Abstract
Purpose
Soil diaspore banks of bryophytes are poorly known in tundra grasslands, yet can be important for the maintenance of local bryophyte assemblages. We examined the effects of fertilization and grazing exclusion on above-ground bryophyte assemblages and soil diaspore banks in a tundra grassland.
Methods
We collected soil diaspore samples and recorded the cover of above-ground bryophytes from a full-factorial experiment with NPK fertilization and grazing exclusion treatments (a Nutrient Network site in NW Finland). Soil diaspore samples were germinated on trays in a greenhouse. We analyzed the compositions of diaspore bank assemblages and of above-ground assemblages and assessed their responses to the experimental treatments.
Results
The diaspore bank contained c. 50% of taxa found in above-ground assemblages; 26 bryophyte taxa germinated from the diaspore bank, while 40 taxa were found in the above-ground assemblages. These communities had distinct species compositions: the diaspore bank was dominated by Pohlia nutans, while above-ground assemblages were dominated by several species. NPK fertilization and grazing exclusion had negative effects on bryophyte richness and cover in above-ground assemblages, and weaker effects on these responses in the diaspore bank.
Conclusion
Soil diaspore banks comprise about half of the bryophyte taxa encountered in above-ground assemblages. Bryophyte diaspore banks are more buffered against nutrient enrichment and grazing exclusion than above-ground assemblages, suggesting that diaspore banks may enhance persistence and recovery of local bryophyte assemblages from environmental changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grassland bryophyte assemblages often comprise tens of taxa and can constitute a significant proportion of the above-ground biomass (Löbel et al. 2006; Michel et al. 2013). Bryophytes contribute to ecosystem functions such as primary production (Rieley et al. 1979), decomposition (Cornelissen et al. 2007), nutrient cycling (Permin et al. 2022; van Tooren et al. 1988; Turetsky 2003), soil stabilization and water retention (Michel et al. 2013). They can play an important role for soil microbiota (Benavent-González et al. 2018), soil microclimate (van der Wal and Brooker 2004), exotic plant invasions (Root et al. 2020), vascular plant recruitment (Gavini et al. 2019; Soudzilovskaia et al. 2011; van Tooren et al. 1985), and habitat provisioning for other organisms (Lagerström et al. 2007; Lindo and Gonzalez 2010). Although bryophytes can be an essential element of grassland ecosystems, they are often neglected.
Many global environmental changes are threatening grassland bryophytes. These bryophytes are sensitive to nutrient pollution (Pearce et al. 2003; Paulissen et al. 2005; van der Wal et al. 2005) and may be lost from ecosystems with increased nutrient deposition (Maskell et al. 2010; Müller et al. 2012; Stevens et al. 2004). Mechanisms leading to bryophyte disappearance under nutrient enrichment include direct toxic effects of fertilizer (Boch et al. 2018; Müller et al. 2012) and increased vascular plant cover and lack of light at ground level (van der Wal et al. 2005). Shifts in grazing pressure can also change bryophyte abundance and diversity (Barbé et al. 2016; Ingerpuu and Sarv 2015; Kaufmann et al. 2021; Moen et al. 1993; Oldén and Halme 2016; Takala et al. 2012, 2014; Virtanen et al. 1997). Reported grazing impacts on grassland bryophytes range from negative (Boch et al. 2018) to neutral (Müller et al. 2012) and positive (Takala et al. 2014). Current evidence suggests that intense land use with severe grazing disturbances causes bryophyte losses whereas moderate levels of grazing may be favorable for bryophytes because it alleviates competition with vascular plants. Grazing disturbances that alter bryophyte-topsoil relationships can be crucial for bryophyte diversity (Concostrina-Zubiri et al. 2014, 2016; Condon and Pyke 2018; Read et al. 2011; Takala et al. 2014).
In the same way that soils contain seed banks of vascular plants, soils contain a diaspore bank composed of buried sexual spores or asexual propagules of bryophytes (During 1997, 2001). Diaspore banks have been found in topsoils of temperate grasslands (During and ter Horst 1983; Bisang 1996), coastal dunes (Callaghan et al. 2020), boreal forests (Jonsson 1993; Rydgren and Hestmark 1997), mires (Sundberg and Rydin 2000; Vellak et al. 2021), mountain rainforests (Bisang et al. 2003), temperate ponds (Eckstein 2006) and subantarctic tundra (Bergstrom and Selkirk 1999). Diaspore banks integrate over temporal and spatial scales: diaspores can be dispersed locally or over long distances and then deposited in soils (Lewis Smith and Ochyra 2006) where they can survive for years or even decades to centuries (Bu et al. 2017; Malta 1922; Vanderpoorten and Goffinet 2009). Diaspore banks may play important role in assemblages in the context of environmental change (Alexander et al. 2012; Leibold and Chase 2018; Shoemaker et al. 2020): they can provide a safeguard for years with unfavorable conditions and enable germination and population growth when conditions become favorable or after disturbance (Caners et al. 2009; During 1997), therefore reducing the probability of local extinctions (also known as the storage effect; Chesson 2000; Wisnoski et al. 2019). Therefore, diaspore banks are presumed to play a key role in species coexistence and the maintenance of bryophyte diversity (Vanderpoorten and Goffinet 2009). Despite these well-established ideas, experimental tests of bryophyte diaspore bank responses to nutrient enrichment and altered grazing pressure are missing.
