Abstract
Aims
Climate change imposes adaptation of viticulture in risk areas, such as the Mediterranean. Mycorrhization is a valid tool to reduce the impact of the expected temperature/drought increase. Aim of this work was to test the effects of mycorrhization on grapevine vegetative growth, element composition of soil/leaves, and microbiota of bulk soil/rhizosphere/endorhiza, in the field, under exacerbated summer stress conditions obtained by planting the rootstocks in June.
Methods
118 rooted cuttings of 1103-Paulsen (Vitis berlandieri × Vitis rupestris) were planted in Salento (Apulia, Southern Italy); about half of them were mycorrhized. Leaf Area Index, shoot growth and survival rate were monitored across two growing seasons. Leaf/shoot weight, chemical analysis of 25 elements, and 16S rRNA gene metabarcoding of bulk soil/rhizosphere/endorhiza were performed on subsamples.
Results
Mycorrhized plants showed significantly higher survival rate and growth, and accumulated significantly higher amounts of 18 elements. 27 endorhizal OTUs (representing ~20% of total sequences) were differently distributed (20 OTUs more abundant in mycorrhized plants); in the rhizosphere, instead, 12 OTUs (~2.5% of total sequences) were differently distributed. A few Actinobacterial OTUs were enriched by mycorrhization in the root endosphere; the same OTUs were the most correlated with the chemical elements, suggesting a role in element dynamics. These OTUs were not hub taxa of the co-occurrence network.
Conclusions
This work shed light onto the interactions between mycorrhiza and microbiome, in the context of plant element dynamics, which is useful to identify potential target candidates for biotechnological applications, thus moving towards a more sustainable, ecosystem-based viticulture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Grapevine is a perennial crop grown in various areas of the world, and viticulture represents a very strategic agricultural sector internationally. Nowadays, viticulture must adapt to new challenges such as climate change and drought increase. Analyzing climate change projections to 2050, global warming levels are assumed to reach + 1.5–2.0 °C (Hristov et al. 2020), which clearly indicates a threat to agriculture in the medium-to-long term. Moreover, in the Southern areas of Europe, the disadvantages of the climate change are expected to be greater than in the Northern areas (Christensen and Christensen 2007; Dinu et al. 2021a; Giorgi et al. 2004; Hristov et al. 2020). The expected decrease in water availability and the increase in extreme weather events (Beniston and Diaz 2004) may cause lower and/or uncertain yields as well as a reduction of areas suitable for cultivation, with the consequence of a general reduction in farm income (Ciais et al. 2005). Losses, especially in Southern Europe, can be reduced by tailored adaptation strategies, for example through the use of resistant varieties and/or by increasing and improving irrigation practices for certain crops (when sustainability requirements are met). However, limitations on sustainable levels of water extraction could become a barrier to increasing irrigation levels, particularly in Mediterranean Countries where the duration of water scarcity during global warming is expected to intensify (Hristov et al. 2020). Both the soil humidity and the water use efficiency of grapevine plants, play a key role for grapevine growth, as well as for grape yield and quality. Often the vineyards are grown in areas with a semi-arid climate, especially during the vegetative season, and this can cause water deficits in the plant. Furthermore, according to global climate models, in the near future, the water deficit could become even more a limiting factor for the development of the vine and for the quality of wines (Pachauri et al. 2014). It has already been shown that climate change affects plant phenology and grape ripeness (Jones and Davis 2000; Webb et al. 2007).
The adaptation of viticulture to water deficit can benefit from the use of arbuscular mycorrhizal fungi (AMF) (Trouvelot et al. 2015), the most widespread fungal symbionts of plants, being associated with over 80% of current terrestrial plants (Smith and Read 1997). AMF are known to improve drought tolerance in grapevines (Augé 2001). This can be attributed to the contribution of hyphae which absorb water more efficiently (Hardie and Leyton 1981; Sieverding 1981), favored by a better soil-root contact (Reid 1978), a greater stimulation of gas exchange through an increase in root penetration force (Allen et al. 1981; Snellgrove et al. 1982), and the effects on the adjustment of the osmotic potential (Ψ) inside the host plant (Allen and Boosalis 1983). In vines colonized by AMF, improved performances in the early stages of the implantation reduce transplant stress, thus increasing the chances of survival for the cuttings (Trouvelot et al. 2015; Van Rooyen et al. 2004). Besides improving plant tolerance to drought, AMF can help plants to acquire mineral nutrients from the soil. Generally, an improved uptake for P, Zn, Cu, S, Ca, K, Fe, Mg, Mn, Cl, Br and N has been reported (Bonfante and Genre 2010; Tinker 1984). Furthermore, AMF modify the plant microbiome, both in the rhizosphere and in the endosphere. These effects are due in first instance to direct recruitment of bacteria, such as the so-called mycorrhiza-helper bacteria (Frey-Klett et al. 2007). Moreover, AMF affect the rest of the microbiome members, through indirect interactions mediated by plant responses (Kroll et al. 2017), further microbe-microbe interactions (Hassani et al. 2018), and redistribution of nutrients and minerals in leaves (Gerlach et al. 2015). In vineyards, inoculation with AMF increases positive associations between grapevine-associated soil microbes, pointing out to the necessity to further explore this aspect (Torres et al. 2021).
Understanding the highly complex nature of the plant microbiome in response to biological, chemical, and physical alterations in the rhizosphere, remains a significant challenge in plant microbiology (Lugtenberg et al. 2002). Within both the rhizosphere (Bhattacharyya and Jha 2012) and the endosphere (Genre et al. 2020), some microbial species, known as Plant Growth-Promoting Rhizobacteria (PGPR), can exert a positive effect on plants with direct growth promotion mechanisms, or indirectly by competitive exclusion or inhibition of pathogens. Improved acquisition of mineral nutrients is among the mechanisms underlying the plant growth promoting effect (Malinowski et al. 2000; Rahman et al. 2005). Notwithstanding the practice of mycorrhization is very common in agriculture and is suggested in grapevine nursery (Aguín et al. 2004), studies in open fields and/or productive vineyards are scarce. In addition, to the best of our knowledge, no study so far considered the complex interactions between grapevine growth, bacterial microbiota and soil nutrients, as affected by AMF inoculation in the field.
Soil microbiome interacts with the grapevine rootstocks, and this interaction is crucial for plant resilience and adaptation to biotic and abiotic stresses (Darriaut et al. 2022). Omics methods have helped in unraveling the complex nature and the potential functions of the grapevine root microbiome (Alaimo et al. 2018). For example, it has been shown that root microbiome in grapevine is involved with growth-defense tradeoff balancing (Nerva et al. 2022). Altogether, the most recent literature points out to the fact that the grapevine plant is involved in intricated relationships with the associated microbes, eventually defining the grapevine holobiont (Bettenfeld et al. 2021).
This applicative research aspires to be a useful support for winegrowers which should deal with extreme growing conditions in viticultural regions where the climate change could compromise the possibility of growing vines soon. To simulate a future climate change scenario, the plantation was established at the beginning of summer. In details, we aimed at investigating the interactions between grapevine rootstocks, bacterial microbiota and dynamics of chemical elements’ absorption and accumulation, in response to mycorrhization treatment in unsterile field conditions. To do this, we monitored the growth parameters of mycorrhized and not mycorrhized grapevine rootstocks across two growing seasons (2020–2021), performed chemical analyses of soil and leaves, and characterized the bacterial microbiota of bulk soil/rhizosphere/endorhiza by 16S rRNA gene metabarcoding. Moreover, we aimed at assessing which bacterial taxa might be involved in the dynamics of element absorption and/or accumulation, by correlating their distribution patterns with those of the elements; we also tested by co-occurrence analysis whether these were also hub taxa of the microbial network.
We hypothesized that: i) mycorrhization will improve both agronomical performances and element absorption and/or accumulation in the grapevine; ii) there will be an enrichment of positive bacterial responders, especially in the root endosphere; III) bacterial responders will be correlated to chemical elements’ dynamics.
Materials and methods
Experimental design, site, and treatments
The experiment was carried out during a commercial vineyard replanting in a field belonging to Tenute Eméra di Claudio Quarta (Lizzano, Apulia, Italy; Lat. 40°21′12.3“N Long. 17°25’32.2”E). Rooted cuttings of 1103 Paulsen (Vitis berlandieri × Vitis rupestris) were used. This genotype is widely used in the experimental area by winegrowers because of its relatively high tolerance to drought. In fact, also in hyper-arid growing conditions it allows an equilibrate plant growth with a good grape yield, despite the difficulties of this genotype in adsorbing Potassium (Verdugo-Vásquez et al. 2021). For the experiment, a total of 118 rooted cuttings were late planted in field on June 8th 2020, in order to exacerbate summer stresses, thus simulating possible future climate change scenarios. Before plantation, the rooted cuttings were placed in water to rehydrate the plant tissues for 24 hours. At this time, 62 plants were inoculated by adding 33 g/L of a commercial powdery formulation of mycorrhiza (Micosat F. SEMI, CCS AOSTA S.r.l., Italy) to the rehydrating water. This product includes five different species of mycorrhizal inoculum (Glomus coronatum GU 53, Glomus caledonium GM 24, Glomus mosseae GP 11, Glomus viscosum GC 41 and Rhizophagus irregularis RI 31), as well as the bacteria Bacillus subtilis, Paenibacillus durus and Pseudomonas fluorescens.
