Abstract
Purpose
The steady rise of atmospheric CO2 concentrations enhances symbiotic N2 fixation and plant growth. However, it is largely unknown whether more P is required to enhance N2 fixation under elevated CO2. We examined if plants under elevated CO2 require more P to support enhanced N2 fixation.
Methods
White lupin (Lupinus albus L. cv. Kiev) was grown in nutrient solution under ambient (380 ppm) and elevated (700 ppm) CO2 levels at 0, 2, 5, 12, 25 and 50 µM P. Nodulation, nodule function, N2 fixation and plant growth were assessed at 4 and 6 weeks post inoculation. Excess cation uptake and proton release were also analysed due to its importance in P acquisition.
Results
Elevated CO2 increased the critical P concentration in nutrient solution for N2 fixation, indicating plants under elevated CO2 need to acquire additional P to support greater N2 fixation. Elevated CO2 improved N2 fixation in P-deficient plants by increasing specific nitrogenase activity. Elevated CO2 also enhanced excess cation uptake which increased proton release by roots.
Conclusions
As elevated CO2 increased the plant demand for P, it increased the critical P concentration in nutrient solution for N2 fixation, thereby plants require more P to mitigate the effect of P deficiency on N2 fixation. Despite this, elevated CO2 could enhance N2 fixation in P-limiting environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric CO2 concentrations are projected to reach 700-800 ppm by the year 2100 (IPCC 2021). Rising CO2 concentrations stimulates photosynthetic activity in many C3 crop species and induces significant increases in plant production (Ainsworth and Long 2005). The elevated CO2-induced increase in plant growth requires plants to enhance N uptake to support this process (Lam et al. 2012). Symbiotic N2 fixation is a process where plants form a symbiotic relationship with N2-fixing bacteria such as Frankia or Rhizobium species. These bacteria are supplied C from the host plant and fix atmospheric N2 into NH3 using nitrogenase enzyme complex, which is then supplied to plants and utilised for plant growth (Sachs et al. 2018). To meet the increase in N demand under elevated CO2, plants commonly increase N2 fixation, particularly in agricultural leguminous (Aranjuelo et al. 2014; Lam et al. 2012) and actinorhizal species (Chen and Markham 2021; Tobita et al. 2010).
Elevated CO2 increases N2 fixation via two main mechanisms. First, elevated CO2 increases nodule formation and nodule development. Li et al. (2017) demonstrated that elevated CO2 could drastically increase nodule number and fresh weight which played a critical role in supporting increased N2 fixation. Improved nodulation under elevated CO2 can be facilitated by enhancing flavonoid exudation which could promote infection of plant roots by rhizobia and hence increase nodule formation (Haase et al. 2007). Second, elevated CO2 enhances photosynthesis, leading to more primary metabolites (malate and sucrose) being allocated to nodules, to increase nitrogenase activity (Haase et al. 2007; Parvin et al. 2020). Therefore, in some plant species elevated CO2 can increase N2 fixation without an increase in nodule number or nodule biomass as found by Butterly et al. (2015). The mechanism by which elevated CO2 enhances N2 fixation will likely depend on plant nutritional status and plant species (Jin et al. 2012; Palit et al. 2020).
Nodules have significantly higher P concentration compared to host plants, which outlines the importance of P for N2 fixation (Schulze et al. 2006). Phosphorus is essential for nodule formation, nodule development and nodule function with P deficiency directly inhibiting N2 fixation in some crop species (Le Roux et al. 2008; Tang et al. 2001). Phosphorus deficiency also decreases host plant growth which reduces plant demand for N and induces a down-regulation in nitrogenase activity (Sulieman et al. 2013). Given that elevated CO2 increases N2 fixation but P deficiency decreases N2 fixation, these two factors play two opposing roles in N2 fixation. The response of N2 fixation to elevated CO2 in P-deficient plants will depend on whether P deficiency directly limits N2 fixation (Almeida et al. 2000; Tang et al. 2001). If nodule function is directly impaired by P deficiency, elevated CO2 may not enhance N2 fixation in P-deficient plants (Sa and Israel 1998). However, Nguyen et al. (2006) found that elevated CO2 could enhance N2 fixation in P-deficient Acacia trees, suggesting that elevated CO2 enhances biomass production in P-deficient plants, and may increase N2 fixation.
Given that elevated CO2 enhances plant biomass and promotes production of P-rich nodules, plants may require more P to support enhanced N2 fixation under elevated CO2 (Rogers et al. 1993). To determine the amount of P required to achieve maximum N2 fixation, P response curves can be generated under different CO2 treatments (Pang et al. 2011). As elevated CO2 can enhance nodulation, greater P supply may be necessary to support greater nodule growth and N2 fixation under elevated CO2 (Lam et al. 2012).
Proton release, excess-cation uptake and N2 fixation are closely linked. When N2 fixation is the main source of N, plants absorb more cation nutrients than anion nutrients which results in net release of protons, thus promoting rhizosphere acidification (Tang et al. 1999). In addition to N2 fixation, P deficiency promotes proton release in many leguminous plant species (Sas et al. 2001). With greater N2 fixation and elevated CO2-induced increases in plant growth, this could lead to greater uptake of cations over anions, particularly in P-limiting environments and hence lead to greater rhizosphere acidification. For plants to access sorbed and precipitated forms of P in soil, transformation is required before root uptake. Enhanced rhizosphere acidification could promote the solubilisation and desorption of non-labile P sorbed to non-crystalline materials or precipitated with metals such as Ca (Barrow 2017; Hinsinger et al. 2003). In soils, optimal P mobilisation occurs at pH 6 which is also the optimal pH for nodulation in Lupinus species (Andersson et al. 2015; Tang and Robson 1993).
