Abstract
Background and aims
As drought threatens the yield and quality of maize (Zea mays L.), it is important to dissect the molecular basis of maize drought tolerance. Flavonoids, participate in the scavenging of oxygen free radicals and alleviate stress-induced oxidative damages. This study aims to dissect the function of flavonoids in the improvement of maize drought tolerance.
Methods
Using far-infrared imaging screening, we previously isolated a drought overly insensitivity (doi) mutant from an ethyl methanesulfonate (EMS)-mutagenized maize library and designated it as doi57. In this study, we performed a physiological characterization and transcriptome profiling of doi57 in comparison to corresponding wild-type B73 under drought stress.
Results
Under drought stress, doi57 seedlings displayed lower leaf-surface temperature (LST), faster water loss, and better performance in growth than B73. Transcriptome analysis reveals that key genes involved in flavonoid biosynthesis are enriched among differentially expressed genes in doi57. In line with these results, more flavonols and less hydrogen peroxide (H2O2) were accumulated in guard cells of doi57 than in those of B73 with the decrease of soil water content (SWC). Moreover, the capacity determined from doi57 seedling extracts to scavenge oxygen free radicals was more effective than that of B73 under the drought treatment. Additionally, doi57 seedlings had higher photosynthetic rates, stomatal conductance, transpiration rates, and water use efficiency than B73 exposed to drought stress, resulting in high biomass and greater root/shoot ratios in doi57 mutant plants.
Conclusion
Flavonoids may facilitate maize seedling drought tolerance by lowering drought-induced oxidative damage as well regulating stomatal movement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought is one of the most serious limiting factors of plant growth and yield. The influences of drought stress on plant growth and development are directly linked to yield (Gray and Brady 2016; Bhaskarla et al. 2020). For example, the decrease of seed/ovule ratio and the increase of maize anthesis-silking interval can occur under drought stress (Semagn et al. 2013; Maiti and Satya 2014; Harrison et al. 2014; Song et al. 2016). Excessive water loss disturbs the electron transport chain, which in turn increases the production of excess reactive oxygen species (ROS), leading to membrane lipid oxidation and damaged membrane structures (Wang et al. 2018; Li et al. 2019a; Chen et al. 2019). In addition, normal metabolic processes are also disrupted under water shortage conditions (Hussain et al. 2018; Lawas et al. 2019). When plants are subjected to drought stress, photosynthesis is severely inhibited, and the synthesis and degradation of biological macromolecules (e.g. proteins, DNA, RNA, etc.) are weakened and strengthened, respectively, which leads to the decrease of their contents in plants (Basu et al. 2016; Li et al. 2018).
Many biological strategies in plants, such as growth and developmental regulation, antioxidant defense, and stomatal movement, have evolved to cope with drought environments (Baxter et al. 2014; Daymi et al. 2016; Seeve et al. 2019; Li et al. 2020). In order to acclimate to water shortage, plants adjust their growth and development to promote water absorption and reduce water loss (Zheng et al. 2016; Kim et al. 2020). The root is the key organ of water uptake from soils. Water uptake capacity of roots can be estimated by such parameters as root biomass, root volume, primary root length, lateral root number, root depth, and root length distribution (Meng 2018). For instance, Tomar et al. (2016) reported that a drought-tolerant genotype of wheat (HW2004) had a compact root system with deeper roots compared to a drought-sensitive genotype of wheat (HD2877). As roots absorb water, shoots lose the water being the dominant organ of transpiration. Generally, under drought conditions, plants trigger several processes to generate more developed root systems and a higher root/shoot ratio. This is evidenced by a more pronounced inhibition of shoot than root production in plants under water deficit (Zheng et al. 2016; Du et al. 2020). Thus, the establishment of reasonable root/shoot ratios under different soil moisture conditions is important to maximize water-use efficiency and crop yield (Masuka et al. 2012).
Apart from the developmental aspects, antioxidant systems, including antioxidant enzymes and non-enzyme components, provide an effective ROS-removal mechanism for protecting plants against oxidative damage induced by water stress (Wang et al. 2016a, 2016b; Ahanger et al. 2017; Li et al. 2019a; Chen et al. 2019). Plants alleviate stress-induced oxidative damage by changing antioxidant enzymes activity and accumulation of non-enzyme antioxidants, such as ascorbic acid, flavonoids, etc. (Wang et al. 2018; ElSayed et al. 2019). In milk thistle (Silybum marianum [L.] Gaertn), the activities of antioxidative enzymes, such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), and peroxidase (POD), increased under drought stress, and the biosynthesis and accumulation of flavonolignans (non-enzyme antioxidants) also increased during drought stress (ElSayed et al. 2019). Flavonoids are secondary metabolites in the phenylpropane pathway and reportedly, they play important roles in plant responses to biological and non-biological stresses (Liu et al. 2011a; Akram et al. 2018; Brunetti et al. 2018). As non-enzymatic antioxidants, hydroxyl groups in the 3′ and 4′ positions of flavonoids participate in the scavenging of oxygen free radicals, and the role of flavonoids in alleviating stress-induced oxidative damage has been widely reported (Wang et al. 2016a, 2016b; Li et al. 2019a; Chen et al. 2019). Other pathways of flavonoid function in stress responses, such as drought, need to be further explored.
Stomatal movement regulation is another important aspect of plant response to drought stress. Water shortage conditions induce stomatal closure to reduce water loss, which is benefit for plant survival under drought stress (Takahashi et al. 2018). Many internal components (protein kinase, protein phosphatase, ion channel, etc.) and environmental factors (light, temperature, CO2 concentration, etc.) regulate stomatal movement (Zhang et al. 2014a; Xu et al. 2016; Zhang et al. 2018). Phytohormones (ABA (abscisic acid), ethylene, etc.) and key signal messengers (H2O2, calcium ions, etc) are well-known to be involved in guard-cell signal transduction and stomatal movement (Sah et al. 2016; Mittler and Blumwald 2015; Qi et al. 2018; Chen et al. 2020; Zhang et al. 2020). Studies have shown that almost all factors of stomatal closure can induce H2O2 accumulation, and the H2O2 level in guard cells directly affects the stomatal closure process (Mittler and Blumwald 2015; Singh et al. 2017; Qi et al. 2018; Islam et al. 2019). Wang et al. (2020) reported that TPPE, Arabidopsis trehalose-6-phosphate phosphatase E, participates in ABA-induced ROS accumulation and stomatal movement regulation, and H2O2 accumulation induced by ABA failed to occur in tppe mutant plants. Flavonols, a group of flavonoids, have recently been shown to be involved in guard cell H2O2 accumulation and stomatal movement (An et al. 2016a; Watkins et al. 2017; Brunetti et al. 2018, 2019). In addition, the phytohormone ethylene has also been reported to positively regulate flavonol accumulation in Arabidopsis and tobacco guard cells, which in turn attenuates ABA-induced H2O2 accumulation and stomatal closure (Watkins et al. 2014, 2017). Stomatal closure inhibits CO2 absorption and photosynthesis, which is not conducive to the developmental adaptability (Ikawa et al. 2018; Endo and Torii 2019). Those conflicting aspects indicate the complicated mechanism of stomatal movement regulation under drought stress.