The field used in this work is traditionally used for viticulture and it is located in a plain area. The soil analyses are reported in Table S1. Furthermore, four samples of bulk soils were chemically and microbiologically analyzed, as described below. The parcel was prepared according to the farm agronomic practices with a soil tillage (preserving the superficial rocks at about 30 cm depth) and a fertilization with 30 kg/ha of P2O5 and 50 kg/ha of K2O. Superficial tillage was performed twice during the experiment to eliminate weeds (in autumn 2020 and in spring 2021). During the first season, two water irrigations were given: 30 L/plant at planting and 20 L/plant as emergency irrigation on July 13th, 2020. Meteorological data of the last 10 years (January 2011–July 2021) are publicly released by the closest meteorological station belonging to ARPA Puglia (Taranto - C/o Colonia San Vito, Lat 40.423332, Long 17.225431 - http://www.webgis.arpa.puglia.it/meteo/index.php) and they were used to describe the local growing conditions.
Grapevine vegetative development
Grapevine growth parameters were observed during one entire year, spanning over the whole first vegetation season and the growth restart after dormancy (first half of the second vegetation season). Different parameters were measured during the site inspections (Table 1).
The total leaf area was measured with the smartphone app ‘Easy Leaf Area Free’ (Easlon and Bloom 2014), already used for grapevine analyses in the field by Dinu et al. (2021b). To avoid interferences caused by the reddish soil, white plastic sheets were placed under the young plants to obtain a white background, thus optimizing the App recording quality. Unfortunately, due to the positioning of reddish stake braces closed to the plants during the dormancy, it was not possible to measure the total leaf area during the second season by using this method.
Numbers and lengths of plants and shoots were recorded by visual inspections and mason’s tape measure, respectively. Data were analyzed statistically by Student’s T test, comparing the mycorrhized vs. not mycorrhized plants for each timepoint.
The 14th of August 2020, a subsample of 5 control and 5 mycorrhized plants were eradicated for fresh weight measurement, elemental and microbiological analyses. These plants were selected based on the total leaf area measured during the previous inspection (8th July 2020), to be representative of the average plant growth of the two experimental groups. Simultaneously, four bulk soil samples (two in between of mycorrhized plants and two in between of control plants) were collected at a depth of about 20 cm (within the tilled soil zone). Roots and bulk soil samples were stored at −20 °C for microbiological analysis; stems and leaves were separated and weighted for each plant, and then frozen at −20 °C. Bulk soil and leaf samples were then used also for elemental analysis.
Elemental analysis
Concentration of 25 elements (Aluminum - Al, Boron - B, Barium - Ba, Beryllium - Be, Bismuth - Bi, Calcium - Ca, Cadmium - Cd, Cobalt - Co, Chrome - Cr, Copper - Cu, Iron - Fe, Gallium – Ga, Potassium - K, Lithium - Li, Magnesium - Mg, Manganese - Mn, Sodium - Na, Nickel - Ni, Phosphorus P, Lead - Pb, Selenium - Se, Strontium - Sr, Tellurium - Te, Thallium - Tl, and Zinc - Zn) was measured in both bulk soil and grapevine leaf samples using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES, Thermo Fisher Scientific, Waltham, Massachusetts). Each sample was weighted (~1 g) and mixed with 4 mL of H2O2 and 6 mL of suprapure HNO3 69%, then treated at 180 °C for 10 min, using a microwave digestion system (Milestone START D). The samples were then cooled, diluted with suprapure water to a final volume of 20 mL, filtered through syringe filters (pore size 0.45 μm), and then measured for element content using an ICP-AES (Thermo Scientific, iCap 6000 Series) spectrometer. An ICP Multi-Element Standard Solution VIII (Merck, Darmstadt, Germany), consisting of 24 elements in dilute nitric acid, was used to construct the calibration lines for spectrometer relatively to 24 analytes (Al, B, Ba, Be, Bi, Ca, Cd, Co, Cr, Cu, Fe, Ga, K, Li, Mg, Mn, Na, Ni, Pb, Se, Sr, Te, Tl, Zn). For quantitative analysis, eight standards were prepared for calibration line, in order to cover a large range of concentrations. The calibration line for P was constructed using a single element standard solution (Carlo Erba Reagenti, Rodano, Italy) and nine standard solutions containing known elemental concentrations of 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 20 and 40 mg/L. Two secondary source standard solutions of 0.05 and 1 ppm concentrations were prepared and used in order to control instrumental efficiency, calibration lines, and standard solution integrity. The calibration lines showed correlation coefficients (r) greater than 0.99 for all measured elements.
Results for each plant were expressed as the average of three different measurements, with elemental concentrations expressed as ppm (mg/kg of fresh weight). The Certified Reference Material used for quality control and quality assurance was the CPAchem (Stara Zagora, Bulgaria) Multi-Element Standard Solution.
The total amount of elements accumulated in the leaves, was calculated by multiplying the element concentration by the total leaf weight of each plant.
To highlight a possible modulation of the absorption selectivity carried out by the interactions between plant and mycorrhiza with respect to the plant itself, the ratio between the total amount of each element with respect to the sum of the total amounts of all the elements analyzed was calculated for each plant.
Data were analyzed statistically by Student’s T test, comparing the mycorrhized vs. not mycorrhized plants/bulk soils for each element. Principal Component Analysis (PCA) was used to calculate the main functions able to describe the data variability.
Microbiological analysis (DNA extraction - Illumina sequencing)
Total DNA was extracted from bulk soil, rhizospheric soil and endosphere using the FastDNA™ Spin Kit for Soil (MP Biomedicals, Solon, OH, USA), according to the manufacturer’s instructions. Prior to DNA extraction, samples of bulk soil (BU-A, BU-B and BU-C samples; Table S2) were solely sieved (diameter: 2 mm), whereas rhizospheric soil was collected by gently scraping and washing the soil adhering to the roots of five mycorrhized (SR-C samples) and five control (SR-A and SR-B samples) plants (Table S2). Bare roots (without soil attached) were treated for obtaining the fractions corresponding to endosphere (A, B and C samples; Table S2). In detail, 2 g of roots were surface-sterilized by immersion (5 min, under stirring) in 16 ml of hydrogen peroxide solution at 15%. Roots were then washed (10 min, under stirring) in 0.1 M phosphate saline buffer (PBS; pH 7), to remove residues of hydrogen peroxide. Afterwards, washed roots were dried using sterile gauze, frozen, and ground using sterile pestle and mortar. Ground roots were added with 16 ml of PBS and homogenized for 3 min in a 400P Bag Mixer. Under laminar flow, the homogenate was twice filtered on two layers of sterile gauze and centrifuged (200 x g for 5 min at 4 °C) to precipitate plant tissue debris. The supernatant was further centrifuged (20,000 x g for 5 min at 4 °C) and the resulting pellet, supposedly containing DNA from microorganisms inhabiting root endosphere, was subjected to DNA extraction. DNA extracted from 300 mg of bulk soil, 300 mg of rhizospheric soil and root endosphere was used as template for high-throughput sequencing performed by Genomix4Life S.r.l. (Baronissi, Salerno, Italy) using the primers 27F (5’-AGRGTTTGATCMTGGCTCAG-3′) and 519R (5’-GTNTTACNGCGGCKGCTG-3′) to amplify the V1-V3 region of the 16S rRNA genes. Libraries were prepared using the following protocol: (i) the PCR amplification reaction mix was composed of 2.5 μl of template DNA (whose concentration was standardized to 5 ng/μl), 5 μl of each forward and reverse primer (1 μM), and 12.5 μl of 2x KAPA HiFi HotStart Ready Mix; (ii) the amplification included initial denaturation (95 °C for 30 s), followed by 25 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and final elongation (72 °C for 5 min). High-throughput sequencing was carried out with the Illumina platform, MiSeq, 2 × 301 bp, V3, paired end.
Bioinformatic analysis
Illumina sequencing data were analyzed with QIIME 1.9 (Caporaso et al. 2010). Paired ends were joined with the “fastq-join” method (Aronesty 2013) and a quality threshold of 25 was used to filter out low-quality reads. Short sequences (< 200 nucleotides) were deleted at this step. Chimeric sequences were detected with Vsearch (Rognes et al. 2016) and eliminated. Operational taxonomic units (OTUs) at 97% sequence similarity were generated with the sumaclust method, which was proven to be more precise than other clustering methods (Jackson et al. 2016), and identified with the uclust method and the SILVA database release 132 (Quast et al. 2013). Unidentified, contaminant (plastidic and mitochondrial) and singleton OTUs were eliminated.