White lupin is a crop species highly adapted to P-deficient soils. In response to P deficiency, white lupin produces dense rootlets along lateral roots, termed cluster roots (Lambers et al. 2006; Wang et al. 2019). Not only do cluster roots increase the surface area of roots for P uptake, these are also the site of intense rhizosphere acidification and organic anion efflux (Neumann et al. 2000). These P acquisition mechanisms allow white lupin to mobilise significant quantities of non-labile P from inorganic sources, particularly under elevated CO2 (Braum and Helmke 1995; O’Sullivan et al. 2020). Furthermore, N2 fixation in white lupin is highly resilient to P-deficient conditions. For example, despite not being supplied with any P, N2 fixation was uninhibited after 21 d of growth (Schulze et al. 2006). As white lupin can sustain N2 fixation in low-P soils, it is unknown if elevated CO2-induced increases in biomass production will affect N2 fixation in P-deficient white lupin (O’Sullivan et al. 2021).
This study aimed to investigate the effects of elevated CO2 on the critical P concentration in nutrient solution, nodules and shoots for N2 fixation in white lupin. The critical P concentration was defined as the concentration of P needed to achieve maximum N2 fixation. We hypothesised that (i) elevated CO2 would increase the N2 fixation in P-deficient plants, (ii) elevated CO2 would increase the P requirement for N2 fixation, and (iii) enhanced plant growth and N2 fixation under elevated CO2 would promote excess cation uptake and proton release.
Materials and methods
Plants were grown in solution culture to facilitate the measurements of nodule formation, nodule function and proton release. Uniform-sized seeds of white lupin (Lupinus albus L. cv. Kiev) were germinated at 20 °C for 5 d in 30-L tubs containing aerated solution of 1 mM CaCl2 and 5 µM H3BO3. After germination, 12 seedlings were transferred to each 5-L container of nutrition solution. The nutrient solution had the following composition (µM): 600 K2SO4; 600 CaCl2; 200 MgSO4; 10 FeNaEDTA; 5 H3BO3; 1 MnSO4; 1 ZnSO4; 0.2 CuSO4; 0.2 CoCl2; 0.03 Na2MoO4 (based on Sas et al. 2001). To generate a P response curve, P was supplied as KH2PO4 at 6 levels: 0, 2, 5, 12, 25 and 50 µM P. These P levels were in range of previous studies to induce P-deficiency and to cover adequate P supply in white lupin (O’Sullivan et al. 2021). After seedling transferring to nutrient solution, a dense water suspension of Bradyrhizobium sp. strain WU425 was applied at approximately 2 × 108 cells L−1. The nutrient solution was renewed after 2 d, and the rhizobial inoculum was reapplied. Uniform seedlings were equally divided amongst 6 CO2-controlled growth chambers with 20 °C days for 16 h and 18 °C nights under 380 ppm and 700 ppm CO2 concentrations for ambient and elevated CO2 levels, respectively (Fitotron SGC 120, Weiss Technik, Loughborough, UK; Climatron 1100-SL-H, Thermoline, Australia). Each treatment was replicated 3 times. In total, there were 36 hydroponic containers distributed amongst 6 growth chambers. Light intensity at the canopy level was around 400 µmol m−2 s−1. Containers were randomised within cabinets every 2 d and were rotated weekly between cabinets. The ambient CO2 concentration was maintained by using a NaOH CO2 scrubber. The elevated CO2 concentration is in range of the predicted CO2 level by 2100 in addition to other CO2 enrichment studies (Jiang et al. 2020; IPCC 2021). To minimize the effect of seed P and induce early P response and stimulate N2 fixation, cotyledons were removed 10 d post inoculation. Nutrient solutions were continuously aerated and renewed every 2 d. Nutrient solution pH was adjusted daily to 6 using standardised 0.1 M KOH, which is the optimal pH for nodulation in Lupinus species (Tang and Robson 1993). The amount of KOH consumed for pH adjustment and the pH of the discarded nutrient solutions were recorded and used to determine proton release.
Nodules were counted after signs of initial emergence at 10, 12, 14, 16 and 20 d, and 4 and 6 weeks after inoculation. Plants were destructively sampled at 4 and 6 weeks after inoculation. These sampling times were chosen because elevated CO2-induced increases in plant growth and nitrogenase activity in soybean began at Week 4 (Sa and Israel 1998). Plant growth of white lupin at Week 6 was relatively rapid which coincided with high plant N-demand, intense nitrogenase activity under P sufficiency and inhibition of nitrogenase activity by P deficiency (O’Sullivan et al. 2021; Schulze et al. 2006). These sampling times represented early (6 leaves emerged) and mid (9 leaves emerged) vegetative growth stages, respectively. At each harvest, 6 plants were harvested from each container. Plants were immediately divided into shoots and roots. To measure nodule nitrogenase activity, roots with intact nodules were placed in sealed 1-L Mason jars and acetylene was injected through a septum. The final concentration of acetylene in the jar was 2.5% (v/v). Gas was sampled from each jar at 15, 30 and 60 min after acetylene addition and ethylene was quantified using a gas chromatograph fitted with a Varian GS-Q capillary column and flame ionisation detector (Varian CP-3800 CA USA). Nitrogenase activity was calculated from the slope of the linear accumulation of ethylene over the 3 sampling times.