Here, we dissect the mechanism underlying improved drought tolerance of maize mutant doi57 (drought overly insensitivity 57) (Li et al. 2017). Our results indicate that flavonoids play important roles in drought tolerance. Flavonoids prevent stomatal closure by reducing the H2O2 level in guard cells. Moreover, flavonoids alleviate drought-induced ROS damage. The doi57 mutant provides an important system in which the function of flavonoids in the regulation of drought tolerance in maize can be further elucidated.
Materials and methods
Plant materials and growth conditions
The doi57 mutant (with an abnormal leaf-temperature phenotype) screened from the EMS maize mutant library and the corresponding inbred line B73 were used for various experiments to obtain different physiological and molecular data to understand maize responses to drought stress. The M3 generation of doi57 mutant was used for subsequent experiments, including phenotypic detection, physiological and biochemical detection. These maize plants were grown in soil in a climate chamber with a photoperiod of 14−/10-h and 27 °C/32 °C night/day cycles at 400 mmol m−2 s−1 of illumination intensity and relative air humidity of 60% (Li et al. 2017). In general, those experiments were as follows. Maize at the three-leaf seedling stage was used for drought treatment by withholding water. Maize seedlings [planted into pots (15*20*10 cm)] in different soil water conditions were used for detection of photosynthetic physiological indicators. To examine the responses of B73 and doi57 exposed to drought stress, we collected samples at different time points after treatments (the levels of drought stress were indicated by the soil water content). Details are as follows: Leaf samples for RNA-seq were collected when the soil water content (SWC) was maintained at 45 ± 2% and 25–30%, which reflected normal growth and moderate drought conditions, respectively. Seedlings grown on soil with ≥45%, 35% (±2.5%) and 25% (±2.5%) water content were used for flavonol and H2O2 imaging in guard cells, photosynthetic parameter detection. Seedlings grown on soil with ≥45% and 15 ± 1% water content were used for damage degree and antioxidant capacity tests. Survival rates were recorded when the SWC decreased to 5–10%.
Measurements of SWC
The total fresh weight of soil used to grow maize seedlings in pots (15 cm × 20 cm × 10 cm) was weighed and recorded. Then the soil was dried at high temperatures (65 °C), and the weight of the dried soil was weighed and recorded. The percentage of the weight lost from the initial fresh weight of the soil was recorded as the SWC.
Infrared thermography imaging
Thermal imaging of drought-stressed seedlings was performed according to the method described by Li et al. (2017) and Dong et al. (2018) with some modifications. The leaf temperatures of maize seedlings at the three-leaf stage in different soil conditions were measured with a ThermaCAMSC1000 infrared imaging instrument (FLIR, Boston, MA, USA). Settings of the instrument were 320 × 240 pixels, long-wave infrared (8–9 mm), and temperature resolution <0.03 °C. Thermal images were saved on a memory card and temperature level was analyzed by an image-analysis program IRW in Reporter version 5.31.
Stomatal complex observation and stomatal density statistics
Epidermal strips from mature leaves of B73 and doi57 plants were peeled for stomatal phenotype determination according to the method of Wang et al. (2019). Epidermal strips were stained with toluidine blue dye (Solarbio, G3668) for 1.5 min and excess toluidine blue was removed with distilled water. The stained epidermal strips were observed with a Zeiss Axioskop II microscope and photographed. Stomatal density was expressed as number of stomata per unit area (mm2).
Water loss, leaf water content, MDA content, and electrolyte leakage
Leaf water loss detection in vitro was performed according to the method of Li et al. (2017) and Wang et al. (2019). B73 and doi57 were grown in pots for 14 d under a well-watered condition. Leaves of B73 and doi57 were cut and placed in weighing dishes for transpirational water-loss measurement. Losses in fresh weight were monitored every 20 min. Water loss was calculated as the percentage of the initial fresh weight. The mean water loss rate (five samples for each material) and the corresponding standard deviation were calculated.
Leaf water content (LWC) was measured as described by Gaxiola et al. (2001). Fresh and dry weights of whole seedlings were measured, and LWC was calculated using the formula [(fresh weight-dry weight)/fresh weight *100%].
The extent of oxidative damage was estimated by the measurement of MDA (malondialdehyde) and ion leakage as described by Quan et al. (2004) and Shou et al. (2004), respectively. Samples (0.1 g) were ground and extracted with 10% (v/v) trichloroacetic acid (10 mL). The mixtures were centrifuged at 4000 rpm for 10 min, and equivalent volumes of the supernatant and 0.67% thiobarbituric acid were mixed and blended in a boiling water bath reaction for 15 min. The solution was centrifuged after rapid cooling, and the absorbance values of reaction liquids were measured at 532, 600, and 450 nm. The content of MDA was calculated using the formula [6.45*(A532-A600)-0.56*A450]. The electrical conductivities of deionized water, deionized water with plant tissue (1 g soaked for 2 h), and deionized water with plant tissue (after boiled and cooled) were measured using an electrical conductivity meter (DDSJ-318, CN) and recorded as E0, E1 and E2, respectively. Electrolyte leakage rate was calculated using the formula [(E1-E0)/ (E2-E0) *100%].
RNA-seq and data analysis
Total RNA from selected samples was isolated with TRIZOL reagent (Invitrogen, USA). One microgram of total RNA from each sample was used as input material for building sequencing libraries. The first and second strands of cDNA were synthesized with M-MuLV Reverse Transcriptase (RNase H-) and DNA Polymerase I with RNase. Remaining overhangs were converted into blunt ends by treatment with exonuclease/polymerase. After adenylation of the 3′ ends of the DNA fragments, NEBNext Adaptors with hairpin loop structures were ligated to prepare for hybridization. After choosing 250 ~ 300 bp cDNA fragments, the library fragments were purified using the AMPure XP system (Beckman Coulter, Beverly, USA). Three microliters of USER Enzyme (NEB, USA) was then used with size-selected, adaptor-ligated cDNA at 37 °C for 15 min and denatured for 5 min at 95 °C before PCR. Reactions of PCR were performed using Phusion High-Fidelity DNA polymerase, Universal PCR primers and Index (X) Primer. Products of PCR were purified (AMPure XP system) and library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using the TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Hiseq platform and 150 bp paired-end reads were generated.