Alpha-diversity measures (Good’s coverage, Shannon, Equitability, Dominance, Simpson, Simpson reciprocal, Simpson evenness, Phylogenetic diversity, Chao1 and Observed OTUs) and beta-diversity metrics (Weighted Unifrac and Bray-Curtis distances) were calculated with the QIIME scripts “parallel_alpha_diversity.py” and “beta_diversity_through_plots.py”, respectively, on a rarefied dataset normalized to an even sequencing depth of 11,100 reads per sample. Statistical comparison of alpha diversity between the two treatments (mycorrhized vs. control) sample groups was performed with SPSS version 20 (IBM Corporation, USA). The T-test of Student or the Mann-Whitney U test (both at p ≤ 0.05) were used to compare the two treatment groups, depending on whether the variable was normally distributed or not, respectively; ANOVA with Tukey post-hoc test was used to compare habitats (bulk soil, rhizosphere, endosphere). Statistical significance of groups in the beta-diversity analysis was tested with ADONIS (McArdle and Anderson 2001), as implemented in Qiime. Significantly different OTUs between treatments were assessed by g-test of independence (at FDR-corrected p ≤ 0.05), as implemented in Qiime; for this test, the endosphere and the rhizosphere were tested separately: for rhizosphere, a deeper dataset rarefaction (45,100 reads per sample) was used.
The software Explicet (Robertson et al. 2013) was used to generate taxonomy bar plots and rarefaction curves. Adobe Photoshop CS6 (Adobe Systems Inc., USA) was used to assemble and label the final figures.
Sequences were submitted to the European Nucleotide Archive (www.ebi.ac.uk/ena) under the accession number PRJEB50609.
Correlation between OTUs and chemical elements
A correlation between the OTUs’ frequency profiles and chemical elements was performed for the rhizosphere and for the endosphere, separately, in order to identify possible responder taxa or drivers of element assimilation in the plants. For this analysis, both element concentrations and total amounts were considered (only for elements having detectable quantity in at least one leaf sample), while the 150 most abundant OTUs of each rhizosphere or endosphere were used. To obtain the correlation matrices, Pearson’s rho correlations (significant at p < 0.05) were calculated in SPSS, and the matrices obtained, after removal of non-significant OTUs, were colored in Microsoft excel to obtain heatmaps. Profiles of OTUs and elements were used to build Unweighted Pair Group Method with Arithmetic mean (UPGMA) trees, which were then used as a guide to rank the heatmaps to show the clustering of both elements and OTUs. A Canonical correspondence analysis (CCA) was performed to further explore the relationships between the bacterial microbiota in the rhizosphere/endorhiza and the element concentrations/amounts in the leaves, by using the software PAST (Hammer et al. 2001). For this analysis, the non-rarefied dataset was used, including all OTUs with more than 5 reads in the corresponding dataset (rhizospheric or endorhizal dataset).
Co-occurrence network analysis
A co-occurrence analysis was performed to find hub OTUs and to verify whether these coincided with the OTUs mostly correlated with the chemical elements. The software CoNet (Faust and Raes 2016), implemented as an add-on in Cytoscape 3.9 (Shannon et al. 2003), was used to compute the connections between OTUs based on non-rarefied dataset of read counts (only OTUs with more than 300 reads). Distribution of pairwise scores was computed for the following similarity measures: Bray-Curtis distances, Kullback-Leibler dissimilarity, Pearson and Spearman correlations. For each measure, 100 permutations and 100 bootstrap-resampling scores were generated (with renormalization and row-shuffling). Unstable edges were removed. The four p-values were merged with the Brown’s method (variances were pooled by combining the p-values of permutations and bootstraps). After FDR-correction using the Benjamini-Hochberg method, only the highly significant correlations (p < 0.01) supported by at least three of the four similarity measures were kept, to account for the biases of the methods (Faust and Raes 2016). The network layout was arranged using the “edge-forced spring embedded” algorithm (Kamada and Kawai 1989), additionally weighted by p-values, to get an unbiased network with interconnected nodes placed closer to each other (Kohl et al. 2011). “Hub” nodes, representing taxa with a large influence in shaping the network, were identified on the basis of degree (number of connections), betweenness centrality and closeness centrality (Agler et al. 2016; Shi et al. 2016). The network legend was created with the Cytoscape-App “Legend creator” (http://apps.cytoscape.org/apps/legendcreator), while Adobe Photoshop (Adobe Systems Inc., USA) was used to assemble the final figure.
Results
Meteorological growing conditions and expected summer stresses
The experimental viticultural region is characterized by a typical Mediterranean climate with the minimum of rainfall occurring in summertime, in concomitance with the highest temperatures. Fig. S1 reports the average meteorological data of the last 10 years (January 2011–July 2021). It is worth noting that the seasons 2020 and 2021 had particularly dry summers. Furthermore, to emphasize the drought stress, the vine plantation was delayed to the 8th of June, to limit natural watering occurring from the rainfalls. Actually, in the experimental vineyard, no rain occurred between the plantation day (June 8th, 2020) and the last site inspection of the year (September 25th, 2020).
Grapevine vegetative development
The number of plants without green leaves (considered as dead plants), was strongly impacted by the mycorrhization treatment, as assessed on June 14th, 2021, in the second vegetative season. In detail, mortality was 17.6% vs. 64.7% in the mycorrhized and control plants, respectively. It is interesting to note that in both vegetative seasons the mycorrhization allowed a plant recovery: in fact, a decrease in the number of plants without leaves was observed in the mycorrhized condition in both date ranges 8th July – 14th August 2020 and 4th May – 14th June 2021 (Fig. 1A).
A) Total number of mycorrhized and not mycorrhized plants without green leaves at each inspection date. B) Average leaf area index per plant; inset photos show examples of mycorrhized and not mycorrhized plants (on 25th September 2020). C) Average shoot number per plant. D) Average length of the longest shoot per plant. Values in panel A indicate total number of plants, while the other graphs indicate averages ± standard errors, calculated over the total plant population (62 mycorrhized and 56 not mycorrhized plants). *** p < 0.001 (Student’s t test, mycorrhized vs. not mycorrhized plants)
In 2020, mycorrhization caused an extension of the vegetative season, allowing an active growth of the shoots during the entire summertime and avoiding the premature senescence of leaves, which was observed in control plants instead (Fig. 1B – decrease in the total leaf area in control plants is due to the abscission of mature leaves between August and September 2020).
Mycorrhized plants grew much more than control vines. The total leaf area at the end of the first season was about fourtimes more expanded in mycorrhized plants with respect to the control ones (Fig. 1B). This difference is related to a significantly higher number of shoots (Fig. 1C), a significantly higher length of the main shoot (Fig. 1D), as well as the sum of the total shoots: in fact, the 25th of September 2020, the lengths of all the shoots of each plant were measured, confirming the significantly different vegetative growth of the mycorrhized plants. In detail, the average shoot length was 27.84 and 6.80 cm for mycorrhized and control plants, respectively, and the sum of the lengths of all the shoots in each plant was 160.96 and 37.46 cm for mycorrhized and control plants, respectively.
It is worth to notice that the differences in the vegetative growth and in the plant vigor were kept also after the plant dormancy, resulting in an improved vegetative growth for mycorrhized plants also in 2021 (Fig. 1C-D).
Leaves and stems weight was destructively measured on subsamples of 5 plants for each condition, the 14th of August 2020. Biomass of mycorrhized plants showed about 3-fold increase compared to control plants. In detail, the average fresh weights of the plant leaves were 10.15 g and 3.08 g in mycorrhized and control plants, respectively (Student’s t test, p < 0.001). Furthermore, the fresh weights of the new formed stems were 2.31 g and 1.04 g in mycorrhized and control plants, respectively (Student’s t test, p < 0.001) (Fig. S2).
Elemental analysis
No significant differences were observed between the bulk soils collected nearby the control- and the mycorrhized plants for any of the considered elements (Table S3). Among the 25 elements considered, 6 were not found in detectable concentrations in plants (Cd, Be, Ni, Se, Tl and Te); among them, Be and Ni were available in the soil, with concentrations of 4.45 ppm and 23.84 ppm respectively. Pb was found in the bulk soils, with an average value 10.22 ppm, but traces of Pb (0.06 ppm) were detected only in 2 out of 10 plants analyzed, both of them not mycorrhized. On the other hand, Bi was not detected in any of the 4 soil samples analyzed, whereas all the mycorrhized plants had detectable concentrations of Bi (average value = 0.98 ppm) (Table S3).
In general, leaves of control vines had a higher concentration of most of the elements considered, with significant differences (T-test, p < 0.05) detected for Al, B, Ba, Ca, Cr, Cu, Co, Fe, Li, Zn and P, while the differences were not significant for Mn, Ga, K, Na, Mg and Pb. Significantly higher concentrations in leaves of mycorrhized plants were obtained only for Bi (undetected in control plants). Sr also showed slightly higher, but not significantly different, concentrations in mycorrhized plants (Fig. 2A; Table S3). However, considering the total amount of the elements absorbed by the plants, the situation was reversed. Except Pb (with a higher, but not significant, content in control vines), mycorrhized plants had higher content of all the elements, with significant differences for B, Ba, Bi, Ca, Mn, Cu, Co, Fe, Ga, K, Na, Li, Mg, Sr, Zn and P (Fig. 2A; Table S3).