After nitrogenase analysis, nodules were counted and then removed from roots. Shoots, roots and nodules were placed in a 70 °C oven for 3 days and their dry weights recorded. Nodule size was calculated by dividing nodule dry weight over nodule number. Shoot and root materials were finely ground and then digested with HNO3 in a microwave digester (Ethos up, Milestone, Milan, Italy). Nodule samples were digested using HNO3 and HClO4 (4:1) in an open block digester (Seal Analytical, USA). Plant digests were analysed for P, S, Ca, Mg, Mn, Zn, K, Na and Fe using inductively coupled plasma optical emission spectrometry (Perkin Elmer Optima 8000, MA, USA). Excess cation uptake (mmol(+) plant−1) was calculated as the number of moles of positive charges acquired as cations (K+, Ca2+, Mg2+, Na+) minus the number of moles of negative charges acquired as anions (PO4−, SO42−). Chloride (Cl−) was not included in the analysis; however, chloride only accounts for small proportion (6%) of excess cation uptake (Tang and Yu 1999). Elements mobilised from seed were not accounted in the excess cation uptake calculations.
Ground shoot and root samples were also analysed for C and N using dry combustion method (PerkinElmer EA2400, CT, USA). As no N was supplied to plants in nutrient solution, the amount of N2 fixed was the content of N in the entire plant minus the contribution of N from the seed before cotyledons were removed.
Statistical analysis
Analysis of variance was performed to determine the effects of CO2 treatment, P supply and their interaction on all measurements except for early nodule formation. The concentrations of P in shoot, root and nodule, and total P content were log10-transformed before analysis to ensure normality of residuals. Linear mixed-effect modelling was used to determine the effects of CO2, P supply, and time of nodule counting on early nodule formation with a random intercept used for each replicate. Normality of residuals for each test was assessed using histograms, Q-Q plots and the Shapiro-Wilk test. To compare means, the least significant difference (LSD) was calculated at the 5% significance level.
A two-phase linear model was used to determine the critical P concentrations in nutrient solution, nodules and shoots for N2 fixation. The breakpoint on the x-axis was considered the critical P concentration which was defined as the P concentration where N2 fixation was not increased by additional P. If the standard error for the critical P concentration did not overlap between CO2 treatments, the difference was considered significant.
Analysis of variance and correlations were performed in R version 4.0.5 (R Core Team 2021) and post-hoc tests and linear mixed effect modelling was carried out using the predictmeans (Luo et al. 2020) and nlme (Pinheiro et al. 2021) packages in R, respectively. The two-phase linear model was performed in GENSTAT (v19; VSN International, Hemel Hempstead, UK).
Results
Plant biomass
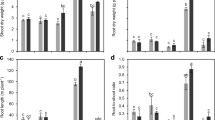
Elevated CO2 significantly increased shoot dry weight at 4 and 6 weeks after transplanting by 13% and 18%, respectively (P <0.001, Fig. 1). Increasing P supply from 0 to 50 µM P increased shoot dry weight by 49% and 157% at 4 and 6 weeks, respectively (P <0.001, Fig. 1a, b). At Week 4, root dry weight was greatest in plants supplied 2 µM P whereas at Week 6, increasing P supply increased root dry weight between 0 and 5 µM P but then plateaued between 5 and 50 µM P. The root dry weight was 76% greater at 50 µM P compared to nil-P control (P <0.001, Fig. 1d).
Dry weights of shoots (a, b) and roots (c, d) of white lupin grown under ambient (380 ppm) and elevated (700 ppm) CO2 concentrations. Plants were supplied with 0, 2, 5, 12, 25 and 50 µM P, and were harvested 4 (a, c) and 6 (b, d) weeks after transplanting. Vertical bar indicates the least significant difference (P = 0.05) for any two means where the main effects and/or interaction are significant. Error bars indicate the standard error of the mean (n = 3)
Nodulation and nitrogenase activity
Elevated CO2 but not P supply influenced early nodule formation. Elevated CO2 enhanced early nodule emergence by 20, 17 and 6% at 12, 14, and 16 days after inoculation (P = 0.016, Fig. S2). The effect of P supply on nodule number was only apparent at Week 6 with plants supplied 50 µM P having 14% more nodules compared to plants without P supply (P = 0.024, Table 1). Nodule dry weight was affected by P supply but not CO2 level at Week 4 (P <0.001) and Week 6 (P <0.001) (Table 1). At Week 4, plants supplied 0 µM P displayed reduced nodule dry weight compared to all other P treatments (Fig. 2a). The effect of P supply on nodule dry weight was stronger at Week 6 compared to Week 4, with increased P supply increasing nodule dry weight up to 5 µM P. Nodule dry weight was over 2-fold greater at 50 µM P than 0 µM P (Fig. 2b). Nodule size was unaffected by CO2 treatment but increased with increasing P supply at both Weeks 4 (P <0.001) and 6 (P <0.001) with nodule size being 68% and 180% larger at 50 µM P than 0 P at Weeks 4 and 6, respectively (Fig. 2e, f).
