at 800 m (Table 2) and concomitant increases in organic layer C and N stocks. The organic carbon concentration (SOC) in the organic layer material increased from 143.7 mg g−1 at 310 m to 304.4 mg g−1 at 800 m, and that of the mineral topsoil from 26 mg g−1 at 310 m to 111.83 mg g−1 at 800 m. In parallel, total N concentration increased in the organic layer from 7.8 mg g−1 at 310 m to 16.7 mg g−1 at 800 m, and in the mineral topsoil from 1.64 mg g−1 at 310 m to 6.05 mg g−1 at 800 m. Soil C/N ratio and P content showed no elevational trends in both layers (Table 2).
Elevational change in fine root biomass and root morphology
FRB in the organic layer increased with elevation largely in parallel with the increasing forest floor depth from 2.2 g m−2 at 310 m to 24.0 g m−2 at 690 m (significant relation; r = 0.51, p < 0.01), revealing a positive relation to MAP and a negative one to MAT (Table 3). FRB in the mineral topsoil (0–10 cm) varied between 9.3 and 48.9 g m−2 without a clear elevational trend (Fig. 2). Both FRB components were negatively related to the P concentrations in the organic layer (Ptot) and mineral soil (Presin) but were unrelated to soil C/N ratio. None of the examined root morphological parameters (SRL, SRA, TD, RTF) changed significantly with elevation, nor were influenced by climatic or soil chemical variables (Table 3) (Fig. 3).
Fine root biomass of beech in the organic layer (grey bars) and the mineral topsoil (0–10 cm) of the seven beech forests in November 2018 (means ± SE of 12 samples per layer). Different letters indicate significantly different means of organic layer samples (capital letters) and mineral soil samples (small letters)
Elevational change in exudation rates and dependence of exudation on climatic and stand structural properties
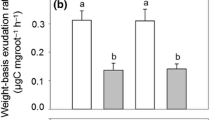
Average mass-specific root exudation rate across the seven sites was 18.34 ± 6 µg g−1 h−1 (Fig. 4a) with site means (averaged over all sampling dates) ranging from 12.2 µg g−1 h−1 at 690 m to 21.6 µg g−1 h−1 at 380 m a.s.l. (peak rates > 37 µg g−1 h−1). While lowest specific exudation rates were measured at the highest sites 690 and 800 m (12.2—15.0 µg g−1 h−1), variation among sites was large and peak rates were recorded at mid elevation (490 m: 22.7 µg g−1 h−1) and not at the lowest sites (Fig. 4a). Yet, the negative relation between mean exudation rate and elevation was highly significant (r = -0.31, p < 0.01; Tab. 3). C flux with exudation per ground area, calculated by multiplying specific exudation with FRB in the organic layer, was significantly higher above 400 m a.s.l. than at lower elevation (Fig. 4b).
Means (and SE) of (a) measured average mass-specific root exudation rate, (b) daily exudation for the fine root mass in the organic layer expressed per m2 ground area, (c) extrapolated annual mass-specific carbon flux with exudation, and (d) estimated annual C flux with exudation per m2 ground area in the topsoil of the seven beech forests along the elevation gradient (averaged over n = 9 samples taken per date and site and n = 3 sampling dates in 2014 and 2015). Annual exudation (c and d) was estimated from the site-specific exudation-temperature relationship, temperature variation across the vegetation period, and the specific length of the vegetation period (number of days with mean temperature ≥ 10 °C) at the sites. As we calculated annual totals with pooled data, no SD can be given in figures c) and d). The exudation flux per ground area was extrapolated using the fine root biomass data from the organic layer (b) or the organic layer and the mineral topsoil (0–10 cm) (d). Different capital letters denote significantly different means at p < 0.05 with p-values adjusted by the Benjamini–Hochberg procedure for multiple comparisons (Kruskal–Wallis test)
Estimated annual cumulative C fluxes with exudation per root mass, derived from site-specific exudation-temperature relationships and recorded temperature variation across the growing season (all days with means > 10 °C), decreased significantly with elevation along the transect, from 80–85 mg g−1 yr−1at 310 m to 40–45 mg g−1 yr−1 at 800 m a.s.l. (mean of the seven sites: 66 ± 29 mg g−1 yr−1; Fig. 4c). Estimating annual C flux per ground area by multiplying the cumulative, mass-specific C flux with the FRB total of the organic layer and mineral topsoil yields a different elevational pattern. Highest stand-level C fluxes were calculated for 380 m (ca. 4.6 g C m−2 yr−1) and 600 m (ca. 3.2 g m−2 yr−1) with highest FRB, and lowest for 310 m (ca. 0.8 g m−2 yr−1) and also 490 m (ca. 1.6 g m−2 yr−1), but without a dependence on elevation (Fig. 4d). Averaged over all sites, we calculated a mean growing season C flux of 2.2 ± 0.2 g m−2.