Species composition of bryophytes often differs between the diaspore bank and above-ground assemblages (Rydgren and Hestmark 1997). Typically, colonist species dominate in the diaspore bank, while perennial species dominate the aboveground community (During and ter Horst 1983; Eckstein 2006; Iglesias et al. 2015; Jonsson 1993; Kövendi-Jakó et al. 2016; Ross-Davis and Frego 2004). However, due to a scarcity of diaspore bank studies, the generality of the differences between diaspore bank vs. above-ground is not well understood.
The purpose of this study is to 1) describe the taxonomic and life history composition of the bryophyte diaspore bank in a tundra grassland, 2) compare the composition of the bryophyte diaspore bank with that of above-ground bryophyte assemblages, and 3) test how diaspore bank and above-ground bryophyte assemblages respond to experimental fertilization and herbivore exclusion treatments. We hypothesized that fertilization and herbivore exclusion will lead to relatively strong and rapid changes in above-ground bryophyte assemblages. We also hypothesized that treatment effects will be weaker in soil diaspore banks because they are not immediately impacted by changes in above-ground vegetation and ecological conditions. To test these hypotheses, we conducted a bryophyte germination trial of soils taken from a factorial fertilization and grazing exclusion experiment in a bryophyte-rich tundra grassland and sampled the above-ground assemblages in the same experimental plots.
Material and methods
Study site
Our study site is located in Kilpisjärvi, in northwest Finnish Lapland, on a treeless oroarctic tundra (69.05°N, 20.87°E) with mountains up to 1029 m high. The experimental site, at an elevation of 730 m a.s.l., is a modestly sloping tundra grassland with relatively thick snow cover during the winter (depth of snow cover typically 1–1.5 m). The snow free period is June–October, and the mean temperature in July is 10 °C, although there is considerable daily variation in summer temperatures and periodically are about 20 °C. Snowmelt water irrigates the soil each spring, and consequently a fluvial soil type with mixed humus and mineral soil prevails at the site. The mean soil pH in the experimental site is 4.7 (SE = 0.05, n = 4). The vegetation is characterized by small graminoids (e.g., Agrostis mertensii, Anthoxanthum alpina, Carex lachenalii and Festuca ovina), low herbs (Antennaria dioica, Sibbaldia procumbens, Solidago virgaurea and Veronica alpina) and creeping dwarf shrubs (Cassiope hypnoides and Salix herbacea). About 50 bryophyte taxa have been observed in an area of 1 ha; bryophytes typically represent c. 26% of total vegetation cover. Above-ground standing crop of these plant communities (litter and live biomass) is 250 g m−2, of which bryophyte biomass is 127 g m−2.
The grassland site is grazed by reindeer (Rangifer tarandus), Norway lemming (Lemmus lemmus) and voles (mainly Myodes rufocanus). During periodic population outbreaks, Norway lemming can consume most of the bryophytes while grazing in sub-nivean spaces. During the study period, notable lemming grazing occurred in winter 2015.
Experimental design, sampling in the field and germination trial
The experimental design follows the methodology of the globally distributed Nutrient Network experiment (Borer et al. 2014a). The experiment consists of 5 × 5 m plots arranged in 3 blocks. Plots are randomly assigned to fertilization and grazing exclusion treatments. In the grazing exclusion treatment, the entire plot was caged with 80 cm tall metal net (mesh size 1.1 cm) and with wires higher to exclude small (Norway lemming, voles) and large (reindeer) mammal herbivores. The fertilized plots received 10 g m−2 of each nutrient (N, P, K) annually from 2014 on, and a mixture of micronutrients in 2014. The micronutrient mixture contained boron (B; 0.1 g/m2), calcium (Ca; 6 g/m2), copper (Cu; 1 g/m2), iron (Fe; 17 g/m2), magnesium (Mg; 3 g/m2), manganese (Mn; 2.5 g/m2), molybdenum (Mo; 0.05 g/m2), sulphur (S; 12 g/m2), and zinc (Zn; 1 g/m2). In this study, we used a subset of experimental plots (n = 12) associated with factorial combinations of NPK fertilization (yes, no) and grazing exclusion (yes, no). After three years of treatments, we visually estimated the percentage cover of each bryophyte taxon in one permanent 1 × 1 m quadrat per plot in early August 2016. Bryophyte nomenclature follows that of the Finnish Biodiversity Info Facility (https://laji.fi/).
In July 2017, we collected four soil cores (10 × 10 cm, 5 cm thick) from an area where biomass was removed in each experimental plot. Above-ground litter and vegetation (vascular plants and bryophytes) were removed at the time of sampling. The soils were dried at room temperature, well-aerated until completely dry, and stored in closed paper bags at room temperature for three months. Dried samples were brought to the greenhouse of the Botanical Gardens of the University of Leipzig for a germination trial.
The germination trial began in late November 2017. For each plot, soil cores were homogenized, and rocks and roots were removed. The soil mixture was then placed on top of a 2-cm layer of sterile, standard potting soil with sand and perlite, within a 28 × 44 cm plastic tray. To assess potential contamination such as species germinating from ambient aerial spore deposition, we prepared 4 additional blank trays filled with the same sterile potting soil (n = 16 trays total). The trays were placed on a greenhouse table, where they were rearranged weekly and watered daily. The greenhouse was kept at c. + 20 °C with 16 h of daily supplemental lighting throughout the trial. The greenhouse lighting included five Philips Master HPI-T Plus, 400 Watt/645 E40 lamps 1.5 m above the bench and 60 cm apart. The air humidity of the greenhouse was on average 70%. We monitored bryophyte germination and recorded %-cover of germinated bryophytes three, five, and seven months after the start of germination (i.e., on 13 February, 10 April, and 9 June 2018). Some spores require a chilling period before they germinate, so we chilled the trays at + 5 °C in June 2018 for six weeks and then returned them to the greenhouse for three months. However, no new species germinated. Five bryophyte species (Barbula unguiculata, Brachythecium rutabulum, Bryum argenteum, an unidentified Bryum, Funaria hygrometrica) germinated in the blank trays, but not in the trays containing soil from experimental plots. Only one species, Ceratodon purpureus, germinated in both blank trays and experimental trays. Since it could have originated from the greenhouse, we excluded Ceratodon from all analyses.