Dry weight (a, b), number (c, d) and size (e, f) of nodules in white lupin grown under ambient (380 ppm) and elevated (700 ppm) CO2 concentrations. Plants were supplied with 0, 2, 5, 12, 25 and 50 µM P, and were harvested 4 (a, c, e) and 6 (b, d, e) weeks after transplanting. Vertical bar indicates the least significant difference (P = 0.05) for any two means where the main effect and/or interaction are significant. Error bars indicate the standard error of the mean (n = 3)
Elevated CO2 increased total nitrogenase activity across all P treatments by 28% at Week 4, but only increased total nitrogenase activity in plants supplied 25 and 50 µM P by 9 and 11%, respectively, at Week 6 (Fig. 3a, b). Nitrogenase activity was enhanced with increasing P supply from 0 to 5 µM P at Week 4 (P <0.001) and from 0 to 12 µM P at Week 6 (P <0.001) before it was plateaued to 50 µM P. Elevated CO2 increased specific nitrogenase activity by 47% and 9% at Week 4 (P <0.001) and Week 6 (P = 0.002) (Fig. 3c, d; Table 1). Specific nitrogenase activity increased with increasing P supply from 0 to 5 µM P at Week 4 and from 0 to 12 µM at Week 6 and then plateaued at higher P supplies (Fig. 3d).
Whole plant nitrogenase activity (a, b) and specific nitrogenase activity (c, d) of white lupin grown for 4 (a, c) and 6 (b, d) weeks under ambient (380 ppm) and elevated (700 ppm) CO2 concentrations. Plants were supplied with 0, 2, 5, 12, 25 and 50 µM P. Vertical bar indicates the least significant difference (P = 0.05) for any two means. Error bars indicate the standard error of the mean (n = 3)
Nitrogen concentration and content
Nitrogen mobilised from the seed to plant was approximately 8 mg and was not affected by CO2 or P treatment (P >0.05), this was determined by calculating the difference between seed N content and removed cotyledon N content (data not shown). Elevated CO2 significantly reduced shoot N concentration by 14% after 4 weeks of growth (P < 0.001) but was unaffected by CO2 level after 6 weeks of growth (P = 0.484, Table 1). The effect of P supply on shoot N was only observed at Week 6 (P <0.001, Table 1). Shoot N concentration decreased by 45% with increasing P supply from 0 to 5 µM P (Fig. 4b). Root N concentration was not affected by CO2, P supply or their interaction at Week 4, and was only affected by P supply at Week 6 where plants supplied 0 µM P had a root P concentration 12% higher than other P supplies (Fig. 4; Table 1).
The concentrations of N in shoots (a, b) and roots (c, d) and total N content (e, f) of white lupin grown under ambient (380 ppm) and elevated (700 ppm) CO2 concentrations. Plants were supplied with 0, 2, 5, 12, 25 and 50 µM P. Plants were harvested 4 (a, c) and 6 (b, d) weeks after transplanting. Vertical bar indicates the least significant difference (P = 0.05) for any two means where the main effect and/or interaction are significant. Error bars indicate the standard error of the mean (n = 3)
Elevated CO2 did not significantly affect the N content of plants at Week 4 (P = 0.201) but increased N content by 15% by Week 6 (P <0.001; Fig. 4e, f). Increasing P supply also increased N content of plants. The 50 µM P resulted in 44% greater N content than those receiving no P.
Nutrient uptake, excess cation uptake and proton release
Elevated CO2 decreased shoot P concentration by 15% at Week 4 (P = 0.004) and decreased it by 3% across all P rates at Week 6 (P = 0.049, Table 2). The shoot P concentration increased linearly with increasing P supply from 0 to 50 µM with plants grown at 50 µM having 9 and 5 times higher shoot P concentration, compared to the 0 µM, at Weeks 4 and 6, respectively (Table 2). Root P concentration was unaffected by elevated CO2. Nodule P was only affected by P supply with nodule P concentrations being 3 and 5 times larger at 50 µM P than 0 µM P at Weeks 4 and 6, respectively. Plant P content was not affected by elevated CO2 at Week 4 (P = 0.061). By Week 6, elevated CO2 increased plant P content only at 25 and 50 µM P, leading to a significant CO2 × P interaction (P = 0.004). Plant P content was influenced by P supply with plants supplied with 50 µM P containing 9 and 14 times more P than plants without P supply at Weeks 4 and 6, respectively (Table 2).
At Week 4, elevated CO2 enhanced excess cation uptake by 4% irrespective of P supply (P = 0.002). By Week 6, elevated CO2 interacted with P supply to increase excess cation uptake with increases only occurring in plants supplied 0 (14%) and 12 µM P (18%) (P = 0.007; Table 3). Elevated CO2 also increased specific excess cation uptake by 4% at Week 4 (P = 0.020) but did not affect it at Week 6 (P = 0.332). At both Weeks 4 and 6, excess cation uptake increased with increasing P supply from 0 to 5 µM but decreased when P supply increased from 5 to 50 µM (Table 3).
Elevated CO2 enhanced proton release by 4% and 3% at Weeks 4 and 6, respectively (Table 3). Increasing P supply decreased proton release with the highest proton release at 2 µM P at both sampling times (P <0.001). Elevated CO2 increased specific proton release only in plants supplied 0 and 25 µM P at Week 4. By Week 6, specific proton release was only affected P supply (P <0.001) with 35% decrease in specific proton release when P supply increased from 0 to 50 µM.
Elevated CO2 increased the molar ratio of proton release to N2 fixation (H+/N) by 5% at Week 4 (P = 0.023) but decreased it by 11% at Week 6 (P <0.001). The highest H+/N ratio was observed at 5 µM P at Week 4, and at 0 µM P at Week 6 (Table 3). Irrespective of CO2 and P treatments, proton release and excess cation uptake were positively correlated (Fig. S1).