The environmental factors with strongest influence on mass-specific root exudation rate were the site-specific daily air and soil temperatures with highly significant positive effects (r = 0.66, p < 0.01; Table 3a), revealing a linear increase of exudation rate at a slope of 1.97 μg g−1 h−1 per 1 °C temperature increase (Fig. 5a; Fig. S1 in the Supplement). In contrast, the relationships to MAT and MST and mean temperature of the measuring year were less tight (r = 0.29–0.31, p < 0.01 or n.s.). Close relations were also found for soil moisture (SWC) and MAP (both negative), and the N content of the upper soil (negative) and soil pH (CaCl2) (positive), while soil P content and C/N ratio were not influential (Table 3a). In contrast to actual exudation rate, the annual stand-level C flux depended only on the Presin concentration of the topsoil (negative relation; r = 0.25; p < 0.05) and specific root area (positive relation; r = 0.25; p < 0.05), but not on any climatic factor. Annual fluxes consequently were related neither to actual (average summer) temperature nor to long-term mean summer temperature (MST) (Fig. 5c and d).
Relationships between site-specific daily air temperature (means of the seven days prior to exudate measurement; left panels) or long-term mean summer air temperature (right panels) and (a) and (d) mass-specific root exudation rates, (b) and (e) cumulated annual C exudation per root mass, and (c) and (f) cumulated annual C exudation per ground area in the seven beech forests along the elevation gradient in summer 2015 (averaged over n = 9 samples per date and site; only 2015 data considered). Temperature data were measured with I-button loggers at the sampling sites. Annual rates take the variable length of the vegetation period (no. of days ≥ 10 °C) at the sites into account. Different colour of symbols indicates elevation of sites
While FRB decreased with increasing stem density (r = 0.36), specific root exudation rate did increase (r = 0.32; Table 3b and Fig. S2 in the Supplement).
Multiple regression analyses on climatic and stand structural drivers of root exudation
Multiple regression analyses on the influence of various climatic and stand structural factors on the exudation rate of beech indicate a dominant positive effect of the site-specific daily air temperature (F = 69.7; p < 0.001), followed by a somewhat weaker negative effect of diameter at breast height (DBH; F = 39.3; p < 0.001). The model explained 41% of the variance in exudation rate (Table 4). Annual C flux per ground area was influenced only by DBH, but not by temperature or any other climatic variable; the explained variance was only 14%.
Discussion
The measured mass-specific root exudation rates of the mature beech trees in our study (site means of 12–22 µg C g−1 h−1) were similar to rates reported in other studies for beech in moist soil (10–23 µg C g−1 h−1, Liese et al. 2018; 16–65 µg C g−1 h−1, Meier et al. 2020) and corresponded also to values found in other tree species in the temperate zone (e.g., black locust: 10–22 µg C g−1 h−1, Uselman et al. 2000; loblolly pine: 12–26 µg C g−1 h−1, Meier et al. 2013). Our simple and multiple regression analyses indicate that the thermal conditions during and 7 days prior to sampling are an important factor controlling exudation of beech across the studied elevational gradient. Exudation per root mass increased by about 2 μg C g−1 h−1 per 1 °C temperature increase. In two Mediterranean tree species, Jakoby et al. (2020) found a linear increase in exudation rate per root surface area of about 0.15 μg C cm−2 d−1 per 1 °C increase, and soil temperature together with soil moisture explained exudation dynamics best in one of the species. Uselman et al. (2000) found a 70% higher root exudation in Robinia pseudoacacia seedlings when temperature was increased from 26 to 30 °C.