Data and statistical analyses
We assembled the composition data in a plot-taxon matrix (i.e., 12 plots × 2 assemblages = 24 rows, 48 species as columns). The elements in this matrix were the %-cover assessed in the field (above-ground bryophyte assemblage) and the maximum %-cover assessed in the greenhouse (diaspore bank). We also characterized the life history strategy (colonist or perennial) of each species and assigned them to broad taxonomic-morphological groups. Life history strategies were based on more detailed life history classes from Dierssen (2001). The ‘colonist’ life history strategy included fugitives (short potential life span, production of numerous small asexual propagules [buds, gemmae, protonemal gemmae, fragments] or sexual spores, f), colonists s. str. (potential life span of a few years, production of numerous small spores, c), pioneer colonists (cp), short-lived shuttles (s), and long-lived shuttles (l) (note: ‘shuttle’ describes a species with large spores that requires a stable environment, such as epiphytes, where end of habitat is predictable; sensu During 1992). The ‘perennial’ life history strategy included perennials s. str. (p), competitive perennials (pc), and stress tolerant perennials (ps). The taxonomic-morphological groups provided another way to synthesize taxa. We used seven groups: 1) Pohlia spp. (almost exclusively Pohlia nutans), 2) Polytrichaceae spp. (five Polytrichum and Polytrichastrum species), 3) Ptychostomum (mainly Ptychostomum cf. moravicum, and some P. algovicum), 4) Dicranaceae (Dicranum spp., Kiaeria starkei, Paraleucobryum enerve), 5) pleurocarpous species (Brachythecium, Heterocladium, Hylocomium, Hylocomiastrum and Sanionia species), 6) liverworts (mainly Barbilophozia, Gymnomitrion, Lophozia species, Pellia sp. and Ptilidium ciliare), and 7) a group of all other taxa (Andreaea rupestris, Conostomum tetragonum, Leptobryum pyriforme, Mniaceae sp., Mnium blyttii, Racomitrium_microcarpon, and Syntrichia norvegica) (Appendix 1).
All analyses were conducted in the R statistical environment (v. 4.1.2). We compared species compositions of diaspore bank and above-ground assemblages with several complementary techniques. First, we compared abundance relationships of taxa using function ‘rankabundcomp’ (R package BiodiversityR; Kindt and Coe 2005). Second, we identified taxa that were strongly associated with one assemblage (i.e., diaspore bank or aboveground) over the other. Here, we used Indicator Species Analysis (ISA) to account for differences in both frequency and abundance between assemblages. ISA was run using ‘indicspecies::multipatt’ function (De Caceres and Legendre 2009; selected association ‘IndVal.g’, significance level = 0.05, 999 permutations). Third, we used permutational multivariate analysis of variance (PERMANOVA, Anderson 2001; implemented in vegan::adonis2; Oksanen et al. 2018) to test whether compositions of the two assemblages differed, and whether they differed in response to experimental treatments. The distance matrix was relativized by plot totals so that compositional differences were not confounded with differences in total abundance, and then expressed as a Bray–Curtis dissimilarity matrix as this measure is well suited to percent cover data. We specified assemblage type (diaspore bank vs. above-ground assemblages), NPK-fertilization, herbivore exclusion, and all interactions as explanatory factors, after including plotID as the first term in the model to account for plot-to-plot variation. We visualized these patterns using non-metric multidimensional scaling (NMDS; implemented in vegan::metaMDS; Oksanen et al. 2018). This function automatically finds the best solution with the lowest stress value for a solution of a given dimensionality. Fourth, we tested whether bryophyte species richness and cover responded to fertilization and herbivore exclusion treatments. We began by testing total richness and total cover, and then tested each taxonomic-morphological group separately. We analyzed the diaspore bank and above-ground assemblages separately. For these tests we used ordinary linear models (‘lm’ function; R Core Team 2018) with fertilization, herbivore exclusion, and their interaction as explanatory factors and experimental block included to account for block-level variation (Crawley 2007). Species richness data were square root transformed, and cover data were arcsine-square root transformed to improve normality of the residual distributions. A few total cover values exceeded 100% and therefore were set to 100% for analysis so that the transformation could be applied.
Results
In total, 48 bryophyte taxa were identified across both above- and below-ground communities (Table 1). Half of the taxa (26 of 48) were colonists, and these accounted for 81% of the cover. In the above-ground assemblages, 40 taxa were identified (Appendix 1). Colonists accounted for 60% of the taxa but only 23% of the cover. In the diaspore bank, 26 taxa were identified. Colonists accounted for 31% of the taxa but 98% of the cover, particularly because of one taxon (Pohlia nutans). Eight taxa were observed only in the diaspore bank (Appendix 1). Rank abundance curves were much more even for the above-ground assemblages than the diaspore bank (Fig. 1).
