Abstract
Background and aims
This study investigated whether root traits at the seedling stage are maintained at the flowering stage in two chickpea (Cicer arietinum) genotypes with contrasting root morphology and physiology; and whether the genotype with greater rhizosheath carboxylates mobilises more poorly-available phosphorus (P) pools to increase shoot P at flowering/podding and seed yield at maturity.
Methods
Two chickpea genotypes were grown in a low P soil with or without P addition (0 and 40 µg P g−1 soil as KH2PO4) under controlled glasshouse conditions and harvested at seedling, flowering/podding, physiological maturity.
Results
At the seedling and flowering/podding stages, ICC2884 had thinner roots and greater root mass ratio, specific root length and rhizosheath carboxylates per root dry weight (DW) than ICC456. Both genotypes had smaller root diameter, higher carboxylates and acid phosphatase activity in rhizosheath soil at flowering/podding than at seedling. In the rhizosheath soil of both genotypes, NaHCO3-Pi concentration was depleted under P0 only; under both P0 and P40, NaHCO3-Po concentration increased while NaOH-Pi and NaOH-Po concentrations decreased at the seedling stage but accumulated at the flowering/podding stage, relative to the bulk soil. ICC2884 did not mobilise more poorly available soil P or acquire more P at the seedling or flowering/podding stages, or produce higher seed yields than ICC456.
Conclusion
ICC2884 and ICC456 maintained the difference in root morphological and physiological characteristics from the seedling stage to the flowering/podding stage. The genotype with greater rhizosheath carboxylates (root DW basis) did not produce higher yield than genotype with less rhizosheath carboxylates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) deficiency is a major constraint to crop production worldwide; therefore, a large amount of P fertiliser is applied to sustain food production for a growing world population. The heavy reliance on P fertiliser is rapidly decreasing non-renewable rock phosphate reserve, which is expected to diminish in the next few centuries (Fixen and Johnston 2012). The applied P fertiliser readily forms complexes with oxides and hydroxides of iron and aluminium in acid soil and calcium in alkaline soil, therefore more than 80% is unavailable for most plants (Pang et al. 2021; Raghothama 1999). Chickpea (Cicer arietinum) is the third most valuable grain legume globally, occupying 14.8 Mha (FAOSTAT 2020; https://www.fao.org/faostat/en/#data/QCL) and a significant part of human food and animal feed for developing countries (Foyer et al. 2016). It is key to nutritional security as it provides an important source of protein, dietary fibre and micronutrients (Varshney et al. 2021). Therefore, it is important to develop chickpea genotypes with high P−acquisition and P−use efficiency.
Plants have evolved root functional traits to enhance P acquisition, such as 1) root morphological changes (e.g., higher root mass ratio, thinner roots, increased specific root length) for enhanced soil exploration and nutrient acquisition at minimal carbon cost; 2) enhanced root exudation of carboxylates and acid phosphatases for increased mobilisation of inorganic and organic P fractions sorbed onto soil particles; and 3) formation of symbiotic relationships with mycorrhizal fungal to explore larger soil volumes (Lambers et al. 2006; Pang et al. 2021; Simpson et al. 2011). All these belowground traits associated with P acquisition require photosynthate (Wen et al. 2022); thus, optimising internal resource allocation requires a cost/benefit balance involving the adjustment of various root functional traits at the individual plant level (Lynch and Ho 2005). For example, our previous study showed that a group of 10 chickpea genotypes with greater amount of carboxylates in rhizosheath soil had relatively thinner roots accompanied by greater specific root length and specific root surface area compared with a group of 10 chickpea genotypes with less rhizosheath carboxylates under low P conditions (Wen et al. 2020).
Most studies on chickpea’s root characteristics (root morphology and physiology) have focused on the vegetative stage. There is only limited information on the carboxylates at different developmental stages. For instance, Wouterlood et al. (2004a) showed that the amount of rhizosheath carboxylates increased with the developmental stage (from 19 − 61 days after sowing) in chickpea cultivar Heera, but not in Tyson. Studies on the dynamics of chickpea root exudation of carboxylates and acid phosphatases and the coordination of root morphology with developmental stage are needed.
Root exudation of carboxylates and acid phosphatases enables plants to access additional soil P pools. Resin P and bicarbonate-extractable (NaHCO3) Pi and Po are considered labile P pools (Linquist et al. 1997), while sodium hydroxide-extractable (NaOH) Pi and Po, acid-extractable (HCl) Pi and residual P are considered less labile pools (Hedley et al. 1982). Organic P can only be acquired by roots or mycorrhizal fungi following hydrolysis by root- or microbial-originated phosphatases (Lambers 2022). Studies on the utilisation of different soil P fractions by crops are quite inconsistent. For example, Vu et al. (2008) found that chickpea and wheat deplete both NaOH-Pi and -Po in rhizosheath soil, implying that the NaOH-Pi and -Po pools provide plant-available P. In contrast, maize (Zea mays) and nine legume crops had higher NaOH-Po in rhizosheath soil than bulk soil under both low and high P supply, possibly reflecting high microbial activity and build-up of stable organic P forms, or low plant Po use efficiency (Kamh et al. 1999). Hassan et al. (2012) found that NaOH-Pi was depleted in the rhizosheath soil of faba bean (Vicia faba) but not chickpea. Rose et al. (2010) showed that four crop species, including wheat (Triticum aestivum), faba bean, canola (Brassica napus) and chickpea, deplete NaOH-Pi when grown in alkaline soil, but not in acidic soils. Similarly, the few studies investigating the relationship between seed yield and carboxylates have revealed the lack of a positive correlation, such as in green gram (Vigna radiata) (Pandey et al. 2014) and wheat (Ryan et al. 2014).
This study used two chickpea genotypes with contrasting root characteristics (ICC2884 and ICC456). ICC2884 has consistently greater rhizosheath carboxylates per root dry weight (DW) and smaller root system with thinner roots and greater specific root length than ICC456 at the seedling stage (Pang et al. 2018a; Sharma et al. 2021; Wen et al. 2020). The study investigated whether: 1) differences in root morphological and physiological characteristics associated with P acquisition at the seedling stage would be maintained at the flowering/podding stage; 2) ICC2884 (genotype with greater carboxylate exudation in the rhizosheath) would mobilise more P from poorly available P pools and result in a greater shoot P content at flowering/podding and a higher seed yield at maturity than ICC456 (genotype with lesser carboxylate exudation in the rhizosheath).
Materials and methods
Plant material and growth conditions
This study used two desi chickpea genotypes, ICC2884 and ICC456, originating from Iran and India, respectively (Pang et al. 2018b). They were selected based on their contrasting amount of rhizosheath carboxylates (root DW basis) at the seedling stage when grown under low P conditions, with ICC2884 ~ sixfold greater than ICC456 (Pang et al. 2018a). The reason why we present the amount of rhizosheath carboxylates on the basis of root DW was to show the amount of carboxylate exudation per gram carbon investment as roots, instead of relating carboxylates to root morphology.