Several explanations of a positive temperature effect on exudation are possible, which relate to the ‘push’ and ‘pull’ hypotheses of the control of C acquisition by roots (Farrar et al. 2003). In the humid climate of our study region, higher summer temperatures are usually related to higher insolation and thus greater carbon assimilation, as beech photosynthesis is primarily limited by radiation at montane elevation (Schulze 1970). Consequently, under warmer conditions, more C should in principle be available for allocation to roots, which might enhance root exudation due to higher levels of non-structural carbohydrates in roots (Prescott et al. 2020) and a steeper concentration gradient of soluble organic compounds between root cells and the soil (Jones et al. 2004). The photosynthetic capacity of beech at montane elevation in Central Europe reveals a pronounced seasonality with a peak in the warmest months (Schulze 1970), which might imprint on the seasonality of exudation, as photosynthetic capacity and root exudation have been found to be closely related (Sun et al. 2017). Another possible explanation focuses on the role of root exudation for the metabolic activity of rhizosphere biota and its stimulating effect on nutrient supply for root uptake (Jones et al. 2004). Warmer weather likely increases the plant demand for nitrogen and other nutrients as photosynthetic capacity is ramped up, which might trigger roots to stimulate soil microbial activity through active secretion of labile C as an energy source for microbes (Pausch and Kuzyakov 2017). Such a mechanism would fit to the ‘pull’ hypothesis, as exudation then were primarily controlled by factors other than C supply from the canopy (Karst et al. 2017). It is possible that both ‘push’ and ‘pull’ mechanisms are underlying the observed increase in exudation with rising temperature.
The fact that exudation was responsive to increases in the actual air and soil temperature at the site but showed no significant relation to mean growing season temperature (MST), i.e. the long-term average thermal conditions of the site, may suggest thermal acclimation of exudation to the mean temperature at the sites. Since other processes of plant carbon turnover such as respiration and photosynthesis are subject to marked thermal acclimation (Atkin and Tjoelker 2003; Hikosaka et al. 2006) and exudation depends on photosynthetic C gain, we would indeed expect that beech trees growing at lower elevation in a warmer climate down-regulate their exudation rate to a certain degree, at least that fraction of exudates that is controlled by the plant. Mean exudation rates would then become more similar across the elevational gradient. More data from field studies along temperature gradients are needed to test this hypothesis.
Since our model considers only several climatic, soil hydrologic and stand structural factors, while ignoring other likely influencing factors (such as photosynthetic activity and mycorrhizal infection) and covering other drivers only marginally due to limited parameter variation in our sample (notably soil N and P availability), we cannot assess the relative importance of temperature as a determinant of root exudation in beech. However, the positive relation of exudation to temperature is remarkably strong with a correlation coefficient of 0.66, suggesting an important role for summer temperatures and the duration of warm summer periods for exudation in beech.
Soil moisture (SWC) is another abiotic factor with a presumably large effect on exudation. Low to moderate drought seems to increase exudation (Jakoby et al. 2020; Liese et al. 2018; Preece et al. 2018), apparently as a stress response similar to the exudation increase observed upon soil cooling to 4 °C (Karst et al. 2017). The seven beech forests in our study represent a precipitation and soil moisture gradient with annual precipitation in the study year 2015 decreasing to nearly a half (1050 – 570 mm) and mean soil moisture in summer to less than a half (ca. 45 to ca. 20 wt.%) from 800 to 310 m elevation. In fact, the highest exudation rates were measured at the driest sites and the negative relationship to mean soil water content was highly significant, which may offer an alternative explanation of the exudation pattern along the slope, apart from a positive temperature effect. However, the correlation of exudation to soil moisture was less tight than to temperature, and SWC was not included in the multiple regression model. Moreover, mid and late-summer moisture contents in the topsoil of 15–25 wt.% likely have exposed the trees to only mild drought, which may question that exudation was stimulated by drought in our sample. It thus remains unclear, how important water availability is for the explanation of the observed exudation patterns.
Soil nutrient availability is another abiotic factor with a possible influence on the exudation rate. For example, P deficiency results in enhanced root secretion of phenolic compounds in certain species (Neumann and Römheld 1999), and exudation of beech is higher at acidic, N-poor sites than in more fertile soils (Meier et al. 2020), which may trigger increases in decomposition rate and thus N availability through a rhizosphere priming effect (Cheng et al. 2014; Jones et al. 2004; Phillips et al. 2011). The negative correlation between topsoil N content and exudation rate in our sample suggests a stimulating effect of N deficiency on exudation. There are, however, also reports of no influence of soil nitrogen deficiency on exudation, or stimulation by N addition (Uselman et al. 2000; Yin et al. 2013b). Along the studied elevational gradient, organic layer mass on the forest floor doubled from 310 to 800 m a.s.l. with the consequence that topsoil C and N pools increased, whereas C/N ratio, Ptot and Presin content as well as soil pH did not change, suggesting that plant-availability of N and P varied only little. Gradients with larger variation in N and P availability are needed to study the relative importance of nutrient availability on the hand, and temperature on the other, on exudation in beech.