Results of the Principal Component Analysis (PCA) of the elemental analysis, showing the different behaviors of mycorrhized and control plants. A) Plot of the first 2 functions obtained considering both concentrations (ppm) and total amounts, as obtained by multiplying the concentrations of elements in leaves by the respective leaf weights (see Table S3). B) Plot of the first 2 functions obtained considering the ratio between the total amount of each element with respect to the sum of the total amounts of all the elements analyzed (absorption selectivity)
These data demonstrated a more active element absorption from the soil, which allowed an improved plant growth with consequent element dilution effect. To build the PCA plot (Fig. 2A), both element concentrations and total amounts were considered. Five functions with eigenvalues higher than 1, representing 96.26% of the total variability (65.64%; 14.78%; 8.31%; 4.60% and 2.94% for PC1, PC2, PC3, PC4 and PC5, respectively) were obtained. The function 1 clearly discriminates the mycorrhization treatment, while the function 2 is characteristic of the plant localization in the field along the rows. To understand the role of each element in the PCA results, the matrix of the components of each function is available in Table S4. A similar data distribution was obtained by performing a PCA considering only the total element content and excluding the concentration values (Fig. S3).
The plant-mycorrhiza interactions did not only affect the total absorption of the elements, but also modulated the absorption selectivity. The results obtained by the ratio between the total amount of each element with respect to the sum of the total amounts of all the elements analyzed were summarized by a PCA (Fig. 2B). Four functions with eigenvalues higher than 1, representing 89.57% of the total variability (53.40%; 20.29%; 10.07% and 5.81% for PC1, PC2, PC3 and PC4 respectively) were obtained. Once again, the function 1 clearly discriminates the mycorrhization treatment, indicating an effect of the plant-fungi interaction in the absorption selectivity. Briefly, among the analyzed macro-elements, control plants showed a relatively preferential absorption of P, while the presence of mycorrhiza promoted the absorption of cations (K, Mg and Ca). Considering the analyzed micro-elements, mycorrhization favored a relatively higher absorption of Mn, with respect to the control plants, which showed relatively higher amounts of Fe, Zn, B and Cu. Considering the other analyzed elements, which could also include phytotoxic elements, mycorrhization created a preferential relative absorption for Bi, Li, Sr, Na and Ga, while control plants showed a relatively higher preference for Cr, Al, Co, Ba and Pb.
Despite the clear data distribution obtained in the PCA graph, it is worth to notice that the difference between the relative amounts of elements (ratios calculated as described above for each plant) obtained in control and mycorrhized plants was not always significantly different (Fig. 2B).
Microbiological analysis (Illumina sequencing)
Two samples of rhizospheric soil and two samples of endosphere did not give a PCR amplicon, therefore remaining with four samples per group for these two habitats. All the four samples of bulk soil gave an amplicon, instead. By Illumina sequencing, a total of 1,062,895 quality-filtered reads were obtained. After removal of 6796 singletons and 1002 contaminant OTUs (298,488 reads), 757,611 reads remained, which were grouped in 9688 OTUs (Tables S2 and S5).
At the rarefaction depth (11,100 reads), all samples reached a coverage >98%, confirming that the majority of the diversity was intercepted (Fig. S4).
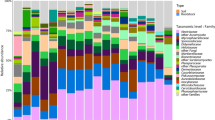
Thirty-one phyla were detected, the major of which were Proteobacteria (2701 OTUs, 55.4% of the total dataset), Actinobacteria (2208 OTUs, 25.7%) and Firmicutes (929 OTUs, 6.2%); all other phyla were below 5% (Table S5). Only 59 OTUs (0.22% of the dataset) remained unidentified at phylum level (Table S5). The dominant orders detected were Pseudomonadales, Rhizobiales and Enterobacterales in the endosphere, Pseudomonadales, Bacillales and Rubrobacterales in the rhizosphere, Rubrobacterales in the bulk soil (Fig. 3A; Table S5).
A) Taxonomical assemblage at order level in the analyzed samples (A- and B-samples: not mycorrhized plants; C-samples: mycorrhized plants). B) Shannon-Wiener index at OTU level across habitats and treatments (similar results were obtained for all the calculated metrics; Table S6). C-D) Weighted Unifrac distances plots with samples colored by treatment (C) or by habitat (D) (similar results were obtained with Bray-Curtis distances; Fig. S5)
Alpha-diversity metrics showed a highly significant effect of the habitat (as expected, bulk soil>rhizosphere>endosphere; ANOVA p < 0.001), while the mycorrhization treatment induced a slight but not statistically significant increase of diversity in both rhizosphere and endosphere (Fig. 3B; Table S6). Beta diversity metrics also showed a clear effect of the habitat (ADONIS, p < 0.001; Fig. 3D) but not of the treatment (Fig. 3C; Fig. S5), even when the habitats were analyzed separately.
The number of OTUs significantly different between mycorrhized and non-mycorrhized plants was notably higher in the endosphere than in the rhizosphere. In the endosphere, 20 OTUs were more abundant in the mycorrhized plants (accounting for 12.7% of the total reads) and 7 in the control plants (6.7% of the total reads); while in the rhizosphere, there were 8 OTUs more abundant in the mycorrhized plants (1.5% of the total reads) and 4 in the control plants (1% of the total reads) (Fig. 4). Among the OTUs enriched by the mycorrhization treatment, several well-known beneficial taxa appeared, especially in the endosphere, such as Burkholderiaceae, Rhizobiaceae, Methylophilaceae, Bacillus, Massilia and Streptomyces. However, after performing a manual BLAST check, no OTU attributable to the bacterial species present in the commercial inoculum used was found enriched in the mycorrhized plants.
OTUs significantly different between mycorrhized and control plants, calculated by G-test of independence, in the endosphere (A) and rhizosphere (B) separately, ordered by decreasing fold change. Pie charts show the percentages with respect to the total number of reads for each dataset. FDR = false discovery rate
Correlation between OTUs and chemical elements
Both the total amount of elements (representative of the plant absorption) and the concentration of elements (representing the plant chemical composition and, thus, the endophytic environment) showed significant correlations with the OTUs in both the rhizosphere and the endosphere habitats. In total, 154 OTUs showed at least one correlation with chemical elements. A higher number of correlations were found between OTUs and concentration of chemical elements with respect to total amount. However, interestingly, when total amount values were used, the number of rhizospheric OTUs with at least one correlation with elements was higher than endospheric OTUs; whereas, when concentration was used, the situation was the opposite (Fig. S6).
Canonical correspondence analysis (CCA; Fig. S7) further showed that concentrations and total amounts of the elements displayed an opposite trend with respect to the sample groups (mycorrhized vs. non mycorrhized), thus conforming the results of the PCA (Fig. 2A).
Eleven OTUs showed a notably higher number of correlations (≥15); in the case of endospheric OTUs, the number of correlations was higher with element concentrations than with total amounts, while for rhizospheric OTUs (except OTU 418) it was the opposite (Fig. 5). Only one of these OTUs, namely OTU-90, identified as Methylophilaceae, order Betaproteobacterales (a manual BLAST did not provide a deeper identification), showed a higher number of correlations in both habitats, while the other OTUs were correlated almost only in the rhizosphere (six OTUs) or in the endosphere (four OTUs). Interestingly, all the OTUs correlated with elements in the endosphere belonged to the phylum Actinobacteria (Fig. 5), and it is worth noting that they were also positive responders of the mycorrhization treatment (Fig. 5, asterisks, and Fig. 4A). OTU-90 also was a positive responder to the mycorrhization in the endosphere. On the other hand, the OTUs correlated in the rhizosphere belonged to different phyla (Actinobacteria, Alpha- and Gammaproteobacteria, and Firmicutes) (Fig. 5), and none of them was a responder of the mycorrhization treatment.
Number of correlations of the eleven OTUs most correlated with chemical elements in each of the four combinations of habitat (rhizosphere/endosphere) and element measurement (concentration/total amount) (bars). The blue line shows the total number of correlations. Taxonomical identification of the OTUs is included, and asterisks indicate the OTUs that were also enriched in the endosphere of mycorrhized plants (Fig. 5)
Heatmaps of the Pearson’s correlations between the concentration of the chemical elements and the endospheric (A) or rhizospheric (B) OTUs, and corresponding clustering (UPGMA trees). The clusters containing the most correlated OTUs in the endosphere and in the rhizosphere (see Fig. 5) are indicated by black and gray arrows, respectively
The clustering of the correlation of chemical elements with the OTUs mainly depended on the type of data (concentrations vs. total amounts) (Figs. 6 and 7).
Heatmaps of the Pearson’s correlations between the total amount of the chemical elements and the endospheric (A) or rhizospheric (B) OTUs, and corresponding clustering (UPGMA trees). The clusters containing the most correlated OTUs in the endosphere and in the rhizosphere (see Fig. 5) are indicated by black and gray arrows, respectively
Considering the concentrations, Bi, Sr and Mg clustered separately from the other elements. Most of the OTUs showed positive correlations with at least one of these elements. These generally positive correlations are particularly evident concerning Bi for the OTUs of the rhizosphere and Bi and Sr for the OTUs of the endosphere.