Plants were grown in polyvinyl chloride cylindrical (PCV) pots (35 cm height and 9 cm diameter). The bottom of each pot had one 8 mm diameter drainage hole. A plastic bag with four holes (8 mm diameter) at the bottom was lined inside each pot, and ~ 300 g of coarse gravel was added at the bottom of each bag for drainage, but this was not included for any measurement. Each pot was then filled with 3 kg of a 3:7 w/w mixture of field soil collected from Cunderdin Agriculture College (31.64°S, 117.24°E) and washed river sand. The soil volume was 2.1 L per pot. Analysis of the soil mixture was performed by The CSBP FutureFarm analytical Laboratories (Bibra Lake, Australia). It contained 0.6 µg g−1 NH4+-N, 0.6 µg g−1 NO3–-N, 141.2 µg g−1 Colwell potassium, 4.41 µg g−1 available sulphur, 7.5 µg g−1 resin P and 77.3 µg g−1 total P, with a pH (CaCl2) of 7.4. The field soil (upper 0.15 m) was a reddish-brown sandy clay loam, comprising silt (9%), clay (27%) and sand (64%) (Pang et al. 2017b). Basal nutrients provided before planting included 11.25 µg g–1 N as Ca(NO3)2·4H2O, 3.75 µg g−1 N as NH4Cl, 50 µg g−1 S as K2SO4, 4 µg g−1 Mn as MnSO4·H2O, 2 µg g−1 Zn as ZnSO4·7H2O, 0.5 µg g−1 Cu as CuSO4·5H2O, 0.4 µg g−1 Mo as Na2MoO4·2H2O and 5 µg g−1 Fe as FeNaEDTA. The low P treatment (P0) received no P and the high P treatment (P40) received 40 mg kg−1 P as KH2PO4.
On 15 May 2019, three seeds were planted 20 mm deep in each pot and inoculated with approximately 1 g of peat-based Group N rhizobium (New Edge Microbials, Albury, NSW, Australia). Seedlings were thinned to one plant per pot at 14 days after sowing. The soil mixture was initially moistened with nutrient solution to 75% of pot capacity (PC). The water content of the soil mixture at 100% PC was 16% (w/w), which was determined before the experiment, as described in Pang et al. (2017b). The water content in each pot was maintained at 75% PC during the experiment by watering pots to weight with deionized (DI) water every two days. Five days after sowing, the soil surface was covered with a thin layer of plastic beads (66 g) to prevent evaporation. The experiment was undertaken from May to July 2019 in a temperature-controlled glasshouse at The University of Western Australia, with an average day and night temperature of 21 °C and 16 °C, respectively, and a mean relative humidity of 62%.
All shed leaves were collected and dried immediately in an oven at 70 °C. Chickpea plants were harvested on three occasions: seedling stage (44 days after sowing); at the flowering/podding stage (75 days after sowing) when > 90% of plants had commenced flowering and some already had young pods, and physiological maturity (161 days after sowing). At maturity, the number of branches was counted. Branch number was the total number of the main stem, primary and secondary branches (≥ 50 mm long).
Plant and soil measurements at the seedling (vegetative) and flowering/podding (reproductive) stages
For the harvests at the seedling and flowering/podding stages, the plastic bags were carefully pulled out of the pots and cut longitudinally. The root systems were gently lifted from the soil and gently shaken to remove excess soil. The soil remaining attached to the roots was defined as rhizosheath soil (Pang et al. 2017a). Remaining soil was considered as bulk soil. Approximately 20 g of the bulk soil and 3 g of the rhizosheath soil were subsampled for analysis of acid phosphatases and soil P fractions.
Acid phosphomonoesterase (EC 3.1.3.2) activity was measured using the method of Tabatabai (1994). About 1 g of the rhizosheath soil was placed in 10 mL plastic tubes containing 0.2 mL toluene, 4 mL modified universal buffer (pH 6.5) and 1 mL of 0.05 M p-nitrophenyl phosphate solution made up in modified universal buffer (pH 6.5). Tubes were swirled for a few seconds and incubated at 37 °C for 1 h. After incubation, 1 mL of 0.5 M CaCl2 and 4 mL of 0.5 M NaOH were added to each tube and gently shaken. A subsample of the soil suspension was filtered, and its absorbance was measured with a spectrophotometer at 410 nm.
After subsampling the rhizosheath soil, the entire root system with adhering rhizosheath was placed in a beaker containing a known volume of 0.2 mM CaCl2 and gently shaken to remove the rhizosheath soil. For the determination of carboxylates in the rhizosheath soil, a subsample of the rhizosheath extract was taken and filtered through a 0.45 µm syringe filter into a 1 mL high-performance liquid chromatography vial containing one drop of orthophosphoric acid (H3PO4) and then frozen at –20 °C until analysis. The analysis of carboxylates followed the protocol of Cawthray (2003). Organic acid standards of acetic, citric, cis‐aconitic, lactic, maleic, malic, malonic, succinic and trans‐aconitic acid (ICN Biomedicals Inc., Aurora, OH, USA) were included. The amount of total carboxylates per plant recovered from the rhizosheath soil was the product of root DW and the amount of carboxylates on a basis of root DW. The rhizosheath soil in the beaker after the extraction of carboxylates was filtered and oven-dried at 105 °C for 7 d, and then DW was recorded.
Plants were then washed and separated into shoots and roots. The roots were placed into a transparent plastic tray filled with water, separated from nodules which were removed from the tray, and roots were then spread out for scanning root images using an Epson 1680 scanner at 300 dpi (Epson America, Long Beach, CA, USA). Root images were analysed for total root length, root surface area, mean root diameter and root volume using WinRHIZO Pro (Regent Instructions, Quebec, Canada). Plant samples (shoots, roots, nodules) were oven-dried at 70 °C for 72 h and then weighed. Root mass ratio was calculated as the ratio of root DW to total plant DW. Specific root length was calculated as total root length per unit root DW. Root length density was calculated as total root length per unit soil volume. Root mass density was calculated as the ratio of root DW to root volume (Wen et al. 2020).
The dried shoot samples were ground to a fine powder using Geno/Grinder 2010 (Spex SamplePrep, Metuchen, NJ, USA). Weighed subsamples of ~ 100 mg were digested in a hot concentrated nitric-perchloric (3: 1) acid mixture (Pang et al. 2015). The malachite green method was used to determine shoot P concentration (Motomizu et al. 1983). Shoot P content was calculated as the product of shoot P concentration and shoot DW. Physiological P-use efficiency was calculated as the ratio of shoot DW to shoot P concentration (Hammond et al. 2009).
Bulk and rhizosheath soil P extraction at the seedling (vegetative) and flowering/podding (reproductive) stages
Soil P fractions were determined via sequential chemical extraction following a modified Hedley fractionation method (Hedley et al. 1982). About 1 g of soil sample was suspended in 10 mL DI water along with four anion exchange membrane resin strips (4 cm × 1 cm) for 16 h shaking. The resin strips were then transferred into 10 ml of 0.5 M HCl for desorption of Pi for 1 h shaking. This fraction is referred to as resin P. The soil samples after resin P extraction were centrifuged at 3,148 g for 15 min and the supernatant was decanted. The soil samples were then extracted with 10 mL of 0.5 M NaHCO3 (pH 8.5) for 16 h shaking and centrifuged at 3148 g for 15 min, and the supernatant was decanted. To obtain the remaining NaHCO3 P extract in the centrifuge tube, 5 mL of 0.5 M NaCl was added to soil samples and centrifuged at 3,148 g for 5 min, before the supernatant was decanted into the supernatant from the NaHCO3 extraction. These extracts were used to determine NaHCO3-extractable Pi and Pt (total P). For NaOH-extractable Pi, the same extraction protocol was followed but substituting 0.1 M NaOH for 0.5 M NaHCO3. For the determination of NaHCO3-Pt and NaOH-Pt, 3 mL NaHCO3 or 1 mL NaOH extract was added to a new tube, together with 5 mL ammonium persulfate (7.5%, w/v) and 0.5 mL sulfuric acid (1:1, v/v). Samples were autoclaved at 121 °C and 212.3 kPa for 3 h. After digestion, milli-Q water was added to make up the volume to 10 mL. All soil P extractions were analysed using the malachite green method (Motomizu et al. 1983). Po was calculated by subtracting Pt from Pi.