Bryophyte composition differed significantly between the diaspore bank and the above-ground assemblages (Table 2; Fig. 2). Above-ground assemblages from different treatments showed large variation in composition (Fig. 2), though PERMANOVA analyses did not identify significant interactions between community type and the experimental treatments (Table 2). The Indicator Species Analysis identified three taxa as being characteristic of the soil diaspore bank and nine taxa as characteristic of above-ground assemblages. The taxa characteristic of the soil diaspore bank were Pohlia nutans (IndVal = 0.999), Pellia sp. (IndVal = 0.764), and Polytrichastrum longisetum (IndVal = 0.645). The taxa characteristic of the above-ground assemblages were Sanionia uncinata (IndVal = 0.954), Kiaeria starkei (IndVal = 0.900), Hylocomiastrum pyrenaicum (IndVal = 0.894), Dicranum spadiceum (IndVal = 0.815), Diplophyllum taxifolium (IndVal = 0.764), Hylocomium splendens (IndVal = 0.761), Trilophozia quinquedentata (IndVal = 0.757), Racomitrium microcarpon (IndVal = 0.645), and Heterocladium dimorphum (IndVal = 0.644).
A NMDS ordination for bryophyte diaspore bank (circles) and above-ground vegetation (squares) assemblages in plots receiving different combinations of fertilizer and herbivore exclusion treatments. B The four strongest indicators of one assemblage over the other. For each species, symbol size is proportional to abundance; grey symbols are absences. All graphs are based on the coordinates of the same two-dimensional ordination (stress = 0.081)
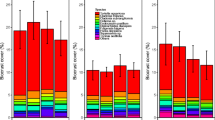
For the diaspore bank, total species richness was unaffected by the experimental treatments while total bryophyte cover was significantly reduced by fencing (Fig. 3, Appendix 2), though biological significance of this pattern is minimal as cover values were very high (> 95%) in all treatments. In contrast, both richness and cover of the above-ground bryophyte assemblages were reduced by the treatments. Cover was reduced by NPK fertilization while richness was reduced by both fertilization and fencing, particularly when applied together.
Responses of total bryophyte cover (%) and richness (number of bryophyte taxa) in assemblages germinated from soil samples (diaspore bank) and observed in the above-ground vegetation to the different treatments. Red bars indicate mean values, and black dots are the values in individual plots. Note that bank data were collected from 28 × 44 cm (0.123 m2) cultivation trays whereas above-ground vegetation data were collected from 1 m2 plots. No significant effects (P > 0.05) are indicated by ‘NS’. Significant effects based on linear models are indicated as follows: experimental block (B), fence (F), NPK fertilization (N), and fence × NPK fertilization interaction (F:N). Detailed statistical results are presented in Appendix 2
For the diaspore bank, all bryophyte groups showed weak responses to treatments (Fig. 4, Appendix 3). In the above-ground bryophyte assemblages, six of seven bryophyte groups responded to NPK-fertilization and/or grazing exclusion; only liverworts showed negligible response to the treatments (Fig. 4, Appendix 3). Pohlia spp. and Ptychostomum spp. showed a negative response to grazing exclusion. NPK fertilization reduced the abundance of Polytrichaceae mosses and Dicranaceae mosses. Grazing exclusion and NPK fertilization had interactive effects on pleurocarpic mosses: grazing exclusion had a positive effect while joint fertilization and grazing exclusion had a strong negative effect. Grazing exclusion and NPK fertilization also had interactive effects on other mosses: this taxonomic-morphological group responded positively to grazing exclusion and to NPK fertilization but not to them in combination.
Cover (%) of bryophyte groups in assemblages germinated from soil samples and observed in the above-ground vegetation in different treatments. The capital letters refer to experimental treatments as described in the caption of Fig. 3. Bryophyte group abbreviations: Pohlia (Pohlia spp.), Poly. (Polytrichaceae spp.), Ptych. (Ptychostomum spp.), Dicran (Dicranaceae spp.), Liver. (Liverworts; Hepaticae spp.), Pleur. (pleurocarpic mosses, Hypnales), and Other. Note strong differences in scale of y-axis among bryophyte groups. Detailed statistical results are presented in Appendix 3
Discussion
We found a diverse diaspore bank of bryophytes in soils of a high-latitude tundra grassland site. While above-ground bryophytes responded strongly and variably to fertilization and herbivore exclusion, soil diaspore bank showed only weak or negligible responses to the same treatments. These findings support our hypothesis that the diaspore bank is buffered against environmental changes and could contribute to species’ persistence under eutrophication and changes in grazing patterns. As bryophytes are a co-dominant element in the vegetation of tundra ecosystem, accounting for 41% of above-ground biomass, and can significantly contribute to essential ecosystem functions, the diaspore bank may be one of the key factors maintaining diversity and ecosystem functioning in tundra grasslands. Integrated analyses of responses of diaspore banks and above-ground bryophyte assemblages reveal novel mechanisms that may be critically important for grassland bryophyte diversity under environmental changes.
We found a rather fast loss of richness in above-ground bryophyte assemblages resulting from three years of fertilization and grazer exclusion. This loss was likely due to increased biomass and reduction in light availability near the soil surface (Borer et al. 2014b) and is consistent with earlier evidence of fertilization-driven decreases in bryophytes (Cusell et al. 2014; Dirkse and Martakis 1992; Virtanen et al. 2000). In total, five primarily initially abundant bryophyte species persisted in plots with NPK fertilization and grazer exclusion, whereas seven species germinated from the diaspore bank in the same plots. These findings provide evidence that diaspore bank can maintain the capacity of local bryophyte assemblages to cope with nutrient enrichment and changes in grazing pressure (see also Ingerpuu and Sarv 2015). In general, while changes in grassland vegetation following nutrient enrichment or grazing regimes may affect diaspore banks (e.g., by preventing diaspores from reaching soil or by altering germination from diaspore banks; Ghorbani et al. 2003), diaspore banks may still act as a dynamically important element contributing to coexistence via storage effect (Chesson 2000; van Tooren et al. 1992), enabling species persistence under environmental changes.