All the high-quality clean reads were mapped to the reference genome Zea mays AGPv4 (Jiao et al. 2017) using Tophat 2.1.1 with default settings (Kim et al. 2013). To measure gene expression level, FPKM (Fragments Per Kilobase of exon model per Million mapped fragments) of each gene was calculated (Anders and Huber 2010). Cufflinks were applied to detect differentially expressed genes between each chosen sample pairs. Significantly differentially expressed transcripts were identified using an adjusted cutoff q-value < 0.05 and fold change ≥2. Genes were annotated according to the maize genome. Gene Ontology (GO) analyses were performed to identify the enrichment of DEGs in GO terms and were enriched using the ClusterProfile package (Yu et al. 2012). A corrected p value (q-value) ≤ 0.05 was chosen as the threshold for significantly enriched GO terms.
Determination of gene expression levels in specific functional pathways was carried out by real-time quantitative PCR amplification with specific primers (Table S3). Relative expression of genes involved in flavonol biosynthesis was calculated based on the method described by Li et al. (2019a) and Bai et al. (2014).
Determination of oxygen radical scavenging ability
The oxygen radical scavenging ability of plants measured from extracts was evaluated as described by Li et al. (2019a). Samples (0.1 g) of B73 and doi57 were fully ground and extracted with 600 μL 1% (v/v) HCl-methanol for 4 h. The extraction mixtures were thoroughly mixed with 600 μL chloroform and 300 μL distilled water. Then the subsequent mixtures were centrifuged at 12,000 rpm for 10 min (4 °C). The supernatants were used as sample extracts for subsequent detection. Equivalent volumes of sample extracts, ferrous sulphate solution (9 mM), salicylic acid-ethanol solution (9 mM), and H2O2 solution (8.8 mM, 400 μL) were mixed and incubated for half an hour at 37 °C. The absorbance of the reaction solution at 512 nm was measured to calculate the scavenging ability of the hydroxyl radical. The same volume of sample extract and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) solution (0.2 mM) were mixed and incubated for half an hour at room temperature. The absorbance of the reaction solution at 517 nm was measured to calculate the inhibition ratio of the DPPH radical.
Measurement of total flavonoids and visualization of flavonol accumulation in guard cells by DPBA staining
Total flavonoid content was measured according to the method described by Jia et al. (1999). Samples were ground and extracted with HCl-methanol (1%, v/v). The extracts were mixed with chloroform (equivalent volume as the supernatants), and the subsequent supernatant was collected by centrifugation (12,000 rpm). The absorption values of the upper aqueous phase were measured at 340 nm, 530 nm, and 657 nm for spectrophotometric quantification.
The visualization of flavonol accumulation in guard cells was performed by DPBA (diphenylboric acid 2-aminoethyl ester, Sigma-Aldrich; D9754) staining according to the method of Watkins et al. (2017). The epidermis of individual leaves were submerged in DPBA dyeing liquid (DPBA, 2.52 mg mL−1; KCl, 50 mM; MES, 10 mM; CaCl2, 0.1 mM; Triton X-100, 0.02%; pH, 6.2) for 3 h. The epidermis were then washed in deionized water and mounted between two coverslips. A Zeiss 710 LSCM (Germany) apparatus was used to excite the epidermis with 20% maximum laser power at 488 nm. The fluorescence emission of DPBA was collected between 475 and 619 nm. All micrographs within each panel were acquired using the same detectors (including offset, gain, and pinhole settings). The relative fluorescence intensity values were determined by the ratio of DPBA fluorescence intensity and chlorophyll fluorescence intensity.
H2DCF-DA imaging and quantification
The H2O2 in guard cells was imaged using H2DCF-DA (3,6-bis (acetyloxy)-2,7-dichloro-9H- xanthen- 9 -yl, Sigma-Aldrich, USA) as described by An et al. (2016a). Epidermal strips of maize seedling growth in different water conditions were placed in the H2DCF-DA dye solution (10 mM Tris and 50 mM KCl, pH 6.5, 50 μM H2DCF-DA) in the dark at 25 °C for 30 min. After staining, excess H2DCF-DA was removed with Tris-KCl buffer under dark conditions. A laser scanning confocal microscope (settings: ex = 488 nm, em = 525 nm) was used for scanning H2DCF-DA fluorescence, and chlorophyll fluorescence was collected in the range of 650 to 700 nm. The relative fluorescence intensity of H2DCF-DA in guard cells was determined by the ratio of H2DCF-DA and chlorophyll fluorescence.
Physiological index measurements during drought treatment
Photosynthetic rate, stomatal conductance, and transpiration rates were measured on fully expanded leaves of three-leaf stage seedlings using a portable photosynthetic measurement system (GFS-3000, HeinzWalz). Water use efficiency was calculated by the ratio of photosynthetic rate to transpiration rate. The statistical analysis was based on data collected from 10 seedlings under each growth condition with three technical replicates.
Determination of stomatal aperture
Stomatal aperture was determined as described previously by Yao et al. (2013). Fully expanded leaves of 14-day-old seedlings (three-leaf seedling stage) grown under different SWCs were harvested and cut into pieces (4 mm × 4 mm), and immediately transferred into fixing solution (containing 2.5% glutaraldehyde and 2% paraformaldehyde) for 8 h. Then after rinsing with a phosphate buffer (0.1 M K2HPO4/KH2PO4, PH 7.0), samples were dehydrated with ascending ethanol series, dried with an Critical Point Dryer (HCP-2, Hitachi, Hitachi Koki Co, Japan), and the whole stomatal structure was observed and photographed with a scanning electronic microscope (FEI Quanta 250, FEI., Ltd., USA).
Statistical analysis
The physiological phenotype was confirmed by more than three repetitions. All statistical analysis was performed using Statistix8.1 software, including water loss rate, total flavonoids content, photosynthetic parameters. Relative fluorescence intensity (DPBA and H2DCF) of guard cells was determined by at least three replicates with at least 25 guard cells observer in each sample. Statistically significant differences were based on P ≤ 0.05 and P ≤ 0.01.
Results
doi57 mutant exhibits lower LST and faster water loss
To study the mechanism of drought-stress response associated with the regulation of stomatal movement in maize, we used infrared thermography to screen a pool of EMS-mutagenized maize for the mutants with alterations in leaf temperature (Gao et al. 2014; Li et al. 2017). Due to the more obvious difference in LST and drought response, doi57 (drought overly insensitive 57) was selected for further study. Drought-stressed doi57 seedlings displayed a phenotype with lower LST than the LST of the wild type (Fig. 1a, b). However, no visible morphological differences were observed between mutant and wild type individuals. As LST is tightly linked with water loss due to transpiration, we compared the rate of water loss in detached leaves (of 15-day-old seedlings). As illustrated in Fig. 1c, the water loss rate was significantly higher in doi57 than that in B73. Plant water loss occurs mainly through stomata; however, we did not find any difference in stomatal morphology, distribution and density between B73 and doi57 (Fig. S1).