Considering the total amounts, Pb was separated from all the other elements. Only few OTUs showed positive (and not always significant) correlations with this element (6 in the rhizosphere and 4 in the endosphere; Table S7). These OTUs were different in the two habitats: in the rhizosphere, they were all belonging to Acidomicrobiales or to Actinobacteria, class Acidimicrobiia, whereas in the endosphere they were belonging to typical beneficial taxa, such as Pseudomonas, Rhizobium and Phyllobacterium (Table S7), and to Klebsiella; the latter (OTU-327) was also significantly enriched in the endosphere of control plants (Fig. 4; Table S7). Furthermore, these OTUs negatively correlated with all other elements, except for slight, and not significant, positive correlations with the metals Al, Cr and Fe of few OTUs (2 in the endosphere and 1 in the rhizosphere).
From the perspective of the OTUs, the heatmaps appeared well structured, as they were composed by a few groups of OTUs characterized by coherent profiles within each group (Figs. 6 and 7). The four actinobacterial OTUs most correlated in the endosphere showed the same pattern of correlations, and in fact were grouped in a single cluster that included also the OTU 90 (Figs. 6A and 7A, black arrow). When element concentrations were used (Fig. 6A), they resulted positively correlated to Bi, Sr and Mg, neutral to Pb, and negatively correlated to all remaining elements. This result was the same in the heatmap with total amount (Fig. 7A, black arrow). When total amounts of elements were used, they resulted negatively correlated only to Pb, positively correlated to Ca, Li, K, Sr, Na, Cu, B, Mg and Bi (Fig. 7A).
On the other hand, the OTUs most correlated in the rhizosphere were clustered in two groups, one containing OTUs 11 and 12, the second one containing the OTUs 4, 157, 357 and 418 (Figs. 6B and 7B, gray arrows). OTU 90 was also included in the second group. These two groups showed opposite patterns in both heatmaps; in particular, when total amounts of elements were used, the OTUs 11 and 12 were negatively correlated to almost all elements, but interestingly they were neutral to Pb.
Co-occurrence network analysis
Of the 400 OTUs considered in this analysis (those with >300 reads), 229 showed at least one highly significant connection. Total number of significant connections was 12,880 (Fig. 8). Statistical analysis of the network indicated four hub OTUs: OTU-75 and OTU-7 (identified as Rhizobiaceae and Steroidobacteriaceae, respectively), which were more abundant in the endosphere (Table S5); OTU-570 and OTU-30 (identified as Solirubrobacterales and Acidobacteria, respectively), which were more abundant in rhizospheric soil (Table S5). With respect to each other, the endophytic hub OTUs were higher in betweenness centrality, while the rhizospheric soil hub OTUs were higher in closeness centrality (Fig. 8). None of the OTUs responders of the mycorrhization treatment (Fig. 4) or mostly correlated to the elements (Fig. 5) were among the most interactive ones in the network; in particular, OTU-90 (the only OTU highly correlated to elements in both rhizosphere and endosphere) appeared very low interactive, with only three connections (Fig. 8); interestingly, one of these was with OTU-7769, which showed the same pattern of correlations with elements (Figs. 5, 6 and 7).
Co-occurrence network showing positive and negative significant connections (green and red lines, respectively) between OTUs (nodes, colored by phylum according to the legend). The graph shows the distribution of betweenness centrality and closeness centrality values as a function of the degree. The four hub OTUs are indicated with numbers 1 to 4
Discussion
Summer stresses and grapevine vegetative development
Salento is a Mediterranean region characterized by hot and dry summers. Because of climate change, future warming in the Mediterranean region is projected to exceed global rates by 25%, particularly with summer warming at a rate 40% faster than the global average (Cramer et al. 2018). Fresh water availability is likely to decrease, with a stretch of the dry seasons and the risk of salt-water intrusion in the aquifers. Drought, heat waves, frost, or heavy rainfall during critical phenological stages may cause unexpected yield losses (Cramer et al. 2018). In this context, the search for useful solutions to implement the resilience of agricultural systems is of crucial importance.
In this work, we demonstrated a strong effect of the mycorrhization on the agronomical performances of a strategical grapevine rootstock (1103 Paulsen) in open field. Our data confirmed the growth promotion related to the symbiotic interaction of the grapevines with the mycorrhiza (Trouvelot et al. 2015). Mycorrhizae are particularly useful in highly stressful conditions. The delayed vineyard plantation emphasized the strongly limiting growing conditions typical of the Salento region, a particularly hot and dry viticultural region (Fig. S1). In this extreme scenario, the beneficial effects of the AMF symbiosis were particularly evident on both the plant survival rate and the growing performances. Nogales et al. (2021) did not obtain significant effects of the grapevine mycorrhization in normal conditions, but only after a heat wave occurrence. Nikolaou et al. (2003) tested the mycorrhization*drought-stress interaction and found a significant improvement of the plants’ growth due to mycorrhization in stressful conditions, while few, and mainly not significant, effects were detected in well-watered conditions. Shreiner and Mihara (2017) showed that the water deficit stimulated the gapevine plants to establish more interactions with AMF, as a way to compensate for lower density of fine roots. It has been found that in cold regions, mycorrhization could even reduce the grapevine water use efficiency (Mikiciuk et al. 2019). Climate change is expected to exacerbate summer stresses, with increased risks of water deficit for agricultural crops both in a global (Döll 2002) and in a local (D’Oria et al. 2018) perspective. In the Mediterranean region, plant-microbe symbioses could play a crucial role, not only in the growth and production performances, but also in the plant survival chances (Singh et al. 2011; Talaat and Shawky 2017).
We can assume that mycorrhiza naturally occur in most of the vineyards (Balestrini et al. 2010), although different vine ages, growing conditions and viticultural management could modify the abundance and diversity of the microbial species (Betancur-Agudelo et al. 2021; Oehl and Koch 2018; Schreiner and Mihara 2017). Thus, a decrease of the impact of the inoculum during time could be expected due to the dominance of naturally equilibrated symbiotic interactions. However, our plants maintained and even increased the gap between the inoculated and the non-inoculated ones during the second growing season. This could be due, on one hand, to the higher vegetative growth of mycorrhized plants during the first season, including the drought-related early leaf abscission avoidance (Prieto et al. 2010) that allowed a prolonged canopy photosynthetic life. As a consequence of this, we can assume a consequent higher non-structural carbon storage at the end of the first vintage in the inoculated plants that promoted a better plant growth during the second year. On the other hand, the plant-microbe interactions induced by the presence of the mycorrhizal inoculum could have influenced the synergies among microorganisms, maintaining the differences during time.
Elemental analysis
Elemental analysis revealed an opposite trend of concentration and total amount of elements accumulated in grapevine leaves, as affected by mycorrhization. Mycorrhized plants experienced a higher accumulation of most of the analyzed elements, with a few exceptions such as Pb. Moreover, mycorrhization also affected the elements’ selective absorbance.
First, the absence of significant differences among bulk soil samples validated the field experimental design, confirming its reliability.
The higher concentration (fresh weight) of most of the elements in control not mycorrhized vines should be ascribed to the reduced plant growth in drought conditions, with the consequently limited dilution effects. In fact, considering the total amount of elements in the plant leaves, the improved absorption induced by the presence of the mycorrhiza clearly appears. Similar considerations were reported also by Trouvelot et al. (2015), who stated that the significant increase of the water flux in mycorrhized plants is independent of changes in the nutrient status of the host plant and our results are in agreement with this assertion.
It is well known that mycorrhiza impacts plant nutrition. Previous research on mycorrhizal symbiosis typically focused on their effect on phosphorous uptake (Plenchette et al. 2005; Püschel et al. 2021). In plants, the uptake of P could be 4–5-fold higher with mycorrhizal symbiosis (Tinker 1984). An increase in P levels was recorded in almost all the grapevine-mycorrhizal associations previously studied; however, it is known that P concentration and plant growth intensity are not strongly linked (Trouvelot et al. 2015). In our conditions, the main limiting factor was surely drought and, thus, despite the higher P total uptake obtained in mycorrhized vines, a higher concentration was recorded in control plants likely due to the reduced dilution ascribable to the limited plant growth, less efficient water absorption, and higher plant dehydration. Van Rooyen et al. (2004) demonstrated that arbuscular mycorrhizal colonization can influence the water relations of transplanted grapevine rootstocks during the initial growth stages, without affecting the mineral nutrition. In fact, in their study, no significant differences were detected concerning the P concentrations in grapevine root, stem or leaf.
Despite the higher total amount of P in mycorrhized plants, we found a lower relative content of this element with respect to the total amount of all the analyzed elements. It has been shown that the presence of the AMF symbiosis could inhibit the direct uptake pathway in colonized roots, and it could induce a P reprioritization in the plant (Cangahuala-Inocente et al. 2011; Smith 2003; Trouvelot et al. 2015). This could explain our results, probably due to a lower selective priority absorption for this element by the mycorrhizal fungi with respect to the plant itself.
Considering the other three macronutrients analyzed in the framework of this experiment, namely K, Ca and Mg, mycorrhiza generally promoted the absorption of all of them, resulting in significantly higher total amounts. Our results are in agreement with those obtained by Nikolaou et al. (2002), who observed an increase in leaf concentrations of K, Ca and Mg in mycorrhized plants, without significant interaction effects of the tested treatments (neither mycorrhizal colonization and P-forms, nor mycorrhizal colonization and rootstock variety). Despite the well-known antagonisms among these nutrients concerning the plant uptake and the genotype absorption specificity (Bianchi et al. 2020), mycorrhiza seemed to not modify the relative content among them.