Critical P concentration for N2 fixation
The critical P concentration is the concentration of P in nutrient solution, nodules or shoots needed to achieve maximum N content. Elevated CO2 increased the critical P concentration in nutrient solution for N2 fixation, with plants requiring 3.7 and 6.4 µM P supply under ambient and elevated CO2, respectively, to reach maximum N content (Fig. 5a). It also increased the critical P concentration in shoots for N2 fixation by 17%. However, elevated CO2 did not affect the critical P concentration in nodules, being 4.95-5.17 mg g−1 to achieve maximum N content (Fig. 5b, Table S1).
Critical P concentrations in nutrient solution (a), nodules (b) and shoots (c) for N content of white lupin grown for 6 weeks under ambient (380 ppm) and elevated CO2 (700 ppm). Solid and dashed lines represent the line of best fit using a two-phase linear model for ambient and elevated CO2, respectively. Ca and Ce represent the critical P concentration (± standard error) for maximum nitrogen fixation. See Table S1 for regression analysis summary
Discussion
P effect on N2 fixation
Phosphorus deficiency inhibited N2 fixation as demonstrated by the positive relationship between P supply and plant N content (Fig. 4f). As no N was supplied to the plants, most of the N was derived from N2 fixation. The reduction in N2 fixation induced by P-deficiency at Week 6 was coupled to reduced host plant growth, impaired nodule function and decreased nodule size (Figs. 1b, 2f and 3b). Phosphorus deficiency may directly inhibit nodule development and function (Tang et al. 2001) or may signal N sufficiency and lead to a reduction in plant N demand (Sulieman et al. 2013). Zhu et al. (2021) proposed that P deficiency induced excessive malate accumulation in nodules which directly impaired nodule metabolism, while Sulieman et al. (2013) suggested that P deficiency decreased host-plant growth and hence plant N demand, leading to downregulation of nitrogenase activity. Given that P deficiency increased shoot and root N concentrations, this would suggest that P deficiency reduces plant demand for N leading to downregulation in nitrogenase for white lupin.
CO2 effect on N2 fixation and nodulation
In the current experiment, elevated CO2 stimulated N2 fixation. This is also supported by a wide range of CO2 enrichment studies. In a meta-analysis, Lam et al. (2012) demonstrated that elevated CO2 increased N2 fixation by 38% which is a similar magnitude to the current experiment (44%) (Fig. 4f). Elevated CO2 increased plant growth, which intensified plant demand for N. Therefore, N2 fixation was enhanced to support the increased plant growth and avoid elevated CO2-induced N deficiency. The increase in N2 fixation under elevated CO2 was supported by the greater specific nitrogenase activity but not increased nodulation (Figs. 2b and 3c). Increased nitrogenase activity without an increase in nodulation indicates that individual nodule activity was enhanced by elevated CO2, which could be achieved through increased allocation of carbohydrates towards nodules (Parvin et al. 2020; Rogers et al. 2006). Elevated CO2 was also shown to increase N2 fixation in field pea (Butterly et al. 2015) and Medicago truncatula (Qiao et al. 2021) without affecting nodulation. Therefore, elevated CO2 enhanced N2 fixation by increasing plant growth and enhancing nitrogenase activity within nodules.
Elevated CO2 but not P supply influenced early nodule formation. In the current study, elevated CO2 enhanced early nodulation between 12 and 16 days after inoculation (Fig. S2). While enhanced nodulation is widely reported in plants grown under elevated CO2, few studies report the effect of elevated CO2 on early nodule formation (Lam et al. 2012; Li et al. 2017). Root infection by rhizobia is driven by flavonoid exudation to attract the free-living rhizobia and to ultimately initiate infection of plant roots. Given that elevated CO2 increased early nodulation, elevated CO2 could have increased flavonoid exudation and aid in plant’s attraction of rhizobia, inducing root infection and early nodule formation (Haase et al. 2007; Wang et al. 2018). The lack of CO2 effect on nodule number at Weeks 4 and 6 after treatment (Fig. 2c, d) could be linked to the final number of nodules being regulated through Autoregulation of Nodulation (Ferguson et al. 2019; Mortier et al. 2012). These results show that elevated CO2 could aid in the attraction and recruitment of beneficial rhizobia during plant establishment even if the plants were grown in low-P conditions.
CO2 effect on the critical P concentration for N2 fixation
Plants grown under elevated CO2 required additional P supply to support increased N2 fixation. Elevated CO2 doubled the critical P concentration in nutrient solution for N2 fixation (Fig. 5a; Table 4). Because elevated CO2 did not enhance nodulation or alter the critical P concentration in nodules, the additional P supply was allocated towards shoot biomass production rather than producing nodules. The additional P was likely utilised to support higher photosynthetic rates and to supply nodules with additional C to enhance nitrogenase activity, and hence N2 fixation (Liu et al. 2018). In a previous study, elevated CO2 increased the critical P concentration for plant growth in cotton (Rogers et al. 1993). The increase in critical P concentration in nutrient solution for N2 fixation is likely to be larger in leguminous species which display enhanced nodulation under elevated CO2. Therefore, to maximise the benefit of rising atmospheric CO2 concentrations on N2 fixation, plants will have to acquire more P. Although it is unclear if improved P acquisition mechanisms or managing soil P through cover cropping will negate the need for additional P inputs under elevated CO2 (Hallama et al. 2019; O’Sullivan et al. 2020, 2021).