Comparison of LST and water loss in vitro between B73 and doi57. a Infrared images of B73 and doi57 seedlings after drought treatment for 2 days when SWC reached 30–35%; b Statistical analysis of LSTs of B73 and doi57 seedlings were calculated based on infrared images, average leaf temperature was calculated from five seedlings; c Water loss from B73 and doi57 seedlings. Rate of water loss is expressed as a percentage of the initial fresh weights of leaves (FW), relative water loss was represent the mean ± SD of five independent samples. Error bars are standard deviations (SDs), statistically significant differences between B73 and doi57 are indicated by asterisks (Student’s t test, **P < 0.01, degree of freedom (df) = 8). Scale bar = 1 cm
doi57 seedlings show increased drought tolerance
Under drought conditions, the LST may be related to either transpiration rate, water retention capacity and/or plant adaptation capacity against drought stress (Li et al. 2017). To examine plant physiological responses to drought, water was withheld for 8 d from 15-day-old seedlings. B73 showed severe wilting while doi57 produced normal-looking turgid leaves at a SWC of 15 ± 2.5% (Fig. 2a). The LWC of doi57 seedlings was higher than that of B73 under drought stress (SWC, 15 ± 2.5%), although no significant difference was observed under the well-watered condition (SWC ≥45%) (Fig. 2b). After 10 d of withholding water (SWC, 5–10%), the survival rate of doi57 was 74% (±2%), which was significantly higher than that of B73, which was about 30% (Fig. 2c). These data demonstrated that doi57 seedlings were more drought-tolerant than B73 seedlings.
The doi57 mutants are more tolerant to drought than B73. a Morphological comparison of B73 and doi57 seedlings under normal and drought (SWC, 15 ± 2.5%) conditions; scale bar = 1 cm. b Comparison of leaf LWC. LWCs were measured after drought treatment for 8 d (SWC, 15 ± 2.5%), WW: Well-watered, DT: Drought treatment; c Comparison of survival rate. Survival rates were calculated after drought treatment for 10 d (SWC, 5–10%). Error bars represent SDs, the relative water content of 5 samples was determined, and then the mean value and SD were obtained; survival rate were calculated from five independent experiments (more than 150 seedlings for each survival rate statistics), and statistically significant differences between B73 and doi57 are indicated by asterisks (Student’s t test, **P < 0.01, df = 8)
doi57 mutant suffers less oxidative damage than B73 under drought stress
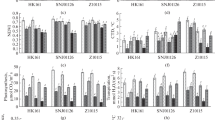
Drought stress results in ROS bursts causing membrane oxidation and altering membrane permeability (Zhang et al. 2014b; Li et al. 2019a). To dissect the mechanism underlying drought tolerance of doi57, we analyzed oxygen free radical (·OH and DPPH) scavenging capacities of maize using their seedling extracts. The oxygen free radical scavenging rate of doi57 seedling extracts was slightly higher than that of B73 under the normal condition. Although the scavenging rates decreased in both B73 and doi57, and the degree was more significant in B73 than in doi57 after drought treatment (Fig. 3a, b). These results suggest that redox balance is differentially maintained in B73 and doi57 under both normal and drought conditions. Respective amounts of membrane oxidation product, MDA and electrolyte leakage were similar between B73 and doi57 seedlings under the normal condition but were distinctly lower in doi57 than in B73 under drought stress (Fig. 3c, d).
Comparisons of scavenging capacities of seedling extracts for oxygen free radicals and oxidation products between B73 and doi57 with or without drought treatment (SWC, 15 ± 1%) for 8 days. a-b Oxygen free radical (·OH and DPPH) scavenging capacity of seedling extracts. Rates of scavenging for ·OH and DPPH were measured from total extracts of 14-day-old seedlings; c Comparison of membrane oxidation products (MDA) and d comparison of electrolyte leakage in leaf extracts of B73 and doi57 after drought treatment. WW: Well-watered. DT: Drought treatment. Data of each experiment represent the mean ± SD of three or more biological replicates. Statistically significant differences between B73 and doi57 are indicated by asterisks. (Student’s t test, **P < 0.01, df = 8)
Gene expression profiling of B73 and doi57 seedlings under drought stress
To further investigate the possible mechanism of altered drought tolerance in the doi57 mutant, we performed RNA-seq analysis to obtain gene expression profiles of B73 and doi57 seedlings under two conditions 45 ± 2% and 25–30% SWCs, which represent normal growth and mild drought conditions, respectively. Differentially expressed genes between B73 and doi57 before and after drought treatment are listed in Table S1 and were used for further analysis.
We performed a GO enrichment analysis to investigate functional categories of DEGs. We classified the DEGs into different functional GO categories, including oxidation reduction and response to stress. Among these categories, the ones associated with redox, water stress, and plant phenylpropane secondary metabolic pathways were further analyzed.
In the well-watered treatment group, up-regulated genes in doi57 compared to those in B73 showed that genes in doi57 covered several categories (Fig. S2), and the category associated with redox was particularly prominent, which supports that intracellular redox balance differs between the two kinds of seedlings. Gene ontology enrichment of down-regulated genes in doi57 were mainly distributed in three biological processes, response to stimulus, response to stress, and response to chemical stimulus (Table S2), indicating that doi57 may be insensitive to stimulation and stress. The functional GO categories between B73 and doi57 under drought stress were similar to those of the normal condition. The up-regulated genes in drought-treated doi57 were mainly distributed in categories related to redox balance (Fig. S3). The GO categories of down-regulated genes in drought-treated doi57 were distributed among 15 GO terms, including response to stress, oxidoreductase activity, and defense response (Fig. S4).
The analysis of DEGs obtained from transcriptome data revealed that the genes involved in the flavonol synthesis pathway (a branch of the phenylpropane biosynthesis pathway) were up-regulated by water shortage. The biosynthetic pathway of flavonols is controlled by key enzymes, including chalcone synthase (CHS), chalcone isomerase (CHI), and flavonol synthase (FLS), and is shown in Fig. 4a (Lepiniec et al. 2006; Stracke et al. 2007). The expression of three ZmCHSs (ZmCHS01 [Zm00001d052673], ZmCHS05 [Zm00001d040479] and ZmCHS10 [Zm00001d052916]), two ZmCHIs (ZmCHI [Zm00001d044683] and ZmCHI1 [Zm00001d034635]), and one ZmFLS (ZmFLS1 [Zm00001d018184]) in doi57 were significantly higher than those in B73 under the normal and drought conditions (Fig. 4b). These expression levels were confirmed by qRT-PCR (Fig. 4c). Overall, results suggest that the biosynthesis of flavonols were promoted by drought stress, especially in doi57.