However, approximately half of the species observed above-ground did not germinate from soils, indicating that the diaspore bank does not fully preserve species present in the above-ground assemblages. This disparity may simply reflect the fact that many species do not form a persistent diaspore bank, perhaps due to limited diaspore production. These species may rely on dispersal from elsewhere in the landscape to recolonize areas. Furthermore, it remains less well known how long bryophyte diaspores persist in the soil, but it is possible that diaspores (especially sexual spores) of some species remain viable for several years or more than a decade or even a century (Bu et al. 2017; Dyer and Lindsay 1992; Vanderpoorten and Goffinet 2009; Wang et al. 2020). For many colonist species in particular, asexual propagules (buds, gemmae, protonemal gemmae, fragmentation) may play an important role for abundance and survival of species in the diaspore bank (Dyer & Lindsay 1992). At least for some species, soil can thus preserve species that have been lost from the above-ground community. Our findings for bryophytes are in line with some evidence for vascular plants: Ma et al. (2014) reported weaker response of soil seed banks to long-term fertilization than above-ground vegetation. However, other studies have found seed banks of vascular plants vulnerable to fertilization (Basto et al. 2015; Eskelinen et al. 2021). Further studies on the long-term impacts of nutrient enrichment and herbivore removal on diaspore banks are clearly needed.
As noted above, the diaspore bank contained a subset of the species occurring in the above-ground vegetation. The diaspore bank was dominated by a pioneer colonist moss Pohlia nutans (cover often > 90% in germination trays), whereas it was scarce in the above-ground assemblage. This moss occurs in diaspore banks of various northern and southern hemisphere ecosystems with non-calcareous soils (Bergstrom and Selkirk 1999; Jonsson 1993). In addition, several Polytrichaceae species that are widely distributed boreal-arctic species and were common in above-ground vegetation also germinated from soil, showing that the diaspore bank also contributes to their persistence in tundra grasslands. One of these, Polytrichastrum longisetum, was not observed in the study plots in the field but has been observed in the surroundings of the study site. This moss is known to colonize disturbed organic soils (Tuittila et al. 2000). Our results show that many perennial mosses such as Dicranum, Hylocomium and Kiaeria also emerge from diaspore bank.
We did not find evidence for commonly observed over-representation of colonist species (During and ter Horst 1983; Jonsson 1993) in the diaspore bank. It is possible that frequent disturbances in this kind of tundra grasslands promote co-existence of colonists and perennials both in above-ground assemblages and in the diaspore bank. Especially, periodic heavy grazing pressure by Norway lemming and voles, and summer-time reindeer trampling can counteract the development of thick moss mats formed by robust perennial species and favor co-existence of colonist and perennial species (Ericson 1977; Moen et al. 1993; Virtanen et al. 1997).
Our finding that bryophyte diaspore banks can affect species persistence in a local community could contribute to metacommunity stability and have implications for species distributions in the context of global climatic changes (Bergstrom and Selkirk 1999; Wisnoski and Shoemaker 2022). The high long-distance dispersal potential of some bryophytes suggests that diaspore banks could, in principle, include diaspores that are currently absent from local assemblages. For example, bryophytes are the first colonizers of volcanic islands by means of long-distance dispersal (Ingimundardóttir et al. 2014), and Lewis Smith and Ochyra (2006) found an exotic moss in Antarctic soils. Although long distance dispersal is relatively uncommon, our results are consistent with recent studies suggesting that some bryophytes disperse most effectively in a scale of tens of kilometers (Lönnell and Hylander 2018; Vanderpoorten et al. 2019), and therefore that soil diaspore banks are likely dominated by species from the local species pool. In that perspective, as also suggested by Ghorbani et al. (2003), larger scale environmental changes or management regimes leading to changes in species pools will likely affect the fate of diaspore banks.