Harvest at physiological maturity
At 161 days after sowing, all plants reached physiological maturity and were cut at ground level. Pods were separated from leaves and stems and then separated into pod walls and seeds. The number of total pods, filled pods, and seeds was recorded. Seed weight was recorded after oven-drying at 30 °C for 7d. Leaves, stems and pod shells were oven-dried at 60 °C for 48 h and weighed. The harvest index was calculated as the ratio of seed weight to total aboveground DW (Pang et al. 2017b). The number of empty pods was calculated as the difference between the number of total pods and filled pods. The percentage of empty pods was the ratio of empty pods to total pods. The number of seeds per pod was calculated as the total number of seeds divided by the total number of filled pods. Mean seed weight was calculated as seed yield divided by the number of seeds.
Statistics
The experiment was a two-factorial (genotype × P level) randomised complete block design with four replicates. General analysis of variance (ANOVA) in GENSTAT v. 19.1 (Lawes Agricultural Trust, Rothamsted Experimental Station, UK, 2018) was used to analyse data. Three sets of analyses were undertaken, 1) A three-way ANOVA for the parameters measured at the seedling and flowering/podding: genotype × P level × growth stage (vegetative, reproductive); 2) Two-way ANOVA for the parameters measured at physiological maturity: genotype × P level; 3) A four-way ANOVA for the soil P fraction data: soil region (rhizosheath and bulk soil) × genotype × P level × growth stage.
Results
Shoot dry weight at the seedling and flowering/podding stages
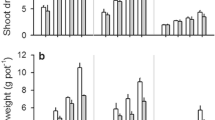
Shoot dry weight was affected by the interactions of genotype × growth stage, and P level × growth stage (Fig. 1, Table 1). These interactions reflect shoot DW being higher for ICC2884 than ICC456, but more so at the flowering/podding stage when shoot DW was highest, and shoot DW being higher at P40 than P0 with this difference being greater at the flowering/podding stage (2.8-fold) than at the seedling stage (2.3-fold).
Shoot P concentration, shoot P content and physiological P-use efficiency at the seedling and flowering/podding stages
Shoot P concentration, shoot P content and physiological P-use efficiency were all affected by the P level × growth stage interaction (Table 1, Fig. 2a, b, c); with no genotype effect. Shoot P concentration and shoot P content under P40 were significantly greater than under P0, more so at the seedling stage than at the flowering/podding stage. Physiological P−use efficiency was low and differed little between P0 and P40 at the seedling stage, however, at the flowering/podding stage it was much higher and this increase was greater at P40 than P0.
Shoot phosphorus (P) concentration (a), shoot P content (b), physiological P-use efficiency (c) and P-utilisation efficiency (d) of two chickpea genotypes (ICC2884 and ICC456) under two levels of P (P0 and P40) at the seedling and flowering/podding stages (means + SE, n = 4). VegP0, ReproP0, VegP40 and ReproP40 represent seedling (vegetative) and flowering/podding (reproductive) stage under P0 and P40, respectively
Root weight and root mass ratio at the seedling and flowering/podding stages
Root DW was affected by the interaction of genotype × growth stage (P < 0.05), P level × growth stage (P < 0.001, Table 1). ICC456 under P0 had 34% and 38% more root DW than ICC2884 at the seedling and flowering/podding stage, respectively, but under P40, root DW differed little between genotypes at the seedling stage and ICC456 had 14% more root DW than ICC2884 at the flowering/podding stage (Fig. 3a). Root DW was similar under P40 and P0 at the seedling stage but 52% greater under P40 than P0 at the flowering/podding stage.
Root dry weight (a), nodule dry weight (b) and root mass ratio (c) of two chickpea genotypes under two levels of phosphorus (P) at the seedling and flowering/podding stages (means + SE, n = 4). The vertical bar in c represents LSD0.05. VegP0, ReproP0, VegP40 and ReproP40 represent seedling (vegetative) and flowering/podding (reproductive) stage under P0 and P40, respectively
Nodule DW was affected by a three-way interaction of genotype × P level × growth stage (P < 0.05, Table 1). Under P0, no nodules were observed at the seedling stage for ICC2884 or ICC456, while at the flowering/podding stage, ICC2884 had 2.6-fold more nodule DW (0.116 g plant‒1) than ICC456 (0.044 g plant‒1) (Fig. 3b). Under P40, nodule DW at the seedling stage did not differ between ICC2884 and ICC456 (~ 0.04 g plant‒1) but increased ~ tenfold for both genotypes at the flowering/podding stage; ICC456 had 26% more nodule DW than ICC2884 at the flowering/podding stage.
Root mass ratio was affected by the interactions of genotype × P level (P < 0.001), genotype × growth stage (P < 0.01), P level × growth stage (P < 0.001, Table 1). Root mass ratio was greatest under P0 at the seedling stage, and lowest under P40 at the flowering/podding stage for both genotypes (Fig. 3c). For both ICC2884 and ICC456, root mass ratio was significantly greater at P0 than their corresponding values at P40, and greater at the seedling stage than their corresponding values at flowering/podding. Under P0, ICC456 had 23% and 46% higher root mass ratios than ICC2884 at the seedling and flowering/podding stages, respectively. Under P40, both genotypes had similar root mass ratios at the seedling stage, but ICC456 had a 24% greater root mass ratio than ICC2884 at the flowering/podding stage.
Root morphology and rhizosheath soil dry weight at the seedling and flowering/podding stages
Total root length was affected by P level, growth stage and their interactions (all P < 0.001), but there was no effects of genotype (Table 1). Total root length at the seedling stage was ~ 10 m and increased significantly from the seedling stage to the flowering/podding stage, more so under P40 (four-fold) than P0 (three-fold) (Fig. 4a). Root surface area followed a similar trend to total root length (data not shown). Root length density also followed a similar trend to total root length (Table 1, Supplementary Fig. S1a).
Total root length (a), mean root diameter (b), specific root length (c) and root mass density (d) of two chickpea genotypes under two levels of phosphorus (P) at the seedling and flowering/podding stages (means + SE, n = 4). VegP0, ReproP0, VegP40 and ReproP40 represent seedling (vegetative) and flowering/podding (reproductive) stage under P0 and P40, respectively
Mean root diameter was affected by genotype (P < 0.01) and growth stage (P < 0.001). ICC2884 had thinner roots (9%) on average than ICC456 (Fig. 4b). Interestingly, the mean root diameter at the seedling stage was 15% greater than that at the flowering/podding stage. Specific root length was affected by the interaction of genotype × P level, P level × growth stage (both P < 0.05). ICC2884 had a greater specific root length than ICC456 under both P0 and P40 at the seedling and flowering/podding stages (Fig. 4c). Specific root length of ICC2884 did not differ significantly between P0 and P40, while ICC456 had a lower specific root length under P0 than P40. Specific root length under P0 and P40 was similar at flowering/podding which was significantly greater than that at P0, but not significant at P40 at the seedling stage.
Significant interactions of genotype × P level (P < 0.01) and genotype × growth stage (P < 0.05) were found for root mass density (Table 1, Fig. 4d). ICC2884 had a significantly lower root mass density than ICC456 at P0, while the opposite trend was found at P40, but the difference between genotypes was not significant. ICC2884 under both P0 and P40 had significantly greater root mass density at the flowering/podding stage than that seedling stage for, while there was little change for ICC456.
Rhizosheath soil DW was affected by growth stage (P < 0.001) and the interaction of genotype × P level (P < 0.05) (Table 1, Supplementary Fig. S1b). Rhizosheath soil DW under P40 was almost twice of that under P0. Rhizosheath soil DW did not differ between ICC2884 and ICC456 under P0, there was little change for ICC2884 but a significant reduction for ICC456 under P40.