Mycorrhized vines had significantly higher total amounts of Fe, B, Mn, Cu and Zn. These results are in agreement with the literature that reports higher concentrations of various micronutrients in mycorrhized plants (Nikolaou et al. 2002; Tinker 1984; Trouvelot et al. 2015). However, considering the proportion among the different elements analyzed, our data showed that control plants generally had a stronger preferential uptake of these elements (especially Fe and Zi) with respect to mycorrhized plants.
Phytotoxic elements should also be considered. Once again, higher concentrations, but lower amounts, were detected for most of these elements in control plants. Bi and Sr were the only two elements with higher concentrations in mycorrhized plants (Fig. 2). Bi deserves a particular attention: despite its presence was not detectable in any soil or control plant samples, all the inoculated plants accumulated small quantities of this element, demonstrating a specific promotion of the Bi uptake by mycorrhiza. Bismuth is a heavy metal not very abundant in Earth’s crust. Bismuth compounds are generally low soluble and are not considered toxic, although some negative effects on different cells have been described (Babula et al. 2008). Thus, it is possible to hypothesize an effect of the mycorrhization on the plant uptake of Bi and Sr with their consequent selective pressure on the plant-associated bacteria.
Pb appeared only in control plants, suggesting an effect of the mycorrhization on the bacteria of the rhizosphere that modulate the plant absorption of this element. Interestingly, one of the OTUs positively correlated with Pb was also found enriched in not mycorrhized plants (OTU-327, identified as Klebsiella). Interestingly, this genus was recently demonstrated to be highly resistant to Pb (Muñoz et al. 2015; Wei et al. 2016), and indeed a Klebsiella strain was used for removal of Pb from contaminated water by biosorption (Muñoz et al. 2016). This suggests that, in our work, the increase of OTU-327 in Pb-containing control plants could be due to its intrinsic resistance, which conferred to this OTU an advantage over the other microbes under the Pb selective pressure.
Microbiological analysis
The characterization of the bacterial microbiota indicated a stronger effect of the mycorrhization in the root endosphere than in the rhizosphere, with the promotion of some beneficial, non-hub taxa, which moreover appeared to be involved in chemical element dynamics.
This is probably due to the fact that arbuscular mycorrhizal fungi penetrate inside the plants, and thus is logical to expect a stronger impact in the relatively closed endophytic habitat as compared to the outer of the plant, where effects could be masked by a stronger influence of further external biotic and abiotic environmental factors. The mechanisms responsible for such enrichment of specific OTUs might be based on direct selective recruitments by mycorrhiza, which are known to be able to specifically interact with certain bacterial taxa (Kobayashi and Crouch 2009). Moreover, mycorrhiza can change the physiology of bacteria, as in the case of those species that show enhanced biofilm-formation ability in presence of ectomycorrhizal symbiosis (van Overbeek and Saikkonen 2016; Hassani et al. 2018); eventually, this can lead to an increased compatibility with a plant niche. Finally, fungal hyphae may be used as “highways” by bacteria, which results in enhanced mobility and, thus, favored the spreading of certain bacteria within the tissues of mycorrhized plants (Kohlmeier et al. 2005). In our study, the taxa enriched in the endosphere (positive responders of the mycorrhization treatment, Fig. 4A) generally belonged to beneficial taxa. Among them, several Actinobacteria appeared. This result is in agreement with Torres et al. (2021) who also found increased Actinobacteria associated with grapevine that had been half-irrigated and inoculated with mycorrhiza. It is also in agreement with Andreolli et al. (2016) who showed that young grapevine plants had a higher incidence of Actinobacteria, and our rootstocks were indeed young.
This result became even more interesting when we found that the same Actinobacterial OTUs were the most correlated to chemical elements (Fig. 5), and moreover showing the same pattern of correlation (Figs. 6 and 7). This suggests common interactions with the element dynamics in the plant, which were strongly impacted by the mycorrhization treatment. Interactions between biotic and abiotic components of the root system, especially of a perennial tree crop like grapevine, are highly complex (Pacifico et al. 2019), and to unravel the molecular mechanisms underlying them is a big challenge for microbial ecologists. However, the involvement of Actinobacteria in the regulation of the nutritional aspects of grapevine that we demonstrated in this work, indicates that members of this phylum should be considered as critical actors of the plant-soil-microbe interactions, as well as promising candidates for applications of plant growth-promoting rhizobacteria (PGPR) in sustainable viticulture. Interestingly, co-occurrence network analysis showed that these taxa were not among the most interconnected ones; notably, the most interesting OTU with respect to the correlations with elements (OTU-90) was even among the less interactive at all (Fig. 8). This interesting result suggests that the bacterial species (or strains) involved in element dynamics are relatively independent from other bacterial members of the root microbiome. This feature might represent an advantage for the perspective of biotechnological applications in viticulture, because it suggests that these taxa, when inoculated, could be less sensitive to the competitive pressure of other members of the grapevine microbiome.
On the other hand, hub OTUs more abundant in the endosphere were Rhizobium and Steroidobacteraceae (Fig. 8). While Rhizobium is a well-known beneficial plant-associated genus, few is known about the recently discovered family Steroidobacteraceae (Liu et al. 2019). To the best of our knowledge, this is the first report of a member of this family being detected as a hub taxon in plant-associated microbiomes.
Conclusions
The results of this study shed light onto the beneficial potential of the mycorrhization in the Mediterranean climate, in the context of the future climate change scenarios, and show the intricated interactions between the microbial actors and the chemical element dynamics.
Mycorrhization had a very strong positive effect on survival and growth of grapevine rootstocks under exacerbated drought stress conditions in open field. Furthermore, mycorrhization significantly affected the bacterial microbiota especially in the root endosphere, where several beneficial taxa were enriched. The elemental analysis showed that mycorrhization modulated element total absorption and absorption selectivity, and correlation between OTUs and elements suggests that the same taxa enriched in the root endosphere by the mycorrhization may play an active role in the elemental absorption by the plant, as modulated by mycorrhization.
Our work opens new perspectives for a multidisciplinary and integrated view of agronomical, chemical, and microbiological aspects that will be of paramount importance for the optimization of successful ecosystem-based strategies for sustainable viticulture, in the context of climate changes and global warming.
Data availability
The datasets generated during and/or analyzed during the current study are available in the European Nucleotide Archive (ENA) repository, www.ebi.ac.uk/ena/browser/view/PRJEB50609.
Change history
25 August 2022
Missing Open Access funding information has been added in the Funding Note.