We found only minor alterations in beech fine root morphology from 310 to 800 m a.s.l.; yet, the structure of the fine root system changed markedly. FRB in the organic layer increased roughly tenfold in parallel with the increasing depth of this layer, while FRB in the mineral topsoil varied without a clear elevational trend. We interpret this pronounced shift of FRB to the surface layer as a consequence of decreasing litter decomposition rates toward higher elevations, prompting the beech trees to concentrate their fine root mass in the organic surface layers with highest mineralization rates. This change in root distribution patterns was associated with apparent change in exudation per unit soil volume: While mass-specific exudation rate was somewhat lower, FRB was much larger in the topsoil of the high-elevation beech forests with the consequence that daily exudation rates per topsoil volume were significantly higher above 400 m elevation where the climate is cooler. Higher root densities in colder environments have been observed in northern as well as high-elevation forests (Helmisaari et al. 2007; Kubisch et al. 2016; Moser et al. 2011). They might serve two purposes, to increase the absorbing root surface area under conditions of reduced nutrient supply and to enhance the stimulation of microbial activity under low temperatures. Lower mineralization rates in cooler soil should increase the necessity for the trees to conduct rhizosphere priming by stimulating soil microbial activity through the provision of labile C as an easily accessible energy source.
Conclusions
Our study of root exudation of mature beech trees in seven forests along an elevation (and associated temperature) gradient provides evidence that mass-specific exudation increases with temperature at the time of measurement, whereas the dependence on the site’s average summer temperatures was weak. Although we fully recognize the limitations of our data set with an only short MST gradient and only three measuring campaigns at the seven sites, our results are convincing with respect to the prominent positive temperature effect on exudation, which was more important than effects of soil moisture, precipitation or stand structure. With data on FRB at the sites and information on the length of the growing season, we were able to compare the seven forests with respect to calculated exudation per ground area and to give rough estimates of cumulative exudation per growing season. Both a higher mass-specific exudation rate and a longer growing season length contribute to a generally higher cumulative exudation at the warmer sites. Future research should investigate whether higher exudation and thus more intense rhizosphere priming is a factor that contributes to the generally higher availability of N, P, and other nutrients in soil when temperature increases. Studies on temperature-dependent changes in the composition of exuded substances are another promising field of future study. In combination with earlier studies in European beech forests across edaphic and climatic gradients (Meier et al. 2020; Tückmantel et al. 2017), this study deepens our understanding of environmental controls of root exudation in this model tree species.
Code availability
Not applicable
References
Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8:343–351
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Bai W, Wan S, Niu S, Liu W, Chen Q, Wang Q, Zhang W, Han X, Li L (2010) Increased temperature and precipitation interact to affect root production, mortality, and turnover in a temperate steppe: Implications for ecosystem C cycling. Glob Change Biol 16:1306–1316
Bowman RA, Cole CV (1978) An exploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci 125:95–101
Brunn M, Hafner BD, Zwetsloot MJ, Weikl F, Pritsch K, Hikino K, Ruehr N, Sayer EJ, Bauerle TL (2022) Carbon allocation to root exudates is maintained in mature temperate tree species under drought. New Phytol 235:965–977
Canarini A, Kaiser C, Merchant A, Richter A, Wanek W (2019) Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front Plant Sci 10:157
Cheng W, Parton WJ, Gonzalez‐Meler MA, Phillips R, Asao S, McNickle GG, Brzostek E, Jastrow JD (2014) Synthesis and modelling perspectives of rhizosphere priming. New Phytol 201:31–44
Farrar J, Hawes M, Jones D, Lindow S (2003) How roots control the flux of carbon to the rhizospere. Ecology 84:827–837