Carboxylates and acid phosphatases in the rhizosheath soil at the seedling and flowering/podding stages
Total amount of rhizosheath carboxylates on the basis of root DW was affected by genotype, and the interaction of P level × growth stage (P < 0.05, Table 1, Fig. 5a). Estimated means for ICC2884 and ICC456 were 137 and 108 µmol g−1 root DW; that is, ICC2884 had 27% more rhizosheath carboxylates than ICC456. The amount of rhizosheath carboxylates (root DW basis) under P0 and P40 at the seedling stage differed little but increased significantly from the seedling stage to the flowering/podding stage, more so under P40 (3.7-fold) than P0 (2.7-fold). The total amount of carboxylates per plant was affected by the P level × growth stage interaction (Table 1, Fig. 5b), but there was no genotype effect. The total amount of carboxylates per plant under P0 and P40 at the seedling stage differed little but increased significantly from the seedling to the flowering/podding stage, more so under P40 (13.6-fold) than P0 (5.6-fold).
Amount of total rhizosheath carboxylates per unit root dry weight (a), amount of total rhizosheath carboxylates per plant (b), composition of carboxylates (c) and activity of acid phosphatases (d) in two chickpea genotypes (ICC2884 and ICC456) under two levels of phosphorus (P) at the seedling and flowering/podding stages (means + SE, n = 4). VegP0, ReproP0, VegP40 and ReproP40 represent seedling (vegetative) and flowering/podding (reproductive) stage under P0 and P40, respectively
Carboxylate composition was dominated by malonate (36 − 64%), citrate (9 − 33%), acetate (21 − 26%) and malate (3 − 8%); other carboxylates including cis-aconitate, fumarate, maleate and shikimate accounted for only 0.1‒0.3% of total carboxylates. The treatment effects on carboxylate composition mostly reflected changes in malonate and the corresponding changes in citrate and malate (Table 1, Fig. 5c). The estimated means showed that the proportion of carboxylates as malonate was 20% greater for ICC2884 than ICC456, 22% greater for P40 than P0, and 25% greater at the flowering/podding stage than at the seedling stage. The corresponding values for the proportion of carboxylates as malate and citrate were 33% and 30% lower for ICC2884 than ICC456, 23% greater and 40% lower for P40 than P0, and 19% and 29% lower at the flowering/podding stage than at the seedling stage, respectively.
Acid phosphatase activity was affected by the interactions of genotype × growth stage, and P level × growth stage (both P < 0.05, Table 1, Fig. 5d). These interactions reflect low activity and little difference among genotypes at the seedling stage but increased activity at the flowering/podding stage, more so for ICC456 than ICC2884; and increased activity from the seedling stage to the flowering/podding stage, more so at P0 than P40.
Soil P fractions at the seedling and flowering/podding stages
Soil resin-P was affected by the interaction of soil region × genotype × P level × growth stage (P < 0.05, Table 2, Fig. 6a). Soil resin P was low under P0 and varied little across genotypes and developmental stages (3.5–5.4 μg g‒1). Soil resin P values were much higher under P40 than P0, in bulk soil than rhizosheath soil, at the seedling stage than the flowering/podding stage, and for ICC2284 than ICC456 at the seedling stage but not at the flowering/podding stage.
Concentrations of soil resin P (a), NaHCO3-Pi (b), NaHCO3-Po (c), NaOH-Pi (d) and NaOH-Po (e) of two chickpea genotypes under two levels of phosphorus (P) at the seedling and flowering/podding stages (means + SE, n = 4). Bulk_veg, Rhizo_veg, Bulk_repro and Rhizo_repro represent bulk soil and rhizosheath soil at seedling (vegetative) and flowering/podding (reproductive) stages, respectively. SR, G, P and S represent soil region (bulk or rhizosheath soil), genotype (ICC2884 and ICC456), P treatments (0 or 40 μg g‒1 P soil) and growth stage (vegetative and reproductive stages). *P < 0.05, **P < 0.01 and ***P < 0.001. The vertical bars in (a) and (e) represent LSD0.05
NaHCO3-Pi concentrations under P0 were lower than under P40 for both genotypes (Fig. 6b). Significant genotype effects occurred for NaHCO3-Pi concentration (P < 0.01, Table 2), with 12% higher estimated means for ICC2884 than ICC456. The soil region × P level interaction was also significant (P < 0.05). The rhizosheath soil had a 37% lower NaHCO3-Pi concentration (3.5 μg g‒1) than the bulk soil (5.7 μg g‒1) under P0, while no difference occurred under P40. NaHCO3-Po concentration was significantly affected by the soil region × genotype × P level interaction (P < 0.05, Table 2, Fig. 6c). The interaction reflects that the NaHCO3-Po concentration in rhizosheath soil was 3.1- and 1.7-fold greater for ICC2884, while it was 1.8- and 2.3-folder greater for ICC456, at P0 and P40, respectively, compared with the bulk soil.
The NaOH-Pi concentration was significantly affected by soil region × P level × growth stage interaction (P < 0.05, Table 2, Fig. 6d), but not affected by genotype (P > 0.05). At the seedling stage, the NaOH-Pi concentration in rhizosheath soil was slightly less under P0 and P40, but slightly higher under P0 and significantly higher (by 38%) under P40 at the flowering/podding stage, relative to the bulk soil. The soil NaOH-Po concentration was significantly affected by the interaction of soil region × genotype × P level × growth stage (P < 0.01, Table 2, Fig. 6e). For both genotypes at the seedling stage, the rhizosheath soil had a lower NaOH-Po concentration than the bulk soil under P0 and P40, with the greatest difference for ICC2884 under P0, followed by ICC456 under P0 and P40, and the smallest difference for ICC2884 under P40. At the flowering/podding stage, both genotypes had significantly higher NaOH-Po concentrations in rhizosheath soil under P0 (17 μg g‒1) and P40 (~ 14.5 μg g‒1) than the bulk soil (8.5‒10.3 μg g‒1).
Yield and yield components at maturity
At maturity, ICC2884 had more total aboveground DW than ICC456 under P0, while the opposite occurred under P40 (P < 0.05, Table 3). ICC456 had more shoot branches than ICC2884 under P0 and P40 (P < 0.01), and more shoot branches were produced under P40 than P0 (P < 0.001). The estimated means of genotypes showed that seed yield and harvest index under P40 were 62% greater (P < 0.001) and 9% lower (P < 0.05) than those at P0, but they were not affected by genotypes (P > 0.05).
The total number of pods per plant was affected by P level (P < 0.001, Table 3), with both genotypes producing ~ 90 pods under P0 and ~ 140 pods under P40. The number of empty pods per plant followed a similar trend, with more empty pods produced under P40 than P0 (P < 0.001), i.e. eight-fold and 4.5-fold greater for ICC2884 and ICC456, respectively. No significant difference occurred between ICC2884 and ICC456 for the number of filled pods per plant (~ 80 pods) under P0, but ICC456 produced 36% more filled pods (99 pods) than ICC2884 (73 pods) under P40 (P < 0.05). A significant genotype × P level interaction occurred for the total number of seeds per plant (P < 0.01); ICC2884 did not significantly differ under P0 and P40 (~ 107 seeds), similar to ICC456 under P40 (110 seeds) but significantly more than ICC456 under P0 (83 seeds). The percentage of empty pods was similar in ICC2884 and ICC456 at P0, and was significantly greater at P40 with 50% empty pods in ICC2884 while this was 30% in ICC456 (P < 0.05). The number of seeds per pod was significantly affected by genotype; ICC2884 produced 30% more seeds per pod than ICC456 under P0 and P40 (P < 0.001). Mean seed weight in ICC2884 and ICC456 was 96 mg and 107 mg per seed under P0, and 140 mg and 163 mg per seed under P40, respectively (P < 0.001). The estimated means showed that ICC2884 had a 14% smaller mean seed size than ICC456 (P < 0.001). The genotype × P level interaction was not significant for the number of shoot branches, seed yield, harvest index, number of total and empty pods, number of seeds per pod or mean seed weight (P > 0.05).