Expression profiles of genes involved in flavonol biosynthesis. a Simplified schematic diagram of flavonol biosynthesis. b A heat map showing expression levels of the key genes involved in flavonol biosynthesis in B73 and doi57 before and after drought stress. Heat maps show relative transcript levels, colors reflect the values of z-score normalization. Labels above the heatmap indicate wild-type and mutants growing in normal (B73 and doi57, SWC ≥ W45%) and moderate moisture conditions (DB73 and Ddoi57, 25–30%). c Expression analysis by qPCR of the key genes involved in flavonol synthesis in B73 and doi57 before and after drought stress. The relative transcript levels were determined by the 2-ΔCт method, Relative expression value represents the mean ± SD of four biological replicates. (Student’s t test, **P < 0.01, df = 6)
doi57 accumulated higher total flavonoids under drought stress
Previous studies have shown that the biosynthesis of flavonoids (including flavonols) is induced by biotic and abiotic stress, such as drought (Winkel-Shirley 2002; Nakabayashi et al. 2014a). Transcriptome data suggest that the expression of some key enzyme genes involved in flavonol synthesis were induced by drought treatment. In order to determine the effect of differential expression of those key enzyme genes on total flavonoid contents, we measured total flavonoid contents in B73 and doi57 seedlings under different drought stress conditions. As shown in Fig. 5, the accumulation of total flavonoids in leaf tissue was induced by drought stress; the degree of induction was relatively higher in doi57 than in B73, which was consistent with the differential expression of key enzyme genes involved in flavonoid biosynthesis obtained from transcriptome data.
Comparison of flavonoid contents between B73 and doi57 under different soil water conditions. The average flavonoid content and standard deviations were calculated from five independent experiments. Statistically significant differences between B73 and doi57 are indicated by asterisks. (Student’s t test, *P < 0.05, **P < 0.01, df = 8)
Guard cells of doi57 maintained higher flavonol and lower H2O2 levels under different SWCs
Flavonols, a class of flavonoids, are widely distributed in various tissues of plants and play important roles in plant responses to biotic and abiotic stress (Winkel-Shirley 2002; Gill and Tuteja 2010; Nakabayashi et al. 2014a, 2014b; Nguyen et al. 2016). Flavonols can be detected in single cells, such as guard cells in Arabidopsis thaliana and Solanum lycopersicum (An et al. 2016a, 2016b; Watkins et al. 2017), using different approaches. To examine whether flavonol accumulation is altered in guard cells of doi57, we used the specific fluorescent dye DPBA to determine flavonol level. As shown in Fig. 6, flavonol accumulation was detected in guard cells, and increased in both doi57 and B73 with the decrease of SWC. However, a slightly higher level of flavonols was observed in doi57 guard cells than in B73 under the normal (SWC ≥45%) (Fig. 6a), and the difference widened with the decrease of SWC (Fig. 6a, b). The average DPBA fluorescence intensity was 1.6-fold higher in doi57 than that in B73 under the 25% (±2.5%) SWC condition (Fig. 6b).
Flavonol and H2O2 imaging in guard cells under different SWCs. a Flavonol accumulation in guard cells of B73 and doi57 seedlings under three SWC conditions; b Comparison of relative DPBA fluorescence intensities under three SWC conditions; c Localization of H2O2 in guard cells in B73 and doi57 seedlings under three SWC conditions. d Comparison of H2DCF-DA fluorescence intensities between B73 and doi57 under three SWC conditions. Relative fluorescence intensity value and SDs were calculated from more than 25 guard cells. Statistically significant differences between B73 and doi57 are indicated by asterisks. (Student’s t test, *P < 0.05, **P < 0.01, df = 24). Scale bar = 5 μm
As a non-enzymatic antioxidant, flavonols may modulate ROS homeostasis in guard cells (Nguyen et al. 2016; Li et al. 2018). To compare the differences in ROS levels caused by flavonol accumulation in B73 and doi57 guard cells, epidermal peels of the seedlings grown under various soil water conditions were stained with H2DCF-DA to detect H2O2 accumulation in guard cells. As shown in Fig. 6c, the accumulation level of H2O2 in guard cells was relatively lower, and no significant differences were found between B73 and doi57 in the well-watered condition (SWC ≥45%). Confocal micrographs showed that relative H2DCF fluorescence intensity increased with the decrease of SWC, but the degree of fluorescence in doi57 guard cells was weaker than that in B73 (Fig. 6c, d).
Effects of different SWCs on stomatal aperture
As a key signal molecule during stomatal closure, H2O2 levels in guard cells affect stomatal movement. Stomatal aperture of B73 and doi57 was detected during the drought treatment. Those results showed stomatal aperture reduced with the decrease of SWC, and the degree of reduction in doi57 was relatively weaker than that in B73 (Fig. 7). Stomatal aperture data are likely related to the data on flavonol accumulation and H2O2 level in guard cells during drought stress, where flavonol accumulation may have partially inhibited H2O2 levels in guard cells and prevented stomatal closure and/or promoted stomatal opening.
doi57 performed better in photosynthetic parameters under drought stress
To further investigate whether flavonols and H2O2 in guard cells affected stomatal movement, we also compared stomatal conductance, transpiration rates, and photosynthesis rates between B73 and doi57 seedlings during drought stress. No significant differences were observed in leaf photosynthetic rates, stomatal conductance, and transpiration rates between B73 and doi57 under the well-watered condition. With the increase of drought stress, the physiological parameters mentioned above declined gradually, but the photosynthesis rate, stomatal conductance, and transpiration rate of doi57 were significantly higher than those of B73 under 35 (±2.5)% and 25 (±2.5)% (Fig. 8a-c). A similar pattern in water use efficiency was also observed (Fig. 8d). These results indicate that doi57 can use the limited amount of water more effectively under drought stress.
Comparison of photosynthetic performance between B73 and doi57 seedlings during drought treatment. a Photosynthetic rate; b Stomatal conductance; c Transpiration rate; d Water-use efficiency. Date of each parameter represent the mean ± SD (n = 10). Statistically significant differences between B73 and doi57 are indicated by asterisks. (Student’s t test, *P < 0.05, **P < 0.01, df = 18)
Water shortage seriously affects the growth and development of plants. Ten-day-old B73 and doi57 seedlings were subjected to drought treatment for 8 days (SWC ≤ 15%). No significant differences were found in shoot dry weights, root dry weights and root/shoot ratios between B73 and doi57 under the normal soil moisture level. Shoot and root dry weights were all significantly lower in both B73 and doi57 after drought stress, but the decrease in doi57 was relatively small. The root/shoot ratio was greater after drought treatment, especially in doi57 (Table 1). These results show that doi57 better tolerated the drought stress, which was also reflected by less-affected LST, stomatal conductance, and photosynthetic rate observed in doi57 during drought stress.