Although elevated CO2 increased the critical P concentration in nutrient solution for N2 fixation, it also partially alleviated the negative effect of P deficiency on N2 fixation. In the current experiment, P deficiency reduced N2 fixation, but elevated CO2 counteracted this effect by stimulating plant growth, nitrogenase activity and N2 fixation under P-limiting conditions (Fig. 4f). Furthermore, the critical P concentration in nodules for N2 fixation was unaffected by CO2 treatment despite enhanced N2 fixation under elevated CO2 (Table S1, Fig. 5). It appears that plants exposed to elevated CO2 could fix the same amount of N2 as that under ambient CO2 but with lower nodule P status. To achieve higher N2 fixation in P-deficient nodules, elevated CO2 could favour the use of carbohydrates in the plant and carboxylates that accumulate in nodules under P deficiency to drive nitrogenase activity (Le Roux et al. 2008; Sa and Israel 1998). Elevated CO2 partially reversed the negative effect of P deficiency on N2 fixation by enhancing nodule function via increased specific nitrogenase activity.
Elevated CO2 or P deficiency did not influence N2 fixation at 4 weeks of growth, and thus the critical P concentration could not be defined. Despite plant growth being limited by P supply, nodule P concentration was significantly higher than shoot P which enabled nodule function and N2 fixation in the low-P environment (Table 3). For example, Shulze et al. (2006) found that N2 fixation in white lupin was unaffected by P deficiency after 3 weeks of plant growth. However, elevated CO2 enhanced shoot dry weight at Week 4 across all P rates but this did not result in enhanced N2 fixation. The results indicate that whilst nodules formed during an early nodulation process could support N2 fixation under ambient CO2, they are unable to enhance N2 fixation under elevated CO2 despite increased shoot dry weight, resulting in a decrease in shoot N concentration (Haase et al. 2007; Sa and Israel 1998). This could be due to nodule dry weight and nodule size being too small, particularly when compared to nodule size at Week 6. A similar effect was observed by Sa and Israel (1998) who found that elevated CO2 did not increase N2 fixation of soybean after 4 weeks of growth. This indicates that elevated CO2 is likely to affect the critical P concentration for N2 fixation when nodules are mature and P deficiency is intensified.
Excess cation uptake and proton release
Elevated CO2 promoted excess cation uptake, resulting in enhanced proton release (Table 2, Fig. S1), which could aid in acquiring non-labile P forms when plants are grown in P-deficient soils. Legumes that rely on N2 fixation for N take up more cations than anions, which results in net proton release into the rhizosphere (Tang et al. 1999). Phosphorus deficiency also leads to rhizosphere acidification to mobilise non-labile P forms (Pueyo et al. 2021). Elevated CO2 might promote excess cation uptake due to enhanced biomass production of N2-fixing species, favouring the uptake of cations over anions (McLay et al. 1997). It also increased specific proton release at Week 4 under low P supply, which would intensify rhizosphere acidification, contributing to mobilisation of non-labile P. Specific excess cation uptake and specific proton release are closely related, indicating that excess cation uptake contributed to the enhanced acidification. However, elevated CO2 can also enhance exudation of organic acid anions under low P, which could further promote proton release (O’Sullivan et al. 2021). The increase in rhizosphere acidification under P deficiency may aid in acquiring non-labile P which could contribute to the increased P demands needed for N2 fixation under elevated CO2.
Conclusions
Elevated CO2 increased N2 fixation rates whereas P deficiency decreased it. Under elevated CO2, plants require greater P supply to support enhanced N2 fixation. To maximise the effect of elevated CO2 on enhancing N2 fixation, plants may have to acquire more P than what is currently required under ambient CO2 concentrations. Therefore, as atmospheric CO2 concentrations rise, there will be greater emphasis on managing soil P levels. Enhanced N2 fixation of P-deficient plants under elevated CO2 was achieved through promoting specific nitrogenase activity and not increasing nodule mass. Elevated CO2 also promoted excess cation uptake and proton release, which would promote rhizosphere acidification and mobilisation of non-labile P in the soil when plants are grown in P-deficient soils. Future research should examine the underlying physioloigical mechanisism of enhanced N2 fixation of P-deficient plants under elevated CO2.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol 165:351–371. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Almeida JPF, Hartwig UA, Frehner M, Nosberger J, Luscher A (2000) Evidence that P deficiency induces N feedback regulation of symbiotic N2 fixation in white clover (Trifolium repens L.). J Exp Bot 51:1289–1297. https://doi.org/10.1093/jexbot/51.348.1289