Increased atmospheric nitrogen deposition and changes in traditional grazing often have negative biodiversity impacts on ecosystems (During and Willems 1986; Stevens et al. 2004; Vitousek et al. 1997). Our work demonstrates the potential of the soil diaspore banks for the restoration of biodiversity (see also Callaghan et al. 2020). Restorative grazing that disturbs topsoil via trampling can lead to recovery of local bryophyte assemblages from the soil diaspore bank (Takala et al. 2012). The soil diaspore bank may play a general role for restoration of bryophyte diversity even when species are absent or very scarce above-ground (see also Vellak et al. 2021), though replenishment often enough from above-ground communities is needed to maintain the storage effect function (Bisang et al. 2021). We nevertheless provide empirical evidence for an assertion of During (2001) that diaspore banks deposited in soil may enhance persistence and recovery of local bryophyte assemblages under environmental pressures.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Alexander HM, Foster BL, Ballantyne F, Collins CD, Antonovics J, Holt RD (2012) Metapopulations and metacommunities: Combining spatial and temporal perspectives in plant ecology. J Ecol 100:88–103
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Barbé M, Chavel ÉE, Fenton NJ, Imbeau L, Mazerolle MJ, Drapeau P, Bergeron Y (2016) Dispersal of bryophytes and ferns is facilitated by small mammals in the boreal forest. Écoscience 23:67–76
Basto S, Thompson K, Phoenix G, Sloan V, Leake J, Rees M (2015) Long-term nitrogen deposition depletes grassland seed banks. Nat Commun 6:1–6
Benavent-González A, Delgado-Baquerizo M, Fernández-Brun L, Singh BK, Maestre FT, Sancho LG (2018) Identity of plant, lichen and moss species connects with microbial abundance and soil functioning in maritime Antarctica. Plant Soil 429:35–52
Bergstrom DM, Selkirk PM (1999) Bryophyte propagule banks in a feldmark on subantarctic Macquarie Island. Arct Ant Alp Res 31:202–208
Bisang I (1996) Quantitative analysis of the diaspore banks of bryophytes and ferns in cultivated fields in Switzerland. Lindbergia 21:9–20
Bisang I, Piippo S, Hedenäs L (2003) Bryophyte diaspore bank in three Malaysian mountain rainforests. J Bryol 25:68–70
Bisang I, Lienhard L, Bergamini A (2021) Three decades of field surveys reveal a decline of arable bryophytes in the Swiss lowlands despite agri-environment schemes. Agric Ecosyst Environ 313:107325
Boch S, Allan E, Humbert JY, Kurtogullari Y, Lessard-Therrien M, Müller J, Prati D, Rieder NR, Arlettaz R, Fischer M (2018) Direct and indirect effects of land use on bryophytes in grasslands. Sci Total Environ 644:60–67
Borer ET, Harpole WS, Adler PB, Lind EM, Orrock JL, Seabloom EW, Smith MD (2014a) Finding generality in ecology: a model for globally distributed experiments. Methods Ecol Evol 5:65–73
Borer ET, Seabloom EW, Gruner DS, Harpole WS, Hillebrand H, Lind EM et al (2014b) Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508:517–520
Bu ZJ, Sundberg S, Feng L, Li HK, Zhao HY, Li HC (2017) The Methuselah of plant diaspores: Sphagnum spores can survive in nature for centuries. New Phytol 214:1398–1402
Callaghan DA, During HJ, Forrest LL, Wilkinson K (2020) Neglected and at risk: bryophyte diaspore banks of coastal dune systems. J Bryol 42:223–234
Caners RT, Macdonald SE, Belland RJ (2009) Recolonization potential of bryophyte diaspore banks in harvested boreal mixed-wood forest. Plant Ecol 204:55–68
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:313–366
Concostrina-Zubiri L, Huber-Sannwald E, Martínez I, Flores JF, Reyes-Agüero JA, Escudero A, Belnap J (2014) Biological soil crusts across disturbance–recovery scenarios: effect of grazing regime on community dynamics. Ecol Appl 24:1863–1877
Concostrina-Zubiri L, Molla I, Velizarova E, Branquinho C (2016) Grazing or not grazing: implications for ecosystem services provided by biocrusts in Mediterranean cork oak woodlands. Land Degrad Dev 28:1345–1353
Condon LA, Pyke DA (2018) Resiliency of biological soil crusts and vascular plants varies among morphogroups with disturbance intensity. Plant Soil 433:271–287
Cornelissen JH, Lang SI, Soudzilovskaia NA, During HJ (2007) Comparative cryptogam ecology: a review of bryophyte and lichen traits that drive biogeochemistry. Ann Bot 99:987–1001
Crawley MJ (2007) The R book. John Wiley Inc., Chichester
Cusell C, Kooijman A, Lamers LP (2014) Nitrogen or phosphorus limitation in rich fens?-Edaphic differences explain contrasting results in vegetation development after fertilization. Plant Soil 384:153–168
De Caceres M, Legendre P (2009) Associations between species and groups of sites: indices and statistical inference. Ecology 90:3566–3574. https://cran.r-project.org/web/packages/indicspecies/indicspecies.pdf. Accessed 21 Apr 2022
Dierssen K (2001) Distribution, ecological amplitude and phytosociological characterization of European bryophytes. Bryoph Bibliot 56:1–289
Dirkse GM, Martakis GFP (1992) Effects of fertilizer on bryophytes in Swedish experiments on forest fertilization. Biol Cons 59:155–161
During HJ (1992) Ecological classifications of bryophytes and lichens. In: Bates JW, Farmer M (eds) Bryophytes and Lichens in a Changing Environment. Oxford University Press, Oxford, pp 1–31
During HJ (1997) Bryophyte diaspore banks. Adv Bryol 6:103–134