Discussion
Drought stress seriously threatens the growth, development and production of crops. Therefore, understanding the mechanisms by which plants respond to drought stress would definitely be beneficial for improving the yield and quality of crops. In this study, we endeavored to dissect the mechanism underlying drought tolerance in doi57, a mutant that exhibited a low leaf temperature and rapid water loss (Fig. 1) and was isolated by far-infrared image screening. Far infrared imaging systems has been widely used in the model plant A. thaliana to isolate mutants with altered LST (Merlot et al. 2002; Mustilli et al. 2002; Belin et al. 2006; Song et al. 2016). Prior to this study, the same imaging system has also been successfully implemented in maize (Liu et al. 2011b; Gao et al. 2014; Li et al. 2017). As expected, the doi57 mutant with abnormal leaf temperature showed different levels of drought resistance compared with the corresponding control, and the differences were consistent with previous studies on mutants with abnormal leaf temperatures (Gao et al. 2014; Li et al. 2017).
Stomatal transpiration regulates LST, and LST can reflect plant water retention ability, especially under drought conditions (Gao et al. 2014; Li et al. 2017). In general, lower leaf temperature is indicative of the plant being sensitive to drought. Miao et al. (2006) reported that deficiency of ATGPX3 (an Arabidopsis glutathione peroxidase) caused lower LST under drought conditions and enhanced sensitivity to drought stress. Transgenic Arabidopsis with overexpression of GhCAS (a calcium-sensing receptor-encoding gene) showed slower leaf water loss in vitro and higher LST (Li et al. 2019b). The lower LST of doi57 seedlings corresponded to the faster leaf water loss rate in vitro (Fig. 1c), which indicated weaker water retention and drought tolerance. Surprisingly, doi57 mutants showed more tolerance to drought stress (Fig. 2a); LWC and survival rate were significantly higher than those of the corresponding control (Fig. 2b, c). Plant responses to drought are very complex processes because they involve water absorption, transportation and dispersal. We assume that the stronger drought tolerance in doi57 than in B73 may be attributed to doi57’s greater root/shoot ratio (Table 1) and higher water use efficiency (Fig. 8d) under drought stress.
Stomata distributed on plant leaf surfaces are main channels for gas (H2O, CO2, etc.) exchange between plants and the environment. Water in plants is lost mainly through stomata, and LST is closely related to water loss and stomatal movement (Kanno et al. 2012). Lower leaf temperature and faster water loss are often associated with higher stomatal conductance under given conditions, which are also reflected by relatively larger stomatal apertures and better photosynthetic performance during drought stress. With the decrease of SWC, stomatal aperture, stomatal conductance and transpiration rate decreased, but the degrees of decrease in doi57 was less than that in B73 (Figs. 7 and 8). On the other hand, larger stomatal aperture and conductance are beneficial to CO2 acquisition and photosynthesis during drought stress. As expected, photosynthetic rate and water use efficiency of doi57 seedlings were significantly higher than those of B73 with the increase in soil water shortage (Fig. 8a, d); higher photosynthetic rate and water use efficiency are beneficial to plant growth and development. Moreover, the high biomass accumulation and root/shoot ratio indicate that doi57 could utilize the limited water supply efficiently and effectively tolerate the water shortage conditions, which may be an important factor contributing to its stronger drought tolerance.
Flavonoids, as important secondary metabolites, play important roles in plant growth and stress response. Agati and Tattini (2010) reported that flavonoids perform multiple functions, for example, they function as antioxidants and developmental regulators in photoprotection. Talbi et al. (2020) reported that drought stress enhanced flavonoid accumulation and antioxidant capacity significantly in the saharan plant Oudeneya africana and thus improved plant adaptability to abiotic stress. Gao et al. (2020) also showed that drought stress promoted the accumulation of secondary metabolites such as flavonoids in two Adonis species, which in turn reduced H2O2 content and promoted drought resistance. Recently, a P450 gene, CsCYT75B1 from Citrus CYTOCHROME, was positively linked to flavonoids and drought resistance (Rao et al. 2020). In this study, our transcriptome analysis revealed that the expression of some key genes involved in flavonoid biosynthesis showed significant differences between B73 and doi57 before and after drought treatment (Fig. 4), suggesting that flavonoids may contribute to the stronger drought-tolerance of doi57. Consistent with the gene expression analysis, total flavonoid content in B73 and doi57 increased with the decrease of SWC (Fig. 5). Greater accumulation of flavonoids, as they are non-enzyme antioxidants, is beneficial to alleviate stress-induced oxidative damage (Li et al. 2019a; Talbi et al. 2020). The doi57 had higher scavenging capacity for oxygen free radicals (·OH and DPPH) than did B73, especially under drought conditions (Fig. 3a, b). Furthermore, lower accumulation of MDA and less membrane structure damage were detected in doi57 (Fig. 3c, d).
Flavonols have been reported to partially inhibit ABA-induced ROS (H2O2) accumulation in guard cells and ABA-mediated stomatal closure (An et al. 2016a, 2016b; Watkins et al. 2014, 2017). Our results show that flavonol production was highly induced by drought stress in guard cells, and the degree of induction was higher in doi57 than in B73 (Fig. 6a, b), which is also consistent with the total flavonoid contents during drought stress (Fig. 5). Due to high levels of flavonols in guard cells, H2O2 levels were relatively lower in the guard cells of doi57 (Fig. 6c, d), which is not conducive to stomatal closure and resulted in greater stomatal aperture/conductance (Fig. 7 and Fig. 8b), better photosynthetic performance, and more biomass accumulation due to drought stress (Fig. 8 and Table 1). Previous reports have shown that expression of key enzyme genes involved in flavonol biosynthesis are regulated by R2R3-MYB transcription factors (Stracke et al. 2007). Overexpression of MYB12 or MYB111 in Arabidopsis promotes flavonol biosynthesis and plant resistance to abiotic stresses, such as drought, salt, etc. (Wang et al. 2016a; Li et al. 2019a). However, no differences in gene expression levels of R2R3-MYB transcription factors were found between B73 and doi57, implying that different regulatory pathways may exist across species.
In addition, the samplings were based on the soil water content, which might be confused for the correlation among the morphology, physiological parameter and molecular events. However, because environment condition is one of the most important factor influencing drought state, soil water contents were regards as a indictor for drought levels suffered by plants, which were used in several studies (Yao et al. 2013; Scharwies and Dinneny 2019).
Taken together, our results demonstrate that the greater drought tolerance of doi57 is attributed to higher flavonoid levels. The increased flavonol levels in guard cells weakened H2O2 accumulation and stomatal closure, and increased antioxidant capacity and adaptive growth under drought stress. This might provide a new avenue for improving drought tolerance by manipulating flavonoid/flavonol levels in maize.