Discussion
Genotypic differences in root growth, morphological and physiological characteristics at the seedling and flowering/podding stages
Consistent with our previous findings showing that ICC456 had a greater root mass ratio than ICC2884 under low P conditions at the seedling stage (Pang et al. 2018a), the present study showed a similar trend at the flowering/podding stage. In addition, the present study showed that relative to P40, root mass ratio of ICC456 (the genotype with a larger root system) under P0 was greater than that of ICC2884 (the genotype with a smaller root system) at both seedling and flowering/podding stages; an increased root mass ratio in response to low P supply is often regarded an acclimation to a low P supply (Lambers et al. 2006). This finding extends our previous knowledge, revealing that ICC456 had greater resilience in root mass ratio at the flowering/podding stage, in addition to that at the seedling stage. However, the difference in root mass ratio between ICC456 and ICC2884 was small under P40 at both seedling and flowering/podding stages which was consistent with results of Wen et al. (2020) showing that the difference in root mass ratio between a group of 10 chickpea genotypes with a greater amount of carboxylates (root DW basis) in the rhizosheath and another group of 10 genotypes with less carboxylates was much smaller under adequate P than under P deficiency at the seedling stage.
Our results show that ICC2884 had thinner roots and greater specific root length than ICC456 under P0 and P40 at the seedling and flowering/podding stages, supporting and extending our previous study under low P (Pang et al. 2018a) and another study under ample or low P (Wen et al. 2020) at the seedling stage. The thinner roots and greater specific root length of ICC2884 than ICC456 regardless of developmental stage or P level, though to different degrees, indicates a persistent genotypic difference in root morphology between ICC2884 and ICC456. Wen et al. (2020) suggested that chickpea genotypes (e.g., ICC2884) with more rhizosheath carboxylates, thinner roots, higher specific root length and faster growth rate have lower construction costs per unit root length, enabling plants to explore a larger soil volume to acquire more P under low P availability (Haling et al. 2016; McCormack et al. 2015; Vance et al. 2003). In contrast, chickpea genotypes (e.g., ICC456) with less rhizosheath carboxylates, thicker roots and a relatively slower growth rate are more aligned with a conservative strategy (Wen et al. 2020).
The present study also shows that genotypes differed in their root morphological parameters dependent on their developmental stage. Both ICC2884 and ICC456 showed a reduced root diameter at the flowering/podding stage relative to that at the seedling stage, regardless of P level; however, the smaller root diameter of ICC2884 at flowering/podding was accompanied with an increased root mass density under both P0 and P40, but not for ICC456. This contrasts with previous findings that chickpea genotypes with thinner roots generally have a lower root mass density at the seedling stage (Wen et al. 2020). The greater root mass density of ICC2884 at the flowering/podding stage might be due to root anatomical and architectural adaptations such as increased frequency and length of lateral roots (Strock et al. 2018; Zhan and Lynch 2015). Thus, the root mass density of the genotype with thinner roots (i.e. ICC2884) was more responsive to developmental stage, regardless of P level.
ICC2884 had 27% more rhizosheath carboxylates (root DW basis) than ICC456 (averaged across P levels and growth stages). This is generally consistent with our previous findings showing that ICC2884 had more carboxylates (root DW basis) than ICC456 under low P (Pang et al. 2018a), or three P levels (i.e. extreme P deficiency, moderate P deficiency and adequate P) (Wen et al. 2022) at the seedling stage. However, the difference in rhizosheath carboxylates (root DW basis) was much smaller in the present study than in Pang et al. (2018a), who reported a six-fold difference between these two genotypes. This discrepancy might be due to the different growth media used. In those studies, the carboxylate amount was the amount recovered in the rhizosheath soil, as affected by the amount of carboxylates exuded by roots, and the amount of organic anions lost by soil sorption and breakdown by soil microorganisms. The soil used in the present study contained more clay than the pure river sand used by Pang et al. (2018a); therefore, more carboxylates might have been sorbed onto soil particles, as different soil properties have different sorption abilities (Wang and Lambers 2020). Soil texture also affects microbial composition and abundance, as preferential microbial degradation of carboxylates reduces the quantities of some carboxylates, affecting the amount of carboxylates recovered from the rhizosheath soil. The sorption of organic anions onto soil particles would decrease extractable carboxylates, and the sorption property of organic anions is often affected by soil properties such as pH, organic matter content and clay content (Jones et al. 2003).
The present study also shows that significantly more rhizosheath carboxylates were produced at the flowering/podding stage than the seedling stage in both genotypes. Wouterlood et al. (2004a) found that the amount of rhizosheath carboxylates increased during the development of chickpea cultivar Heera, but not in cultivar Tyson. Dynamic rhizosheath carboxylates during plant development have been reported in other cereal species, but in much lower amounts than chickpea, e.g., in wheat, the amount increased before stem elongation and then decreased until after flowering (Wang et al. 2017), and rice (Oryza sativa) having the lowest amount at the seedling stage, increasing until flowering and then decreasing until maturity (Aulakh et al. 2001). Plants tend to grow faster during earlier growth stages, leaving little carbon for later carboxylate release (Prescott 2022). Both ICC2884 and ICC456 at the flowering/podding stage had more rhizosheath carboxylates under P40 than P0. Wouterlood et al. (2004b) also found an increased amount of carboxylates (root DW basis) in response to increased P levels in chickpea cultivar Heera, but not Tyson. Wen et al. (2020) showed that rhizosheath carboxylates (root DW basis) increased with increasing soil P availability during the seedling stage, in contrast with other studies showing the typical increase in carboxylate release and ‘P-mining strategy’ under low P (Lambers et al. 2015b). The increase in carboxylate exudation under P-sufficient conditions might 1) function as a mechanism to dispose of carbon excess to growth requirements (He et al. 2021; Prescott 2022; Prescott et al. 2020), 2) function as a defence strategy to protect roots against pathogens, insects or competing neighbours (Li and Copeland 2000), or 3) provide critical substrate to shape the composition and function of microbial communities in the rhizosheath to sustain plant health (Martin et al. 2016; Zhalnina et al. 2018).
There was a large shift in the composition of carboxylates in the rhizosheath soil in response to low P. Both genotypes had a greater proportion of citrate and smaller proportion of malonate under P0 than under P40 at the seedling and flowering/podding stages. Citrate is the only tri-carboxylate identified in chickpea, and is generally more effective in P mobilisation than di-carboxylates such as malonate and malate (Jones and Brassington 1998; Wouterlood et al. 2004a).
The present study also showed that plant developmental stage affected the composition of carboxylates, with 25% more malonate and 41% less citrate at the flowering/podding stage than at the seedling stage. Wouterlood et al. (2004a) also showed that the carboxylate composition of two chickpea cultivars (Heera and Tyson) changed during plant development; however, the amount of carboxylates comprising malonate decreased with development (from 19 to 61 days after sowing) under all four P levels, accompanied with an increased proportion of citrate which contrasts with the present findings. The difference in the direction of the proportion of malonate and citrate with developmental stage might be due to differences in genotypes or soil texture, which needs further investigation. The lower proportion of citrate at the flowering/podding stage implies that the increased total amount of carboxylates relative to that at the seedling stage may not lead to more effective mobilisation of P from insoluble and organic P sources as citrate is generally more effective than di-carboxylates such as malonate and malate (Jones and Brassington 1998; Wouterlood et al. 2004a).