Fransson PMA, Johansson EM (2010) Elevated CO2 and nitrogen influence exudation of soluble organic compounds by ectomycorrhizal root systems. FEMS Microb Ecol 71:186–196
Freschet GT, Pagès L, Iversen CM, Comas LH, Rewald B, Roumet C, Klimešová J, Zadworny M, Poorter H, Postma JA, Adams TS et al (2021) A starting guide to root ecology: strengthening ecological concepts and standardizing root classification, sampling, processing and trait measurements. New Phytol 232:973–1122
Gougherty SW, Bauer JE, Pohlman JW (2018) Exudation rates and δ13C signatures of tree root soluble organic carbon in a riparian forest. Biogeochemistry 137:235–252
Graham SL, Hunt JE, Millard P, McSeveny T, Tylianakis JM, Whitehead D (2014) Effects of soil warming and nitrogen addition on soil respiration in a New Zealand tussock grassland. PLoS One 9:91204
Helmisaari H-S, Derome J, Nöjd P, Kukkola M (2007) Fine root biomass in relation to site and stand characteristic in Norway spruce and Scots pine stands. Tree Physiol 27:1493–1504
Hikosaka K, Ishikawa K, Brojigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:313–347
Jakoby G, Rog I, Megidish S, Klein T (2020) Enhanced root exudation of mature broadleaf and conifer trees in a Mediterranean forest during the dry season. Tree Physiol 44:1595–1605
Jones DL, Hodge A, Kuzyakov Y (2004) Plant and mycorrhizal regulation of rhizodeposition. New Phytol 163:459–480
Jones DL, Nguyen C, Finlay RD (2009) Carbon flow in the rhizosphere: carbon trading at the soil-root interface. Plant Soil 321:5–33
Kannenberg SA, Phillips RP (2017) Plant responses to stress impacts: the C we do not see. Tree Physiol 37:151–153
Karst J, Gaster J, Wiley E, Landhäusser SM (2017) Stress differently causes roots of tree seedlings to exude carbon. Tree Physiol 37:154–164
Kaspar F, Mächel H, Jacob D, Kottmeier C (2017) Beobachtung von Klima und Klimawandel in Mitteleuropa und Deutschland. In Brasseur GDJ, Schuck-Zöller S (eds.) Klimawandel in Deutschland. Springer Spektrum, Berlin, pp. 17–26. https://doi.org/10.1007/978-3-662-50397-3_3
Kubisch P, Leuschner C, Coners H, Gruber A, Hertel D (2016) Fine root abundance and dynamics of Stone pine (Pinus cembra) at the alpine treeline is not impaired by self-shading. Front Plant Sci 7:1233
Kuzyakov Y (2002) Review: factors affecting rhizosphere priming effects. J Plant Nutr Soil Sci 165:382–396
Liang J, Xia J, Liu L, Wan S (2013) Global patterns of the responses of leaf-level photosynthesis and respiration in terrestrial plants to experimental warming. J Plant Ecol 6:437–447
Liese R, Lübbe T, Albers NW, Meier IC (2018) The mycorrhizal type governs root exudation and nitrogen uptake of temperate tree species. Tree Physiol 38:83–95
Majdi H, Ohrvik J (2004) Interactive effects of soil warming and fertilization on root production, mortality, and longevity in a Norway spruce stand in Northern Sweden. Glob Change Biol 10:182–188
Meier IC, Leuschner C (2008) Belowground drought response of European beech: fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob Change Biol 14:2081–2095
Meier IC, Avis PG, Phillips RP (2013) Fungal communities influence root exudation rates in pine seedlings. FEMS Microbiol Ecol 83:585–595
Meier IC, Tückmantel T, Heitkötter J, Müller K, Preusser S, Wrobel TJ, Kandeler E, Marschner B, Leuschner C (2020) Root exudation of mature beech forests across a nurient availability gradient: the role of root morphology and fungal activity. New Phytol 226:583–594
Moser G, Leuschner C, Hertel D, Graefe S, Soethe N, Iost S (2011) Elevation effects on the carbon budget of tropical mountain forests (S Ecuador): The role of the belowground compartment. Glob Change Biol 17:2211–2226
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nakayama M, Tateno R (2018) Solar radiation strongly influences the quantity of forest tree exudates. Trees 32:871–879
Neumann G, Römheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130
Neumann G, Römheld V (2007) The release of root exudates as affected by the plant physiological status. In: Pinton R, Varanini Z, Nannipieri Z (eds) The rhizosphere: biochemistry and organic substances at the soil-plant interface, 2nd edn. CRC Press, Boca Raton, pp 23–72
Nguyen C (2003) Rhizodeposition of organic C by plants: Mechanisms and controls. Agronomie 23:375–396