References
Agati G, Tattini M (2010) Multiple functional roles of flavonoids in photoprotection. New Phytol 186(4):786–793
Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal RM (2017) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants 23(4):731–744
Akram NA, Iqbal M, Muhammad A, Ashraf M, Al-Qurainy F, Shafiq S (2018) Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 255:163–174
An Y, Liu L, Chen L, Wang L (2016a) ALA inhibits ABA-induced stomatal closure via reducing H2O2 and Ca2+ levels in guard cells. Front Plant Sci 7:482
An Y, Feng X, Liu L, Xiong L, Wang L (2016b) ALA-induced flavonol accumulation in guard cells is involved in scavenging H2O2 and inhibiting stomatal closure in Arabidopsis cotyledons. Front Plant Sci 7:1713
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11:R106
Bai L, Ma X, Zhang G, Song S, Zhou Y, Gao L, Miao Y, Song CP (2014) A receptor-like kinase mediates ammonium homeostasis and is important for the polar growth of root hairs in Arabidopsis. Plant Cell 26(4):1497–1511
Basu S, Ramegowda V, Kumar A, Pereira A (2016) Plant adaptation to drought stress. F1000 Res 5:1554
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240
Belin C, de Franco PO, Bourbousse C, Chaignepain S, Schmitter JM, Vavasseur A, Giraudat J, Barbier-Brygoo H, Thomine S (2006) Identification of features regulating OST1 kinase activity and OST1 function in guard cells. Plant Physiol 141(4):1316–1327
Bhaskarla V, Zinta G, Ford R, Jain M, Varshney RK, Mantri N (2020) Comparative root transcriptomics provide insights into drought adaptation strategies in chickpea (Cicer arietinum L.). Int J Mol Sci 21(5):1781
Brunetti C, Fini A, Sebastiani F, Gori A, Tattini M (2018) Modulation of phytohormone signaling: a primary function of flavonoids in plant-environment interactions. Front Plant Sci 9:1042
Brunetti C, Sebastiani F, Tattini M (2019) Review: ABA, flavonols, and the evolvability of land plants. Plant Sci 280:448–454
Chen S, Wu F, Li Y, Qian Y, Pan X, Li F, Wang Y, Wu Z, Fu C, Lin H, Yang A (2019) NtMYB4 and NtCHS1 are critical factors in the regulation of flavonoid biosynthesis and are involved in salinity responsiveness. Front Plant Sci 10:178
Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y (2020) Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol 62:25–54
Daymi C, Guzmán-Cedeño Á, Moreno A (2016) Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol Biochem 103:10–23
Dong H, Bai L, Zhang Y, Zhang G, Mao Y, Min L, Xiang F, Qian D, Zhu X, Song CP (2018) Modulation of guard cell turgor and drought tolerance by a peroxisomal acetate-malate shunt. Mol Plant 11(10):1278–1291
Du Y, Zhao Q, Chen L, Yao X, Zhang W, Zhang B, Xie F (2020) Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol Biochem 146:1–12
ElSayed AI, El-Hamahmy MAM, Rafudeen MS, Mohamed AH, Omar AA (2019) The impact of drought stress on antioxidant responses and accumulation of flavonolignans in Milk Thistle (Silybum marianum (L.) Gaertn). Plants (Basel) 8(12):611
Endo H, Torii KU (2019) Stomatal development and perspectives toward agricultural improvement. Cold Spring Harb Perspect Biol 11(5):a034660
Gao ZY, Liu H, Wang HL, Li N, Wang DJ, Song YW, Miao YC, Song CP (2014) Generation of the genetic mutant population for the screening and characterization of the mutants in response to drought in maize. Chin Sci Bull 59:766–775
Gao SS, Wang YL, Yua S, Huang YQ, Liu H, Chen W, He XY (2020) Effects of drought stress on growth, physiology and secondary metabolites of two Adonis species in Northeast China. Sci Hortic 259:108795
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR (2001) Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci U S A 98(20):11444–11449
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gray SB, Brady SM (2016) Plant developmental responses to climate change. Dev Biol 419(1):64–77
Harrison MT, Tardieu F, Dong Z, Messina CD, Hammer GL (2014) Characterizing drought stress and trait influence on maize yield under current and future conditions. Glob Chang Biol 20:867–878
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L (2018) Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front Plant Sci 9:393
Ikawa H, Chen CP, Sikma M, Yoshimoto M, Sakai H, Tokida T, Usui Y, Nakamura H, Ono K, Maruyama A, Watanabe T, Kuwagata T, Hasegawa T (2018) Increasing canopy photosynthesis in rice can be achieved without a large increase in water use-a model based on free-air CO2 enrichment. Glob Chang Biol 24(3):1321–1341
Islam MM, Ye W, Matsushima D, Rhaman MS, Munemasa S, Okuma E, Nakamura Y, Biswas MS, Mano J, Murata Y (2019) Reactive carbonyl species function as signal mediators downstream of H2O2 production and regulate [Ca2+] cyt elevation in ABA signal pathway in Arabidopsis guard cells. Plant Cell Physiol 60(5):1146–1159
Jia Z, Tang M, Wu J (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Jiao Y, Peluso P, Shi J, Liang T, Stitzer MC, Wang B, Campbell MS, Stein JC, Wei X, Chin CS, Guill K, Regulski M, Kumari S, Olson A, Gent J, Schneider KL, Wolfgruber TK, May MR, Springer NM, Antoniou E, McCombie WR, Presting GG, McMullen M, Ross-Ibarra J, Dawe RK, Hastie A, Rank DR, Ware D (2017) Improved maize reference genome with single-molecule technologies. Nature 546:524–527
Kanno Y, Hanada A, Chiba Y, Ichikawa T, Nakazawa M, Matsui M, Koshiba T, Kamiya Y, Seo M (2012) Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci U S A 109(24):9653–9658
Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14(4):R36
Kim Y, Chung YS, Lee E, Tripathi P, Heo S, Kim KH (2020) Root response to drought stress in rice (Oryza sativa L.). Int J Mol Sci 21(4):1513
Lawas LMF, Li X, Erban A, Kopka J, Jagadish SVK, Zuther E, Hincha DK (2019) Metabolic responses of rice cultivars with different tolerance to combined drought and heat stress under field conditions. Gigascience 8(5):giz050
Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57:405–430
Li B, Fan R, Huang S, Lei P, Guo JX, Zhan QD, Zhao X, Song CP (2017) Far infrared imaging, an effective way to screen maize seedling mutants for drought stress response. Biologia 72:1010–1016