The release of acid phosphatases and phytases is important for mobilising organic P (Richardson et al. 2011, 2022; Tarafdar et al. 2001). Our study showed that significantly greater acid phosphatase activities at the flowering/podding stage than at the seedling stage under P0 and P40 for both genotypes. In Brassica napus, acid phosphatase activities differed during the five whole-life developmental stages, with the greatest value at the flowering stage in one season and at leaf development stage in the following season which was significantly correlated with seed yield at maturity under low P availability (Duan et al. 2020). Joshi et al. (2021) found that maize plants during early vegetative growth under P deficiency had increased acid phosphatase activities, improved microbial community structure and reduced Po pool compared with those at a later vegetative growth stage. The increased acid phosphatase activities during the flowering/podding stage in the present study suggests that the lower resin-Pi availability possibly triggered its production; the enhanced activity of phosphatases at flowering/podding in the present study did not deplete but instead accumulated Po (i.e., NaHCO3-Po and NaOH-Po) in rhizosheath soil. Increased acid phosphatase activity does not necessarily mean Po mineralisation because the accessibility of compounds hydrolysable by phosphatases might limit Po mineralisation (Giles et al. 2017; Tarafdar and Claassen 1988).
It should also be noted that the present study only assessed genotypic differences at one low P level, which may not capture the differences in root traits that might occur across a P-responsive range, since acclimation may also depend on the degree of P stress experienced by the genotypes.
Changes in P fractions in the rhizosheath soil
Both ICC2884 and ICC456 under P40 depleted more resin-P than under P0. The P level affected NaHCO3-Pi depletion in rhizosheath soil, being 37% lower than in bulk soil under P0, with little difference under P40. This is consistent with Kamh et al. (1999), who found that maize and nine leguminous cover crops depleted more resin-P under high P supply, but more NaHCO3-Pi under low P supply, suggesting that NaHCO3-Pi is more important under low P conditions. Similarly, Hassan et al. (2012) reported the strong depletion of NaHCO3-Pi in rhizosheath soil by faba bean and chickpea, indicating their strong ability to access this labile P.
Both ICC2884 and ICC456 had significantly higher NaHCO3-Po concentrations in rhizosheath soil than in bulk soil under P0 and P40 at the seedling and flowering/podding stages, consistent with the findings of Hassan et al. (2012). NaHCO3-Po concentration, which includes the microbial P component (Maher and Thorrold 1989), is generally considered the most labile and easily mineralisable Po pool (Bowman and Cole 1978; Linquist et al. 1997). Kamh et al. (1999) reported higher NaHCO3-Po concentrations in rhizosheath soil than in bulk soil for Lupinus albus, suggesting enhanced microbial activity in the rhizosphere or the formation of a Fe-citrate-P complex. In contrast, other studies found significantly lower NaHCO3-Po concentration in rhizosheath soil than in bulk soil for chickpea and wheat (Vu et al. 2008) and for perennial ryegrass (Lolium perenne) and radiata pine (Pinus radiata), which was related to increased microbial biomass levels (Chen et al. 2002).
For the first time, the present study has shown that chickpea developmental stage affects the dynamics of NaOH-Pi and NaOH-Po concentrations in rhizosheath soil during the seedling and flowering/podding stages, regardless of P treatment. At the seedling stage, NaOH-Pi and NaOH-Po concentrations in rhizosheath soil were lower than in bulk soil, indicating that chickpea roots depleted NaOH-Pi and NaOH-Po, such that chickpea mobilised NaOH-Pi and NaOH-Po followed by Pi uptake. Chickpea roots release large quantities of carboxylates and acid phosphatases, as carboxylates mobilise both Pi and Po sorbed onto soil particles, and acid phosphatases break down Po into Pi (Lambers et al. 2015a). Interestingly, while rhizosheath carboxylates and acid phosphatases significantly increased at the flowering/podding stage relative to the seedling stage, both NaOH-Pi and NaOH-Po concentrations in rhizosheath soil significantly increased at flowering/podding relative to those in the bulk soil, which was opposite to that at the seedling stage. Increased NaOH-Pi and NaOH-Po concentrations in rhizosheath soil might be associated with the transformation of inorganic P into organic P by soil microbes due to the increased carbon released from roots, including carboxylates and polysaccharide mucilage for microbes that colonise the rhizosheath (Richardson et al. 2011, 2022; Wang and Lambers 2020). In addition, the increased NaOH-Pi and NaOH-Po pools in the rhizosheath soil at the flowering/podding stage might be due to the conversion of P from more recalcitrant fractions by carboxylates or other root exudates. It is worth noting that the present study did not quantify HCl-extractable P and residue P, but should be investigated in a future study. Studies have reported the depletion of acid-extractable fractions (HCl-Pi) and labile P pools (resin- and NaHCO3-Pi), but it is unclear whether this was due to direct depletion or accelerated P dissolution (Rose et al. 2010; Vu et al. 2008).
More rhizosheath carboxylates did not equate to higher seed yield
ICC2884 produced slightly higher seed yield than ICC456 under P0 but lower seed yield under P40; however, the differences were not significant. This was because the greater seed number in ICC2884 under P0 was compromised by its smaller seed size than that of ICC456. In green gram (Vigna radiata), greater carboxylate exudation from roots did not always result in higher grain yield (Pandey et al. 2014). Similarly, enhanced citrate exudation in wheat near-isogenic lines showed no consistent advantage for biomass production or yield (Ryan et al. 2014). In the present study, ICC2884 did not acquire more P at the seedling or flowering/podding stages under P0 or P40 than ICC456. This is consistent with the findings of Pearse et al. (2007), who showed that the ability of a crop species to use P from aluminium, iron or calcium phosphate does not correlate consistently with carboxylate exudation. In chickpea, Wen et al. (2020) found that shoot DW and shoot P content in a group of 10 genotypes with more carboxylates in their rhizosheath did not differ from a group of 10 genotypes with less carboxylates at the seedling stage at 10 µg g−1 P soil, supplied as insoluble FePO4. In the present study, while ICC2884 had more rhizosheath carboxylates on the basis of root DW than ICC456, both genotypes had a similar amount of total carboxylates per plant and total root length under P0 and P40 at the seedling and flowering/podding stages. In addition, the present study only included two chickpea genotypes, which were not bred for high yield. Thus, the contribution of carboxylates to seed yield needs further investigation in chickpea. Crossing chickpea genotypes containing large amounts of carboxylates in the rhizosheath soil with high-yielding commercial varieties should be undertaken to investigate the potential benefit of carboxylates for P acquisition and yield improvement.
Conclusions
Both ICC2884 and ICC456 reduced root diameter and increased carboxylates and acid phosphatase activities in rhizosheath soil at the flowering/podding stage, relative to at the seedling stage. However, ICC2884 at the seedling and flowering/podding stages maintained thinner roots and greater root mass ratio, specific root length and rhizosheath carboxylates (root DW basis) relative to ICC456. In the rhizosheath soil of both genotypes, the NaHCO3-Pi concentration was depleted under P0 only; under both P0 and P40 the NaHCO3-Po concentration increased; NaOH-Pi and NaOH-Po concentrations decreased at the seedling stage but accumulated at the flowering/podding stage, relative to the bulk soil. ICC2884, the genotype with greater carboxylate exudation (root DW basis), did not mobilise more poorly available soil P or acquire more P at the seedling or flowering/podding stage or increased yield, compared with ICC456 (genotype with less carboxylate exudation).