Norby RJ, Rustad LE, Dukes JS, Ojima DS, Parton WJ, Del Grosso SJ, McMurtrie RE, Pepper DA (2007) Ecosystem responses to warming and interacting global change factors. In: Canadell JG, Pataki DE, Pitelka LF (eds) Terrestrial Ecosystems in a Changing World. Springer, Berlin, pp 23–36
Pausch J, Kuzyakov Y (2017) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Chang Biol 24:1–12
Persson H (1978) Root dynamics in a young Scots pine stand in Central Sweden. Oikos 30:508–519
Phillips RP, Erlitz Y, Bier R, Bernhardt ES (2008) New approach for capturing soluble root exudates in forest soils. Funct Ecol 22:990–999
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:1887–1894
Pinton R, Varanini Z, Nannipieri P (2007) The Rhizosphere Biochemistry and Organic Substances at the Soil-Plant Interface. Taylor & Francis Group, New York
Preece C, Farré-Armengol G, Llusia J, Penuelas J (2018) Thirsty tree roots exude more carbon. Tree Physiol 38:690–695
Prescott CE, Grayston SJ, Helmisaari H-S, Kaštovská E, Körner C, Lambers H, Meier IC, Millard P, Ostonen I (2020) Surplus carbon drives allocation and plant–soil interactions. Trends Ecol Evol 35:1110–1118
Price SP, Bradford MA, Ashton MS (2012) Characterizing organic carbon stocks and flows in forest soils. In: Ashton MS, Tyrrell ML, Spalding D, Gentry B (eds) Managing Forest Carbon in a Changing Climate. Springer, Dordrecht, pp 7–30
Schulze E-D (1970) CO2-Gaswechsel der Buche (Fagus sylvatica L.) in Abhängigkeit von den Klimafaktoren im Freiland. Flora 159:177–232
Sibbesen E (1977) A simple ion-exchange resin procedure for extracting plant-available elements from soil. Plant Soil 46:665–669
Sun L, Kominami Y, Yoshimura K, Kitayama K (2017) Root-exudate flux variations among four co-existing canopy species in a temperate forest, Japan. Ecol Res 32:331–339
Tückmantel T, Leuschner C, Preusser S, Kandeler E, Angst G, Mueller CW, Meier IC (2017) Root exudation patterns in a beech forest: Dependence on soil depth, root morphology and environment. Soil Biol Biochem 107:188–197
Uselman SM, Qualls RG, Thomas RB (2000) Effects of increased atmospheric CO2, temperature, and soil N availability on root exudation of dissolved organic carbon by a N-fixing tree (Robinia pseudoacacia L.). Plant Soil 222:191–202
von Lützow M, Kögel-Knabner I (2009) Temperature sensitivity of soil organic matter decomposition—what do we know? Biol Fertil Soils 46:1–15
Wang Q, Chen L, Xu H, Ren K, Xu Z, Tang Y, Xiao J (2021) The effects of warming on root exudation and associated soil N transformation depend on soil nutrient availability. Rhizosphere 17:100263
Yang L, Wang X, Mao Z, Jiang Z, Gao Y, Chen X, Aubrey DP (2020) Root exudation rates decrease with increasing latitude in some tree species. Forests 11:1045
Yin H, Xiao J, Li Y, Chen Z, Cheng X, Zhao C, Liu Q (2013a) Warming effects on root morphological and physiological traits: the potential consequences on soil C dynamics as altered root exudation. Agric For Meteorol 180:287–296
Yin H, Li Y, Xiao J, Xu Z, Cheng X, Liu Q (2013b) Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Glob Chang Biol 19:2158–2167
Yin HJ, Wheeler E, Phillips RP (2014) Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biol Biochem 78:213–221
Acknowledgements
We thank Dietrich Hertel, Mechthild Stange and Irmgard Gerstmann for their skillful support in the root analyses and Hessen Forst for the permit to work on the study sites.
Funding
Open Access funding enabled and organized by Projekt DEAL. The work was funded by the Department of Plant Ecology, University of Göttingen.
Author information
Authors and Affiliations
Contributions
The research idea and study design were conceived by CL and ICM, data collection and analysis were conducted by TT, the writing was conducted by CL with contributions by TT and ICM, all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Does not apply.
Consent to participate
Does not apply.
Consent for publication
Does not apply.
Conflict of interest
Authors declare no conflict of interest.
Additional information
Responsible Editor: Hans Lambers.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leuschner, C., Tückmantel, T. & Meier, I.C. Temperature effects on root exudation in mature beech (Fagus sylvatica L.) forests along an elevational gradient. Plant Soil 481, 147–163 (2022). https://doi.org/10.1007/s11104-022-05629-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05629-5