References
Agler MT, Ruhe J, Constanze Morhenn SK, Kim ST, Weigel D, Keme EM (2016) Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol 14:e1002352. https://doi.org/10.1371/journal.pbio.1002352
Aguín O, Mansilla JP, Vilariño A, Sainz MJ (2004) Effects of mycorrhizal inoculation on root morphology and nursery production of three grapevine rootstocks. Am J Enol Viticul 55:108–111
Alaimo S, Marceca GP, Giugno R, Ferro A, Pulvirenti A (2018) Current knowledge and computational techniques for grapevine meta-omics analysis. Front Plant Sci 8:2241. https://doi.org/10.3389/fpls.2017.02241
Allen MF, Boosalis MG (1983) Effects of two species of VA mycorrhizal fungi on drought tolerance of winter wheat. New Phytol 93:67–76. https://doi.org/10.1111/j.1469-8137.1983.tb02693.x
Allen MF, Smith WK Jr, Moore TS, Christensen M (1981) Comparative water relations and photosynthesis of mycorrhizal and non-mycorrhizal Bouteloua gracilis H.B.K. New Phytol 88:683–693. https://doi.org/10.1111/j.1469-8137.1981.tb01745.x
Andreolli M, Lampis S, Zapparoli G, Angelini E, Vallini G (2016) Diversity of bacterial endophytes in 3 and 15 year-old grapevines of Vitis vinifera cv. Corvina and their potential for plant growth promotion and phytopathogen control. Microbiol Res 183:42–52. https://doi.org/10.1016/j.micres.2015.11.009
Aronesty E (2013) Comparison of sequencing utility programs. Open Bioinforma J 7:1–8. https://doi.org/10.2174/1875036201307010001
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42. https://doi.org/10.1007/s005720100097
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6:189–213. https://doi.org/10.1007/978-1-4020-9654-9_14
Balestrini R, Magurno F, Walker C, Lumini E, Bianciotto V (2010) Cohorts of arbuscular mycorrhizal fungi (AMF) in Vitis vinifera, a typical Mediterranean fruit crop. Environ Microbiol Rep 2:594–604. https://doi.org/10.1111/j.1758-2229.2010.00160.x
Beniston M, Diaz HF (2004) The 2003 heat wave as an example of summers in a greenhouse climate? Observations and climate model simulations for Basel, Switzerland. Glob Planet Change 44:73–81. https://doi.org/10.1016/j.gloplacha.2004.06.006
Betancur-Agudelo M, Meyer E, Lovato PE (2021) Arbuscular mycorrhizal fungus richness in the soil and root colonization in vineyards of different ages. Rhizosphere 17:100307. https://doi.org/10.1016/j.rhisph.2021.100307
Bettenfeld P, Cadena i Canals J, Jacquens L, Fernandez O, Fontaine F, van Schaik E, Courty PE, Trouvelot S (2021). The microbiota of the grapevine holobiont: a key component of plant health. J Adv Res in press. https://doi.org/10.1016/j.jare.2021.12.008. Accessed 19 May 2022
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350. https://doi.org/10.1007/s11274-011-0979-9
Bianchi D, Grossi D, Simone Di Lorenzo G, Yinga YZ, Rustioni L, Brancadoro L (2020) Phenotyping of the “G series” Vitis hybrids: first screening of the mineral composition. Sci Horticul 264:109155. https://doi.org/10.1016/j.scienta.2019.109155
Bonfante P, Genre A (2010) Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nature Comm 1:48. https://doi.org/10.1038/ncomms1046
Cangahuala-Inocente GC, Da Silva MF, Johnson J-M, Manga A, van Tuinen D, Henry C, Lovato PE, Dumas-Gaudot E (2011) Arbuscular mycorrhizal symbiosis elicits proteome responses opposite of P-starvation in SO4 grapevine rootstock upon root colonisation with two Glomus species. Mycorrhiza 21:473–493. https://doi.org/10.1007/s00572-010-0352-0
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Christensen JH, Christensen OB (2007) A summary of the PRUDENCE model projections of changes in European climate by the end of this century. Clim Chang 81:7–30. https://doi.org/10.1007/s10584-006-9210-7
Ciais P, Reichstein M, Viovy N, Granier A, Ogée J, Allard V, Aubinet M, Buchmann N, Bernhofer C, Carrara A, Chevallier F, De Noblet N, Friend AD, Friedlingstein P, Grünwald T, Heinesch B, Keronen P, Knohl A, Krinner G et al (2005) Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437:529–533. https://doi.org/10.1038/nature03972
Cramer W, Guiot J, Fader M, Garrabou J, Gattuso JP, Iglesias A, Lange MA, Lionello P, Llasat MC, Paz S, Peñuelas J, Snoussi M, Toreti A, Tsimplis MN, Xoplaki E (2018) Climate change and interconnected risks to sustainable development in the Mediterranean. Nat Clim Chang 8(972):972–980 www.nature.com/natureclimatechange
D’Oria M, Tanda MG, Todaro V (2018) Assessment of local climate change: historical trends and rcm multi-model projections over the Salento area (Italy). Water 10:978. https://doi.org/10.1038/nature03972
Darriaut R, Lailheugue V, Masneuf-Pomarède I, Marguerit E, Martins G, Compant S, Ballestra P, Upton S, Ollat N, Lauvergeat V (2022) Grapevine rootstock and soil microbiome interactions: keys for a resilient viticulture. Horticul Res 9:uhac019. https://doi.org/10.1093/hr/uhac019
Dinu DG, Ricciardi V, Demarco C, Zingarofalo G, De Lorenzis G, Buccolieri R, Cola G, Rustioni L (2021a) Climate change impacts on plant phenology: grapevine (Vitis vinifera) bud break in wintertime in southern Italy. Foods 10:2769. https://doi.org/10.3390/foods10112769
Dinu DG, Bianchi D, Mamasakhlisashvili L, Quarta C, Brancadoro L, Maghradze D, Cardinale M, Rustioni L (2021b) Effects of genotype and environmental conditions on grapevine (Vitis vinifera L.) shoot morphology. Vitis 60:85–91. https://doi.org/10.5073/vitis.2021.60.85-91
Döll P (2002) Impact of climate change and variability on irrigation requirements: a global perspective. Clim Chang 54:269–293. https://doi.org/10.1023/A:1016124032231
Easlon HM, Bloom A (2014) Easy leaf area: automated digital image analysis for rapid and accurate measurement of leaf area. Appl Plant Sci 2:1400033. https://doi.org/10.3732/apps.1400033
Faust K, Raes J (2016) CoNet app: inference of biological association networks using Cytoscape. F1000 Res 5:1519. https://doi.org/10.12688/f1000research.9050.2
Frey-Klett P, Garbaye J, Tarkka M (2007) The mycorrhiza helper bacteria revisited. New Phytol 176:22–36. https://doi.org/10.1111/j.1469-8137.2007.02191.x
Genre A, Lanfranco L, Perotto S, Bonfante P (2020) Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol 18:649–660. https://doi.org/10.1038/s41579-020-0402-3
Gerlach N, Schmitz J, Polatajko A, Schlüter U, Fahnenstich H, Witt S, Fernic AR, Uroic K, Scholz U, Sonnewald U, Bucher M (2015) An integrated functional approach to dissect systemic responses in maize to arbuscular mycorrhizal symbiosis. Plant Cell Environ 38:1591–1612. https://doi.org/10.1111/pce.12508
Giorgi F, Bi X, Pal J (2004) Mean, interannual and trends in a regional climate change experiment over Europe. II: climate change scenarios (2071-2100). Clim Dyn 23:839–858. https://doi.org/10.1007/s00382-004-0467-0
Hammer Ø, Harper DA, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:4
Hardie K, Leyton L (1981) The influence of vesicular–arbuscular mycorrhiza on growth and water relations of red clover in phosphate deficient soil. New Phytol 89:599–608. https://doi.org/10.1111/j.1469-8137.1981.tb02339.x
Hassani MA, Durán P, Hacquard S (2018) Microbial interactions within the plant holobiont. Microbiome 6:58. https://doi.org/10.1186/s40168-018-0445-0
Hristov J, Toreti A, Domínguez Pérez I, Dentener F, Fellmann T, Elleby C, Ceglar A, Fumagalli D, Niemeyer S, Cerrani I, Panarello L, Bratu M (2020) Analysis of climate change impacts on EU agriculture by 2050 : JRC PESETA IV project : Task 3, Publications Office of the European Union 10:121115. https://doi.org/10.2760/121115
Jackson MA, Bell JT, Spector TD, Steves CJ (2016) A heritability-based comparison of methods used to cluster 16S rRNA gene sequences into operational taxonomic units. PeerJ 4:e2341. https://doi.org/10.7717/peerj.2341
Jones GV, Davis RE (2000) Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. Am J Enol Viticul 51:249–261
Kamada T, Kawai S (1989) An algorithm for drawing general undirected graphs. Inf Process Lett 3:7–15. https://doi.org/10.1016/0020-0190(89)90102-6
Kobayashi DY, Crouch JA (2009) Bacterial/fungal interactions: from pathogens to mutualistic endosymbionts. Annu Rev Phytopathol 47:63–82. https://doi.org/10.1146/annurev-phyto-080508-081729
Kohl M, Wiese S, Warscheid B (2011) Cytoscape: software for visualization and analysis of biological networks. In: Hamacher M., Eisenacher M., Stephan C. (eds) Data Mining in Proteomics. Methods in Molecular Biology (Methods and Protocols), vol 696 (pp. 291–303). Humana Press. https://doi.org/10.1007/978-1-60761-987-1_18
Kohlmeier S, Smits TH, Ford RM, Keel C, Harms H, Wick LY (2005) Taking the fungal highway: mobilization of pollutant-degrading bacteria by fungi. Environ Sci Technol 39:4640–4646. https://doi.org/10.1021/es047979z
Kroll S, Agler MT, Kemen E (2017) Genomic dissection of host–microbe and microbe–microbe interactions for advanced plant breeding. Curr Opin Plant Biol 36:71–78. https://doi.org/10.1016/j.pbi.2017.01.004
Liu Q, Liu HC, Zhou YG, Xin YH (2019) Stenotrophobium rhamnosiphilum gen. Nov., sp. nov., isolated from a glacier, proposal of Steroidobacteraceae fam. Nov. in Nevskiales and emended description of the family Nevskiaceae. Int J Syst Evol Microbiol 69:1404–1410. https://doi.org/10.1099/ijsem.0.003327
Lugtenberg BJ, Chin-A-Woeng TF, Bloemberg GV (2002) Microbe–plant interactions: principles and mechanisms. Antonie Van Leeuwenhoek 81:373–383. https://doi.org/10.1023/A:1020565523615