References
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol 3:139–148. https://doi.org/10.1055/s-2001-12905
Bowman RA, Cole CV (1978) Transformations of organic phosphorus substrates in soils as evaluated by NaHCO3 extraction. Soil Sci 125:49–54. https://doi.org/10.1097/00010694-197801000-00008
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr 1011:233–240. https://doi.org/10.1016/S0021-9673(03)01129-4
Chen CR, Condron LM, Davis MR, Sherlock RR (2002) Phosphorus dynamics in the rhizosphere of perennial ryegrass (Lolium perenne L.) and radiata pine (Pinus radiata D. Don.). Soil Biol Biochem 34:487–499. https://doi.org/10.1016/S0038-0717(01)00207-3
Duan XJ, Jin KM, Ding GD, Wang C, Cai HM, Wang SL, White PJ, Xu FS, Shi L (2020) The impact of different morphological and biochemical root traits on phosphorus acquisition and seed yield of Brassica napus. Field Crops Res 258. https://doi.org/10.1016/j.fcr.2020.107960
Fixen PE, Johnston AM (2012) World fertilizer nutrient reserves: a view to the future. J Sci Food Agric 92:1001–1005. https://doi.org/10.1002/jsfa.4532
Foyer CH, Lam H-M, Nguyen HT, Siddique KHM, Varshney RK, Colmer TD, Cowling W, Bramley H, Mori TA, Hodgson JM et al (2016) Neglecting legumes has compromised human health and sustainable food production. Nat Plants 2:16112. https://doi.org/10.1038/nplants.2016.112
Giles CD, George TS, Brown LK, Mezeli MM, Richardson AE, Shand CA, Wendler R, Darch T, Menezes-Blackburn D, Cooper P et al (2017) Does the combination of citrate and phytase exudation in Nicotiana tabacum promote the acquisition of endogenous soil organic phosphorus? Plant Soil 412:43–59. https://doi.org/10.1007/s11104-016-2884-3
Haling RE, Yang Z, Shadwell N, Culvenor RA, Stefanski A, Ryan MH, Sandral GA, Kidd DR, Lambers H, Simpson RJ (2016) Root morphological traits that determine phosphorus-acquisition efficiency and critical external phosphorus requirement in pasture species. Funct Plant Biol 43:815–826. https://doi.org/10.1071/FP16037
Hammond JP, Broadley MR, White PJ, King GJ, Bowen HC, Hayden R, Meacham MC, Mead A, Overs T, Spracklen WP et al (2009) Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J Exp Bot 60:1953–1968. https://doi.org/10.1093/jxb/erp083
Hassan HM, Marschner P, McNeill A, Tang C (2012) Growth, P uptake in grain legumes and changes in rhizosphere soil P pools. Biol Fertil Soils 48:151–159. https://doi.org/10.1007/s00374-011-0612-y
He H, Zhang Z, Peng Q, Chang C, Su R, Cheng X, Li Y, Pang J, Du S, Lambers H (2021) Increasing nitrogen supply to phosphorus-deficient Medicago sativa decreases shoot growth and enhances root exudation of tartrate to discharge surplus carbon dependent on nitrogen form. Plant Soil 469:193–211. https://doi.org/10.1007/s11104-021-05161-y
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976. https://doi.org/10.2136/sssaj1982.03615995004600050017x
Jones DL, Brassington DS (1998) Sorption of organic acids in acid soils and its implications in the rhizosphere. Eur J Soil Sci 49:447–455. https://doi.org/10.1046/j.1365-2389.1998.4930447.x
Jones DL, Dennis PG, Owen AG, van Hees PAW (2003) Organic acid behavior in soils – misconceptions and knowledge gaps. Plant Soil 248:31–41. https://doi.org/10.1023/a:1022304332313
Joshi SR, Morris JW, Tfaily MM, Young RP, McNear DH (2021) Low soil phosphorus availability triggers maize growth stage specific rhizosphere processes leading to mineralization of organic P. Plant Soil 459:423–440. https://doi.org/10.1007/s11104-020-04774-z
Kamh M, Horst WJ, Amer F, Mostafa H, Maier P (1999) Mobilization of soil and fertilizer phosphate by cover crops. Plant Soil 211:19. https://doi.org/10.1023/a:1004543716488
Lambers H (2022) Phosphorus acquisition and utilization in plants. Annu Rev Plant Biol 73: null. https://doi.org/10.1146/annurev-arplant-102720-125738.
Lambers H, Hayes PE, Laliberte E, Oliveira RS, Turner BL (2015a) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90. https://doi.org/10.1016/j.tplants.2014.10.007
Lambers H, Martinoia E, Renton M (2015b) Plant adaptations to severely phosphorus-impoverished soils. Curr Opin Plant Biol 25:23–31. https://doi.org/10.1016/j.pbi.2015.04.002
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Li J, Copeland L (2000) Role of malonate in chickpeas. Phytochemistry 54:585–589. https://doi.org/10.1016/s0031-9422(00)00162-x
Linquist BA, Singleton PW, Cassman KG (1997) Inorganic and organic phosphorus dynamics during a build-up and decline of available phosphorus in an ultisol. Soil Sci 162:254–264. https://doi.org/10.1097/00010694-199704000-00003
Lynch J, Ho M (2005) Rhizoeconomics: Carbon costs of phosphorus acquisition. Plant Soil 269:45–56. https://doi.org/10.1007/s11104-004-1096-4
Maher FM, Thorrold BS (1989) Accumulation of phosphorus fractions in yellow-brown pumice soils with development. New Zealand J Agric Res 32:53–62. https://doi.org/10.1080/00288233.1989.10423477
Martin BC, George SJ, Price CA, Shahsavari E, Ball AS, Tibbett M, Ryan MH (2016) Citrate and malonate increase microbial activity and alter microbial community composition in uncontaminated and diesel-contaminated soil microcosms. Soil 2:487–498. https://doi.org/10.5194/soil-2-487-2016
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB et al (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207:505–518. https://doi.org/10.1111/nph.13363
Motomizu S, Wakimoto T, Toei K (1983) Spectrophotometric determination of phosphate in river waters with molybdate and malachite green. Analyst 108:361–367. https://doi.org/10.1039/AN9830800361
Pandey R, Meena SK, Krishnapriya V, Ahmad A, Kishora N (2014) Root carboxylate exudation capacity under phosphorus stress does not improve grain yield in green gram. Plant Cell Rep 33:919–928. https://doi.org/10.1007/s00299-014-1570-2
Pang J, Bansal R, Zhao H, Bohuon E, Lambers H, Ryan MH, Ranathunge K, Siddique KHM (2018a) The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529. https://doi.org/10.1111/nph.15200
Pang J, Ryan MH, Siddique KH, Simpson RJ (2017a) Unwrapping the rhizosheath. Plant Soil 418:129–139. https://doi.org/10.1007/s11104-017-3358-y
Pang J, Turner NC, Khan T, Du Y-L, Xiong J-L, Colmer TD, Devilla R, Stefanova K, Siddique KHM (2017b) Response of chickpea (Cicer arietinum L.) to terminal drought: leaf stomatal conductance, pod abscisic acid concentration, and seed set. J Exp Bot 68:1973–1985. https://doi.org/10.1093/jxb/erw153
Pang J, Wen Z, Kidd D, Ryan MH, Yu RP, Li L, Cong WF, Siddique KHM, Lambers H (2021) Advances in understanding plant root uptake of phosphorus. In: Gregory PJ (ed) Understanding and improving crop root function. Burleigh Dodds Science Publishing Limited, Cambridge
Pang J, Yang J, Lambers H, Tibbett M, Siddique KHM, Ryan MH (2015) Physiological and morphological adaptations of herbaceous perennial legumes allow differential access to sources of varyingly soluble phosphate. Physiol Plant 154:511–525. https://doi.org/10.1111/ppl.12297