Andersson KO, Tighe MK, Guppy CN, Milham PJ, McLaren TI (2015) Incremental acidification reveals phosphorus release dynamics in alkaline vertic soils. Geoderma 259–260:35–44. https://doi.org/10.1016/j.geoderma.2015.05.001
Aranjuelo I, Cabrerizo PM, Aparicio-Tejo PM, Arrese-Igor C (2014) Unravelling the mechanisms that improve photosynthetic performance of N2-fixing pea plants exposed to elevated [CO2]. Environ Exp Bot 99:167–174. https://doi.org/10.1016/j.envexpbot.2013.10.020
Barrow NJ (2017) The effects of pH on phosphate uptake from the soil. Plant Soil 410:401–410. https://doi.org/10.1007/s11104-016-3008-9
Braum SM, Helmke PA (1995) White lupin utilizes soil phosphorus that is unavailable to soybean. Plant Soil 176:95–100. https://doi.org/10.1007/BF00017679
Butterly CR, Tang C, Armstrong R, Chen D (2015) Free-air CO2 enrichment (FACE) reduces the inhibitory effect of soil nitrate on N2 fixation of Pisum sativum. Ann Bot 117:177–185. https://doi.org/10.1093/aob/mcv140
Chen H, Markham J (2021) The interactive effect of elevated CO2 and herbivores on the nitrogen-fixing plant Alnus incana ssp. rugosa. Plants 10:440. https://doi.org/10.3390/plants10030440
Ferguson BJ, Mens C, Hastwell AH, Zhang M, Su H, Jones CH, Chu X, Gresshoff PM (2019) Legume nodulation: The host controls the party. Plant Cell Environ 42:41–51. https://doi.org/10.1111/pce.13348
Haase S, Neumann G, Kania A, Kuzyakov Y, Römheld V, Kandeler E (2007) Elevation of atmospheric CO2 and N-nutritional status modify nodulation, nodule-carbon supply, and root exudation of Phaseolus vulgaris L. Soil Biol Biochem 39:2208–2221. https://doi.org/10.1016/j.soilbio.2007.03.014
Hallama M, Pekrun C, Lambers H, Kandeler E (2019) Hidden miners – the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant Soil 434:7–45. https://doi.org/10.1007/s11104-018-3810-7
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 248:43–59. https://doi.org/10.1023/A:1022371130939
IPCC (2021) Climate Change 2021: The physical science basis. Contribution of working group I to the sixth assessment report of the Intergovernmental Panel on Climate
Jiang M, Caldararu S, Zhang H, Fleischer K, Crous KY, Yang J, De Kauwe MG, Ellsworth DS, Reich PB, Tissue DT, Zaehle S, Medlyn BE (2020) Low phosphorus supply constrains plant responses to elevated CO2: A meta-analysis. Global Change Biol 26:5856–5873. https://doi.org/10.1111/gcb.15277
Jin J, Tang C, Armstrong R, Sale P (2012) Phosphorus supply enhances the response oflegumes to elevated CO2 (FACE) in a phosphorus-deficient vertisol. Plant Soil 358:91–104. https://doi.org/10.1007/s11104-012-1270-z
Lam SK, Chen D, Norton R, Armstrong R, Mosier AR (2012) Nitrogen dynamics in grain crop and legume pasture systems under elevated atmospheric carbon dioxide concentration: A meta-analysis. Global Change Biol 18:2853–2859. https://doi.org/10.1111/j.1365-2486.2012.02758.x
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Le Roux MR, Khan S, Valentine AJ (2008) Organic acid accumulation may inhibit N2 fixation in phosphorus-stressed lupin nodules. New Phytol 177:956–964. https://doi.org/10.1111/j.1469-8137.2007.02305.x
Li Y, Yu Z, Liu X, Mathesius U, Wang G, Tang C, Wu J, Liu J, Zhang S, Jin J (2017) Elevated CO2 increases nitrogen fixation at the reproductive phase contributing to various yield responses of soybean cultivars. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.01546
Liu A, Contador CA, Fan K, Lam H-M (2018) Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.01860
Luo D, Ganesh S, Koolaard J (2020) predictmeans: Calculate predicted means for linear models. R package version 1.0.4 https://CRAN.R-project.org/package=predictmeans
McLay CDA, Barton L, Tang C (1997) Acidification potential of ten grain legume species grown in nutrient solution. Aust J Agric Res 48:1025–1032. https://doi.org/10.1071/A96174
Mortier V, Holsters M, Goormachtig S (2012) Never too many? How legumes control nodule numbers. Plant Cell Environ 35:245. https://doi.org/10.1111/j.1365-3040.2011.02406.x
Neumann G, Massonneau A, Langlade N, Dinkelaker B, Hengeler C, Römheld V, Martinoia E (2000) Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.). Ann Bot 85:909–919. https://doi.org/10.1006/anbo.2000.1135
Nguyen NT, Mohapatra PK, Fujita K (2006) Elevated CO2 alleviates the effects of low P on the growth of N2-fixing Acacia auriculiformis and Acacia mangium. Plant Soil 285:369–379
O’Sullivan JB, Jin J, Tang C (2020) Elevated CO2 promotes the acquisition of phosphorus in crop species differing in physiological phosphorus-acquiring mechanisms. Plant Soil 455:397–408. https://doi.org/10.1007/s11104-020-04698-8
O’Sullivan JB, Plozza T, Stefanelli D, Jin J, Tang C (2021) Elevated CO2 and phosphorus deficiency interactively enhance root exudation in Lupinus albus L. Plant Soil 465:229–243. https://doi.org/10.1007/s11104-021-04991-0
Qiao Y, Miao S, Jin J, Mathesius U, Tang C (2021) Differential responses of the sunn4 and rdn1-1 super-nodulation mutants of Medicago truncatula to elevated atmospheric CO2. Ann Bot 128:441–452. https://doi.org/10.1093/aob/mcab098