During HJ (2001) Diaspore banks. Bryologist 104:92–97
During HJ, ter Horst B (1983) Diaspore bank of bryophytes and ferns in chalk grassland. Lindbergia 9:57–64
During HJ, Willems JH (1986) The impoverishment of the bryophyte and lichen flora of the Dutch chalk grasslands in the thirty years 1953–1983. Biol Cons 36:143–158
Dyer AF, Lindsay S (1992) Soil spore banks of temperate ferns. Amer Fern J 82:89–122
Eckstein J (2006) Die Moosdiasporenbanken einiger Teiche im Ostthüringer Buntsandsteingebiet. Herzogia 19:341–351
Ericson L (1977) The influence of voles and lemmings on the vegetation in a coniferous forest during a 4-year period in northern Sweden. Wahlenbergia 4:1–114
Eskelinen A, Elwood E, Harrison S, Beyen E, Gremer JR (2021) Vulnerability of grassland seed banks to resource-enhancing global changes. Ecology 102:e03512
Gavini SS, Suárez GM, Ezcurra C, Aizen MA (2019) Facilitation of vascular plants by cushion mosses in high-Andean communities. Alp Bot 129:137–148
Ghorbani J, Das PM, Das AB, Hughes JM, McAllister HA, Pallai SK, Pakeman RJ, Marrs RH, Le Duc MG (2003) Effects of restoration treatments on the diaspore bank under dense Pteridium stands in the UK. Appl Veg Sci 6:189–198
Iglesias N, Delgado V, Ederra A (2015) A comparison between the diaspore bank and above-ground bryoflora in the beech forests of Navarra (Northern Spain). Cryptog Bryol 36:19–40
Ingerpuu N, Sarv M (2015) Effect of grazing on plant diversity of coastal meadows in Estonia. Ann Bot Fenn 52:84–92
Ingimundardóttir GV, Weibull H, Cronberg N (2014) Bryophyte colonization history of the virgin volcanic island Surtsey, Iceland. Biogeosciences 11:4415–4427
Jonsson BG (1993) The bryophyte diaspore bank and its role after small-scale disturbance in a boreal forest. J Veg Sci 4:819–826
Kaufmann R, Mayer R, Schallhart N, Erschbamer B (2021) Effects of climate change vs. grazing exclusion on species diversity over 18 years along an elevation gradient in the European Alps. Front Ecol Evol 9: https://doi.org/10.3389/fevo.2021.640103
Kindt R, Coe R (2005) Tree diversity analysis. A manual and software for common statistical methods for ecological and biodiversity studies. World Agroforestry Centre (ICRAF), Nairobi
Kövendi-Jakó A, Márialigeti S, Bidló A, Ódor P (2016) Environmental drivers of the bryophyte propagule bank and its comparison with forest-floor assemblage in Central European temperate mixed forests. J Bryol 38:118–126
Lagerström A, Nilsson MC, Zackrisson O, Wardle DA (2007) Ecosystem input of nitrogen through biological fixation in feather mosses during ecosystem retrogression. Funct Ecol 21:1027–1033
Leibold MA, Chase JM (2018) Metacommunity ecology. Princeton University Press, Princeton
Lewis Smith RI, Ochyra R (2006) High altitude Antarctic soil propagule bank yields an exotic moss and potential colonist. J Hattori Bot Lab 100:325–331
Lindo Z, Gonzalez A (2010) The bryosphere: an integral and influential component of the Earth’s biosphere. Ecosystems 13:612–627
Löbel S, Dengler J, Hobohm C (2006) Species richness of vascular plants, bryophytes and lichens in dry grasslands: the effects of environment, landscape structure and competition. Folia Geobot 41:377–393
Lönnell N, Hylander K (2018) Calcicolous plants colonize limed mires after long-distance dispersal. J Biog 45:885–894
Ma Z, Ma M, Baskin JM, Baskin CC, Li J, Du G (2014) Responses of alpine meadow seed bank and vegetation to nine consecutive years of soil fertilization. Ecol Eng 70:92–101
Malta N (1922) Über die Lebensdauer der Laubmoossporen. Acta Univ Latv 4:235–246
Maskell LC, Smart SM, Bullock JM, Thompson K, Stevens CJ (2010) Nitrogen deposition causes widespread loss of species richness in British habitats. Global Ch Biol 16:671–679
Michel P, Payton IJ, Lee WG, During HJ (2013) Impact of disturbance on above-ground water storage capacity of bryophytes in New Zealand indigenous tussock grassland ecosystems. New Zealand J Ecol 37:114–126
Moen J, Lundberg PA, Oksanen L (1993) Lemming grazing on snowbed vegetation during a population peak, northern Norway. Arct Alp Res 25:130–135
Müller J, Klaus VH, Kleinebecker T, Prati D, Hölzel N, Fischer M (2012) Impact of land-use intensity and productivity on bryophyte diversity in agricultural grasslands. PLoS One 7:e51520
Oksanen J, Blanchet GF, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2018) vegan: Community Ecology Package. R package version 2.5–3. https://cran.r-project.org/web/packages/vegan/index.html. Accessed 21 Apr 2022
Oldén A, Halme P (2016) Grazers increase β-diversity of vascular plants and bryophytes in wood-pastures. J Veg Sci 27:1084–1093
Paulissen MP, Besalú LE, de Bruijn H, van der Ven PJ, Bobbink R (2005) Contrasting effects of ammonium enrichment on fen bryophytes. J Bryol 27:109–117
Pearce ISK, Woodin SJ, Van der Wal R (2003) Physiological and growth responses of the montane bryophyte Racomitrium lanuginosum to atmospheric nitrogen deposition. New Phyt 160:145–155
Permin A, Michelsen A, Rousk K (2022) Direct and indirect effects of warming on moss abundance and associated nitrogen fixation in subarctic ecosystems. Plant Soil 471:343–358
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07–0, http://www.R-project.org. Accessed 21 Apr 2022
Read CF, Duncan DH, Vesk PA, Elith J (2011) Surprisingly fast recovery biological soil crusts following livestock removal in southern Australia. J Veg Sci 22:905–916