Pang J, Zhao H, Bansal R, Bohuon E, Lambers H, Ryan MH, Siddique KHM (2018b) Leaf transpiration plays a role in phosphorus acquisition among a large set of chickpea genotypes. Plant Cell Environ 41:2069–2079. https://doi.org/10.1111/pce.13139
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MDA, Lambers H (2007) Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol 173:181–190. https://doi.org/10.1111/j.1469-8137.2006.01897.x
Prescott CE (2022) Sinks for plant surplus carbon explain several ecological phenomena. Plant Soil. https://doi.org/10.1007/s11104-022-05390-9
Prescott CE, Grayston SJ, Helmisaari H-S, Kaštovská E, Körner C, Lambers H, Meier IC, Millard P, Ostonen I (2020) Surplus carbon drives allocation and plant-soil interactions. Trends Ecol Evol 35:1110–1118. https://doi.org/10.1016/j.tree.2020.08.007
Raghothama KG (1999) Phosphate acquisition. Annu Rev Plant Physiol Plant Mol Biol 50:665–693. https://doi.org/10.1146/annurev.arplant.50.1.665
Richardson A, Lynch J, Ryan P, Delhaize E, Smith F, Smith S, Harvey P, Ryan M, Veneklaas E, Lambers H et al (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156. https://doi.org/10.1007/s11104-011-0950-4
Richardson AE, George TS, Hens M, Delhaize E, Ryan PR, Simpson RJ, Hocking PJ (2022) Organic anions facilitate the mobilization of soil organic phosphorus and its subsequent lability to phosphatases. Plant Soil. https://doi.org/10.1007/s11104-022-05405-5
Rose TJ, Hardiputra B, Rengel Z (2010) Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant Soil 326:159–170. https://doi.org/10.1007/s11104-009-9990-4
Ryan PR, James RA, Weligama C, Delhaize E, Rattey A, Lewis DC, Bovill WD, McDonald G, Rathjen TM, Wang E et al (2014) Can citrate efflux from roots improve phosphorus uptake by plants? Testing the hypothesis with near-isogenic lines of wheat. Physiol Plant 151:230–242. https://doi.org/10.1111/ppl.12150
Sharma M, Pang J, Wen Z, De Borda A, Kim HS, Liu Y, Lambers H, Ryan MH, Siddique KHM (2021) A significant increase in rhizosheath carboxylates and greater specific root length in response to terminal drought is associated with greater relative phosphorus acquisition in chickpea. Plant Soil 460:51–68. https://doi.org/10.1007/s11104-020-04776-x
Simpson R, Oberson A, Culvenor R, Ryan M, Veneklaas E, Lambers H, Lynch J, Ryan P, Delhaize E, Smith F et al (2011) Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant Soil 349:89–120. https://doi.org/10.1007/s11104-011-0880-1
Strock CF, Morrow de la Riva L, Lynch JP (2018) Reduction in root secondary growth as a strategy for phosphorus acquisition. Plant Physiol 176:691–703. https://doi.org/10.1104/pp.17.01583
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Angle S, Bottomley P, Smith S, Tabatabai MA, Wollum A (eds) Methods of soil analysis Part 2 Microbiological and biochemical properties. Soil Science Society of America, Madison
Tarafdar JC, Claassen N (1988) Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biol Fertil Soils 5:308–312. https://doi.org/10.1007/bf00262137
Tarafdar JC, Yadav RS, Meena SC (2001) Comparative efficiency of acid phosphatase originated from plant and fungal sources. J Plant Nutr Soil Sci 164:279–282. https://doi.org/10.1002/1522-2624(200106)164:3%3c279::AID-JPLN279%3e3.0.CO;2-L
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447. https://doi.org/10.1046/j.1469-8137.2003.00695.x
Varshney RK, Roorkiwal M, Sun S, Bajaj P, Chitikineni A, Thudi M, Singh NP, Du X, Upadhyaya HD, Khan AW et al (2021) A chickpea genetic variation map based on the sequencing of 3,366 genomes. Nature 599:622–627. https://doi.org/10.1038/s41586-021-04066-1
Vu DT, Tang C, Armstrong RD (2008) Changes and availability of P fractions following 65 years of P application to a calcareous soil in a Mediterranean climate. Plant Soil 304:21–33. https://doi.org/10.1007/s11104-007-9516-x
Wang Y, Krogstad T, Clarke N, Øgaard AF, Clarke JL (2017) Impact of phosphorus on rhizosphere organic anions of wheat at different growth stages under field conditions. AoB PLANTS 9. https://doi.org/10.1093/aobpla/plx008
Wang Y, Lambers H (2020) Root-released organic anions in response to low phosphorus availability: recent progress, challenges and future perspectives. Plant Soil 447:135–156. https://doi.org/10.1007/s11104-019-03972-8
Wen Z, Pang J, Tueux G, Liu Y, Shen J, Ryan MH, Lambers H, Siddique KHM (2020) Contrasting patterns in biomass allocation, root morphology and mycorrhizal symbiosis for phosphorus acquisition among 20 chickpea genotypes with different amounts of rhizosheath carboxylates. Funct Ecol 34:1311–1324. https://doi.org/10.1111/1365-2435.13562
Wen Z, White PJ, Shen J, Lambers H (2022) Linking root exudation to belowground economic traits for resource acquisition. New Phytol 233:1620–1635. https://doi.org/10.1111/nph.17854
Wouterlood M, Cawthray GR, Scanlon TT, Lambers H, Veneklaas EJ (2004a) Carboxylate concentrations in the rhizosphere of lateral roots of chickpea (Cicer arietinum) increase during plant development, but are not correlated with phosphorus status of soil or plants. New Phytol 162:745–753. https://doi.org/10.1111/j.1469-8137.2004.01070.x
Wouterlood M, Cawthray GR, Turner S, Lambers H, Veneklaas EJ (2004b) Rhizosphere carboxylate concentrations of chickpea are affected by genotype and soil type. Plant Soil 261:1–10. https://doi.org/10.1023/B:PLSO.0000035568.28893.f6
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, Cho H, Karaoz U, Loqué D, Bowen BP et al (2018) Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3:470–480. https://doi.org/10.1038/s41564-018-0129-3
Zhan A, Lynch JP (2015) Reduced frequency of lateral root branching improves N capture from low-N soils in maize. J Exp Bot 66:2055–2065. https://doi.org/10.1093/jxb/erv007
Acknowledgements
The work was funded by the Australian Research Council (LP200100341) and the UWA Institute of Agriculture. We thank Mr Greg Cawthray for technical help with the quantification of carboxylates, and Mr. Rob Creasy and Mr. Bill Piasini for help in maintaining the plants in the glasshouse.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
J.P., K.H.M.S. and H.S.K. designed the study; H.S.K., G.B., Z.W., M.S., B.M., G.G. and J.P. performed the experiments and collected the data; J.P. and H.S.K. analysed and interpreted the data; J.P. led the writing of the manuscript. M.H.R., H.L. and K.H.M.S. contributed critically to the drafts and all authors gave final approval for publication.
Corresponding author
Ethics declarations
The authors declare that we have no known competing financial interests.
Additional information
Responsible Editor: Tim S. George.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pang, J., Kim, H.S., Boitt, G. et al. Root diameter decreases and rhizosheath carboxylates and acid phosphatases increase in chickpea during plant development. Plant Soil 476, 219–238 (2022). https://doi.org/10.1007/s11104-022-05579-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05579-y