Palit P, Ghosh R, Tolani P, Tarafdar A, Chitikineni A, Bajaj P, Sharma M, Kudapa H, Varshney RK (2020) Molecular and physiological alterations in chickpea under elevated CO2 concentrations. Plant Cell Physiol 61:1449–1463. https://doi.org/10.1093/pcp/pcaa077
Pang J, Tibbett M, Denton MD, Lambers H, Siddique KHM, Ryan MH (2011) Soil phosphorus supply affects nodulation and N: P ratio in 11 perennial legume seedlings. Crop Pasture Sci 62:992–1001. https://doi.org/10.1071/CP11229
Parvin S, Uddin S, Tausz-Posch S, Armstrong R, Tausz M (2020) Carbon sink strength of nodules but not other organs modulates photosynthesis of faba bean (Vicia faba) grown under elevated [CO2] and different water supply. New Phytol 227:132–145. https://doi.org/10.1111/nph.16520
Pinheiro J, Bates D, DebRoy S, Sarkar D, Core Team R (2021) nlme: Linear and nonlinear mixed effects models. R package version 3.1-152 https://CRAN.R-project.org/package=nlme
Pueyo JJ, Quiñones MA, Coba de la Peña T, Fedorova EE, Lucas MM (2021) Nitrogen and phosphorus interplay in lupin root nodules and cluster roots. Front Plant Sci 12. https://doi.org/10.3389/fpls.2021.644218
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Rogers A, Gibon Y, Stitt M, Morgan PB, Bernacchi CJ, Ort DR, Long SP (2006) Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant Cell Environ 29:1651–1658. https://doi.org/10.1111/j.1365-3040.2006.01549.x
Rogers GS, Payne L, Milham P, Conroy J (1993) Nitrogen and phosphorus requirements of cotton and wheat under changing atmospheric CO2 concentrations. Plant Soil 155:231–234. https://doi.org/10.1007/bf00025026
Sa TN, Israel DW (1998) Phosphorus-deficiency effects on response of symbiotic N2 fixation and carbohydrate status in soybean to atmospheric CO2 enrichment. J Plant Nutr 21:2207–2218. https://doi.org/10.1080/01904169809365555
Sachs JL, Quides KW, Wendlandt CE (2018) Legumes versus rhizobia: a model for ongoing conflict in symbiosis. New Phytol 219:1199–1206. https://doi.org/10.1111/nph.15222
Sas L, Rengel Z, Tang C (2001) Excess cation uptake, and extrusion of protons and organic acid anions by Lupinus albus under phosphorus deficiency. Plant Sci 160:1191–1198. https://doi.org/10.1016/S0168-9452(01)00373-9
Schulze J, Temple G, Temple SJ, Beschow H, Vance CP (2006) Nitrogen fixation by white lupin under phosphorus deficiency. Ann Bot 98:731–740. https://doi.org/10.1093/aob/mcl154
Sulieman S, Ha CV, Schulze J, Tran L-SP (2013) Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J Exp Bot 64:2701–2712. https://doi.org/10.1093/jxb/ert122
Tang C, Unkovich MJ, Bowden JW (1999) Factors affecting soil acidification under legumes. III. Acid production by N2-fixing legumes as influenced by nitrate supply. New Phytol 143:513–521. https://doi.org/10.1046/j.1469-8137.1999.00475.x
Tang C, Robson AD (1993) pH above 6.0 reduces nodulation in Lupinus species. Plant Soil 152:269-276. https://doi.org/10.1007/BF00029097
Tang C, Yu Q (1999) Impact of chemical composition of legume residues and initial soil pH on pH change of a soil after residue incorporation. Plant Soil 215:29–38. https://doi.org/10.1023/A:1004704018912
Tang C, Hinsinger P, Drevon JJ, Jaillard B (2001) Phosphorus deficiency impairs early nodule functioning and enhances proton release in roots of Medicago truncatula L. Ann Bot 88:131–138. https://doi.org/10.1006/anbo.2001.1440
Tobita H, Uemura A, Kitao M, Kitaoka S, Utsugi H (2010) Interactive effects of elevated CO2, phosphorus deficiency, and soil drought on nodulation and nitrogenase activity in Alnus hirsuta and Alnus maximowiczii. Symbiosis 50:59–69. https://doi.org/10.1007/s13199-009-0037-7
Wang Q, Liu J, Zhu H (2018) Genetic and molecular mechanisms underlying symbiotic specificity in legume-rhizobium interactions. Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.00313
Wang X, Ding W, Lambers H (2019) Nodulation promotes cluster-root formation in Lupinus albus under low phosphorus conditions. Plant Soil 439:233–242. https://doi.org/10.1007/s11104-018-3638-1
Zhu S, Chen Z, Xie B, Guo Q, Chen M, Liang C, Bai Z, Wang X, Wang H, Liao H, Tian J (2021) A phosphate starvation responsive malate dehydrogenase, GmMDH12 mediates malate synthesis and nodule size in soybean (Glycine max). Environ Exp Bot 189:104560. https://doi.org/10.1016/j.envexpbot.2021.104560
Acknowledgements
We wish to thank anonymous reviewers for their constructive comments, Dr Christine Frisina from Agriculture Victoria for her assistance with the gas chromatograph and Mr Mark Richards in New South Wales Department of Primary Industry for providing the white lupin seeds. JBO was supported by an Australian Government Research Training Program Scholarship and the work was partly supported by Australian Research Council (DP210100775).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Honghua He.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 180 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Sullivan, J.B., Jin, J. & Tang, C. White lupin (Lupinus albus L.) exposed to elevated atmospheric CO2 requires additional phosphorus for N2 fixation. Plant Soil 476, 477–490 (2022). https://doi.org/10.1007/s11104-022-05311-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05311-w