Rieley JO, Richards PW, Bebbington ADL (1979) The ecological role of bryophytes in a North Wales woodland. J Ecol 67:497–527
Root HT, Miller JE, Rosentreter R (2020) Grazing disturbance promotes exotic annual grasses by degrading soil biocrust communities. Ecol Appl 30:e02016
Ross-Davis A, Frego KA (2004) Propagule sources of forest floor bryophytes: spatiotemporal compositional patterns. Bryologist 107:88–97
Rydgren K, Hestmark G (1997) The soil propagule bank in a boreal old-growth spruce forest: changes with depth and relationship to aboveground vegetation. Can J Bot 75:121–128
Shoemaker LG, Sullivan LL, Donohue I, Cabral JS, Williams RJ, Mayfield MM, Chase JM, Chu CW, Harpole WS, Huth A, HilleRisLambers J, James ARM, Kraft NJB, May F, Muthukrishnan R, Satterlee S, Taubert F, Wang X, Wiegand T, Yang Q, Abbott KC (2020) Integrating the underlying structure of stochasticity into community ecology. Ecology 101:e02922
Soudzilovskaia NA, Graae BJ, Douma JC, Grau O, Milbau A, Shevtsova A, Wolters L, Cornelissen JH (2011) How do bryophytes govern generative recruitment of vascular plants? New Phytol 190:1019–1031
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876–1879
Sundberg S, Rydin H (2000) Experimental evidence for a persistent spore bank in Sphagnum. New Phytol 148:105–116
Takala T, Tahvanainen T, Kouki J (2012) Can re-establishment of cattle grazing restore bryophyte diversity in abandoned mesic semi-natural grasslands? Biod Cons 21:981–992
Takala T, Tahvanainen T, Kouki J (2014) Grazing promotes bryophyte species richness in seminatural grasslands. Ann Bot Fenn 51:148–160
Tuittila ES, Rita H, Vasander H, Laine J (2000) Vegetation patterns around Eriophorum vaginatum L. tussocks in a cut-away peatland in southern Finland. Can J Bot 78:47–58
Turetsky MR (2003) The role of bryophytes in carbon and nitrogen cycling. Bryologist 106:395–409
van der Wal R, Brooker RW (2004) Mosses mediate grazer impacts on grass abundance in arctic ecosystems. Funct Ecol 18:77–86
van der Wal R, Pearce IS, Brooker RW (2005) Mosses and the struggle for light in a nitrogen-polluted world. Oecologia 142:159–168
van Tooren BF, During HJ, Lensink MJ (1985) The influence of the bryophyte layer on the microclimate in chalk grasslands. Abstr Bot 9:219–230
van Tooren BF, Hertog JD, Verhaar J (1988) Cover, biomass and nutrient content of bryophytes in Dutch chalk grasslands. Lindbergia 14:47–54
van Tooren BF, Ode B, During HJ, Bobbink R (1992) Regeneration of the species richness of the bryophyte layer of Dutch chalk grasslands. Lindbergia 16:153–160
Vanderpoorten A, Goffinet B (2009) Introduction to bryophytes. Cambridge University Press, Cambridge
Vanderpoorten A, Patiño J, Désamoré A, Laenen B, Górski P, Papp B, Holá E, Korpelainen H, Hardy O (2019) To what extent are bryophytes efficient dispersers? J Ecol 107:2149–2154
Vellak K, Samson T, Rikka M, Ingerpuu N (2021) Above-and below-ground species richness of bryophytes in Estonian mires: diversity and differences. J Bryol 43:224–233
Virtanen R, Henttonen H, Laine K (1997) Lemming grazing and structure of a snowbed plant community: a long-term experiment at Kilpisjärvi, Finnish Lapland. Oikos 79:155–166
Virtanen R, Johnston AE, Crawley MJ, Edwards GR (2000) Bryophyte biomass and species richness on the Park Grass Experiment, Rothamsted, UK. Plant Ecol 151:129–141
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW et al (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Wang LZ, Feng L, Bu ZJ, Sundberg S, Zhang M, Chen X, Yang YH, Yu XY, Liu WJ (2020) Sphagnum spore banks in two montane peatlands at different elevations. Wetlands Ecol Manag 28:825–835
Wisnoski NI, Shoemaker LG (2022) Seed banks alter metacommunity diversity: The interactive effects of competition, dispersal and dormancy. Ecol Lett. 25:740–754. https://doi.org/10.1111/ele.13944
Wisnoski NI, Leibold MA, Lennon JT (2019) Dormancy in metacommunities. Amer Nat 194:135–151
Acknowledgements
This project was funded by Academy of Finland (project 297191) to A.E. Kilpisjärvi Biological Station provided facilities during the field work, and the Botanical Gardens of University of Leipzig provided greenhouse facilities for the germination trial. We are grateful to Alvin Barth and Daniel Uhlig for their help with the germination trial. Soil pH analyses were supported, in part, by USDA-ARS grant 58-3098-7-007 to Elizabeth Borer (University of Minnesota).
Funding
Open Access funding provided by University of Oulu including Oulu University Hospital. This project was funded by Academy of Finland (project 297191) to A.E.
Author information
Authors and Affiliations
Contributions
The initial study conception and design was conducted by Anu Eskelinen. W. Stanley Harpole, Lauren Sullivan and Risto Virtanen. Material preparation, data collection and analysis were performed by Jonathan Bakker, Anu Eskelinen, Maria-Theresa Jessen, and Risto Virtanen. The first draft of the manuscript was written by Risto Virtanen, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Jeffrey Walck.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Virtanen, R., Bakker, J.D., Jessen, MT. et al. Is the bryophyte soil diaspore bank buffered against nutrient enrichment and grazing exclusion?. Plant Soil 477, 487–499 (2022). https://doi.org/10.1007/s11104-022-05450-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05450-0