Malinowski DP, Alloush GA, Belesky DP (2000) Leaf endophyte Neotyphodium coenophialum modifies mineral uptake in tall fescue. Plant Soil 227:115–126. https://doi.org/10.1023/A:1026518828237
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data. A comment on distance-based redundancy analysis. Ecology 82:290–297. https://doi.org/10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2
Mikiciuk G, Sas-Paszt L, Mikiciuk M, Derkowska E, Trzcinski P, Ptak P, Chylewska U, Statkiewicz M, Liseket A (2019) Physiological response of three grapevine cultivars grown in North-Western Poland to mycorrhizal fungi. S Afr J Enol Vitic 40:1–14
Muñoz AJ, Espínola F, Moya M, Ruiz E (2015) Biosorption of Pb (II) ions by Klebsiella sp. 3S1 isolated from a wastewater treatment plant: kinetics and mechanisms studies. Biomed Res Int 2015:719060. https://doi.org/10.1155/2015/719060
Muñoz AJ, Espínola F, Ruiz E (2016) Removal of Pb (II) in a packed-bed column by a Klebsiella sp. 3S1 biofilm supported on porous ceramic Raschig rings. J Ind Eng Chem 40:118–127. https://doi.org/10.1016/j.jiec.2016.06.012
Nerva L, Giudice G, Quiroga G, Belfiore N, Lova PR, Volpe MG, Moffa L, Sandrini M, Gaiotti F, Balestrini R, Chitarra W (2022) Mycorrhizal symbiosis balances rootstock-mediated growth-defence tradeoffs. Biol Fertil Soils 58:17–34. https://doi.org/10.1007/s00374-021-01607-8
Nikolaou N, Karagiannidis N, Koundouras S, Fysarakis I (2002) Effects of different P sources in soil on increasing growth and mineral uptake of mycorrhizal Vitis vinifera L. (cv. Victoria) vines. J Int Sci Vigne Vin 36:195–204. https://doi.org/10.20870/oeno-one.2002.36.4.1687
Nikolaou N, Angelopoulos K, Karagiannidis N (2003) Effects of drought stress on mycorrhizal and non-mycorrhizal cabernet sauvignon grapevine, grafted onto various rootstocks. Experim Agric 39:241–252. https://doi.org/10.1017/S001447970300125X
Nogales A, Rottier E, Campos C, Victorino G, Costa JM, Coito JL, Pereira HS, Viegas W, Lopes C (2021) The effects of field inoculation of arbuscular mycorrhizal fungi through rye donor plants on grapevine performance and soil properties. Agric Ecosyst Envir 313:107369. https://doi.org/10.1016/j.agee.2021.107369
Oehl F, Koch B (2018) Diversity of arbuscular mycorrhizal fungi in no-till and conventionally tilled vineyards. J App Bot Food Qual 91:56–60. https://doi.org/10.5073/JABFQ.2018.091.008
Pachauri RK, Allen MR, Barros VR, Broome J, Cramer W, Christ R, Dubash NK et al. (2014) Climate change 2014: synthesis report. In: Core Writing Team, R.K., Pachauri and L.A. Meyer (eds) Contribution of Working Groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change: p. 151. Geneva, Switzerland. hdl:10013/epic.45156.d001
Pacifico D, Squartini A, Crucitti D, Barizza E, Lo Schiavo F, Muresu R, Carimi F, Zottini M (2019) The role of the endophytic microbiome in the grapevine response to environmental triggers. Front Plant Sci 10:1256. https://doi.org/10.3389/fpls.2019.01256
Plenchette C, Clermont-Dauphin C, Meynard JM, Fortin JA (2005) Managing arbuscular mycorrhizal fungi in cropping systems. Can J Plant Sci 85:31–40. https://doi.org/10.4141/P03-159
Prieto JA, Lebon E, Ojeda H (2010) Stomatal behavior of different grapevine cultivars in response to soil water status and air water vapor pressure deficit. OENO One 44:9–20. https://doi.org/10.20870/oeno-one.2010.44.1.1459
Püschel D, Bitterlich M, Rydlová J, Jansa J (2021) Drought accentuates the role of mycorrhiza in phosphorus uptake. Soil Biol Biochem 157:108243. https://doi.org/10.1016/j.soilbio.2021.108243
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project. Improved data processing and web-based tools. Nucl Ac Res 41:D590–D596. https://doi.org/10.1093/nar/gks1219
Rahman H, Sabreen S, Alam S, Kawai S (2005) Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J Plant Nutr 28:393–404. https://doi.org/10.1081/PLN-200049149
Reid CPP (1978) Mycorrhizae and water stress. In: Reidacher, A., Gagnaire-Michard, G. (eds) Root physiology and symbiosis: 392–408. Nancy, France
Robertson CE, Harris JK, Wagner BD, Granger D, Browne K, Tatem B, Feazel LM, Park K, Pace NR, Frank DN (2013) Explicet: graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics 29:3100–3101. https://doi.org/10.1093/bioinformatics/btt526
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH. A versatile open source tool for metagenomics. PeerJ 4:e2584. https://doi.org/10.7717/peerj.2584
Schreiner RP, Mihara KL (2017) The diversity of arbuscular mycorrhizal fungi amplified from grapevine roots (Vitis vinifera L.) in Oregon vineyards is seasonally stable and influenced by soil and vine age. Mycologia 101:599–611. https://doi.org/10.3852/08-169
Schreiner RP, Tarara JM, Smithyman RP (2007) Deficit irrigation promotes arbuscular colonization of fine roots by mycorrhizal fungi in grapevines (Vitis vinifera) in an arid climate. Mycorrhiza 17:551–562. https://doi.org/10.1007/s00572-007-0128-3
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape. A software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504. https://doi.org/10.1101/gr.1239303
Shi S, Nuccio EE, Shi ZJ, He Z, Zhou J, Firestone MK (2016) The interconnected rhizosphere. High network complexity dominates rhizosphere assemblages. Ecol Lett 19:926–936. https://doi.org/10.1111/ele.12630
Sieverding E (1981) Influence of soil water regimes on VA mycorrhizae. Effect on plant growth, water utilization and development of mycorrhiza. J Agron Crop Sci 150:400–411
Singh LP, Gill SS, Tuteja N (2011) Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Sign Behav 6:175–191. https://doi.org/10.4161/psb.6.2.14146
Smith SE (2003) Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol 133:16–20. https://doi.org/10.1104/pp.103.024380
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, vol Ed. 2. Academic Press, London
Snellgrove RC, Splittstoesser WE, Stribley DP Tinker PB (1982) The distribution of carbon and the demand of the fungal symbiont in leek plants with vesicular-arbuscular mycorrhizas. New Phytol 92:75–87. https://doi.org/10.1111/j.1469-8137.1982.tb03364.x
Talaat NB, Shawky BT (2017) Microbe-mediated induced abiotic stress tolerance responses in plants. In: Plant-microbe interactions in agro-ecological perspectives. Springer, Singapore, pp 101–133. https://doi.org/10.1007/978-981-10-6593-4_5
Tinker PB (1984) The role of microorganisms in mediating and facilitating the uptake of plant nutrients from soil. Plant Soil 76:77–91. https://doi.org/10.1007/978-94-009-6101-2_7
Torres N, Yu R, Kurtural SK (2021) Inoculation with mycorrhizal fungi and irrigation management shape the bacterial and fungal communities and networks in vineyard soils. Microorganisms 9:1273. https://doi.org/10.3390/microorganisms9061273
Trouvelot S, Bonneau L, Redecker D, Van Tuinen D, Adrian M, Wipf D (2015) Arbuscular mycorrhiza symbiosis in viticulture: a review. Agron Sustain Dev 35:1449–1467. https://doi.org/10.1007/s13593-015-0329-7
van Overbeek LS, Saikkonen K (2016) Impact of bacterial–fungal interactions on the colonization of the endosphere. Trends Plant Sci 21:230–242. https://doi.org/10.1016/j.tplants.2016.01.003
Van Rooyen M, Valentine A, Archer E (2004) Arbuscular mycorrhizal colonization modifies the water relations of young, transplanted grapevines (Vitis). S Afr J Enol Vitic 25:37–42. https://doi.org/10.21548/25-2-2137
Verdugo-Vásquez N, Gutiérrez-Gamboa G, Díaz-Gálvez I, Ibacache A, Zurita-Silva A (2021) Modifications induced by rootstocks on yield, vigor and nutritional status on Vitis vinifera Cv Syrah under hyper-arid conditions in Northern Chile. Agronomy 11:979. https://doi.org/10.3390/agronomy11050979
Webb LB, Whetton PH, Barlow EWR (2007) Modelled impact of future climate change on the phenology of winegrapes in Australia. Austr J Grape Wine Res 13:165–175. https://doi.org/10.1111/j.1755-0238.2007.tb00247.x
Wei W, Wang Q, Li A, Yang J, Ma F, Pi S, Wu D (2016) Biosorption of Pb (II) from aqueous solution by extracellular polymeric substances extracted from Klebsiella sp. J1: adsorption behavior and mechanism assessment. Sci Rep 6:1–10. https://doi.org/10.1038/srep31575
Funding
Open access funding provided by Università del Salento within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Massimiliano Cardinale, Laura Rustioni, Claudio Quarta, Fabio Minervini and Maria De Angelis contributed to the study conception and design. Study site (vineyard) was provided by Claudio Quarta. Material preparation and data collection were performed by Massimiliano Cardinale, Laura Rustioni, Matteo Dimaglie, Daniel Grigorie Dinu, Fabio Minervini, Mirco Vacca, Paride Papadia, Danilo Migoni, Filippo Selleri and Alessandro Caccioppola. Data analysis was performed by Massimiliano Cardinale and Laura Rustioni. The first draft of the manuscript was written by Massimiliano Cardinale, Fabio Minervini, Matteo Dimaglie and Laura Rustioni, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Janusz J. Zwiazek.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cardinale, M., Minervini, F., De Angelis, M. et al. Vineyard establishment under exacerbated summer stress: effects of mycorrhization on rootstock agronomical parameters, leaf element composition and root-associated bacterial microbiota. Plant Soil 478, 613–634 (2022). https://doi.org/10.1007/s11104-022-05495-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05495-1