Li L, Gu W, Li J, Li C, Xie T, Qu D, Meng Y, Li C, Wei S (2018) Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol Biochem 129:35–55
Li BZ, Fan RN, Guo SY, Wang PT, Zhu XH, Fan YT, Chen YX, He KY, Kumar A, Shi JP, Wang Y, Li LH, Hu ZB, Song CP (2019a) The Arabidopsis MYB transcription factor, MYB111 modulates salt responses by regulating flavonoid biosynthesis. Environ Exp Bot 166:103807
Li S, Chen H, Hou Z, Li Y, Yang C, Wang D, Song CP (2019b) Screening of abiotic stress-responsive cotton genes using a cotton full-length cDNA overexpressing Arabidopsis library. J Integr Plant Biol 62(7):998–1016
Li S, Li X, Wei Z, Liu F (2020) ABA-mediated modulation of elevated CO2 on stomatal response to drought. Curr Opin Plant Biol S1369-5266(19):30117–30117
Liu D, Pei ZF, Naeem MS, Ming DF, Liu HB, Khan F (2011a) 5-Aminolevulinic acid activates antioxidative defence system and seedling growth in Brassica napus L. under water-deficit stress. J Agron Crop Sci 197:284–295
Liu Y, Subhash C, Yan JB, Song CP, Zhao JR, Li JS (2011b) Maize leaf temperature responses to drought: thermal imaging and quantitative trait loci (QTL) mapping. Environ Exp Bot 71(2):158–165
Maiti RK, Satya P (2014) Research advances in major cereal crops for adaptation to abiotic stresses. GM Crops Food 5:259–279
Masuka B, Araus JL, Das B, Sonder K, Cairns JE (2012) Phenotyping for abiotic stress tolerance in maize. J Integr Plant Biol 54:238–249
Meng LS (2018) Compound synthesis or growth and development of roots/stomata regulate plant drought tolerance or water use efficiency/water uptake efficiency. J Agric Food Chem 66(14):3595–3604
Merlot S, Mustilli AC, Genty B, North H, Lefebvre V, Sotta B, Vavasseur A, Giraudat J (2002) Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J 30:601–609
Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18(10):2749–2766
Mittler R, Blumwald E (2015) The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 27:64–70
Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14(12):3089–3099
Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K (2014a) Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77:367–379
Nakabayashi R, Mori T, Saito K (2014b) Alternation of flavonoid accumulation under drought stress in Arabidopsis thaliana. Plant Signal Behav 9:e29518
Nguyen NH, Kim JH, Kwon J, Jeong CY, Lee W, Lee D, Hong SW, Lee H (2016) Characterization of Arabidopsis thaliana flavonol synthase 1 (FLS1) -overexpression plants in response to abiotic stress. Plant Physiol Biochem 103:133–142
Qi J, Song CP, Wang B, Zhou J, Kangasjärvi J, Zhu JK, Gong Z (2018) ROS signaling and stomatal movement in plant responses to drought stress and pathogen attack. J Integr Plant Biol 60(9):805–826
Quan RD, Shang M, Zhang H, Zhao YX, Zhang JR (2004) Improved chilling tolerance by transformation with betA gene for the enhancement of glycinebe- taine synthesis in maize. Plant Sci 166:141–149
Rao MJ, Xu Y, Tang X, Huang Y, Liu J, Deng X, Xu Q (2020) CsCYT75B1, a Citrus CYTOCHROME P450 gene, is involved in accumulation of antioxidant flavonoids and induces drought tolerance in transgenic Arabidopsis. Antioxidants (Basel) 9(2):161
Sah SK, Reddy KR, Li J (2016) Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci 7:571
Scharwies JD, Dinneny JR (2019) Water transport, perception, and response in plants. J Plant Res 132(3):311–324
Seeve CM, Sunkar R, Zheng Y, Liu L, Liu Z, McMullen M, Nelson S, Sharp RE, Oliver MJ (2019) Water-deficit responsive microRNAs in the primary root growth zone of maize. BMC Plant Biol 19(1):447
Semagn K, Beyene Y, Warburton ML, Tarekegne A, Mugo S, Meisel B, Sehabiague P, Prasanna BM (2013) Meta-analyses of QTL for grain yield and anthesis silking interval in 18 maize populations evaluated under water-stressed and well-watered environments. BMC Genomics 14:313
Shou H, Bordallo P, Fan JB, Yeakley JM, Bibikova M, Sheen J, Wang K (2004) Expression of an active tobacco mitogen–activated protein kinase kinase kinase enhances freezing tolerance in transgenic maize. Proc Natl Acad Sci U S A 101:3298–3303
Singh R, Parihar P, Singh S, Mishra RK, Singh VP, Prasad SM (2017) Reactive oxygen species signaling and stomatal movement: current updates and future perspectives. Redox Biol 11:213–218
Song Y, Xiang F, Zhang G, Miao Y, Miao C, Song CP (2016) Abscisic acid as an internal integrator of multiple physiological processes modulates leaf senescence onset in Arabidopsis thaliana. Front Plant Sci 7:181
Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B (2007) Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J 50(4):660–677
Takahashi F, Kuromori T, Sato H, Shinozaki K (2018) Regulatory gene networks in drought stress responses and resistance in plants. Adv Exp Med Biol 1081:189–214
Talbi S, Rojas J, Sahrawy M, Rodríguez-Serrano M, Cárdenas K, Debouba M, Sandalio L (2020) Effect of drought on growth, photosynthesis and total antioxidant capacity of the Saharan plant Oudeneya Africana. Environ Exp Bot 176:104099
Tomar RS, Tiwari S, Vinod NBK, Chand S, Deshmukh R, Mallick N, Singh S, Singh NK, Tomar SM (2016) Molecular and morpho-agronomical characterization of root architecture at seedling and reproductive stages for drought tolerance in wheat. PLoS One 11(6):e0156528
Wang F, Kong W, Wong G, Fu L, Peng R, Li Z, Yao Q (2016a) AtMYB12 regulates flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol Gen Genomics 291(4):1545–1559
Wang F, Zhu H, Kong W, Peng R, Liu Q, Yao Q (2016b) The Antirrhinum AmDEL gene enhances flavonoids accumulation and salt and drought tolerance in transgenic Arabidopsis. Planta 244(1):59–73
Wang J, Zhang L, Cao Y, Qi C, Li S, Liu L, Wang G, Mao A, Ren S, Guo YD (2018) CsATAF1 positively regulates drought stress tolerance by an ABA-dependent pathway and by promoting ROS scavenging in cucumber. Plant Cell Physiol 59(5):930–945
Wang H, Guo S, Qiao X, Guo J, Li Z, Zhou Y, Bai S, Gao Z, Wang D, Wang P, Galbraith DW, Song CP (2019) BZU2/ZmMUTE controls symmetrical division of guard mother cell and specifies neighbor cell fate in maize. PLoS Genet 15(8):e1008377
Wang W, Chen Q, Xu S, Liu WC, Zhu X, Song CP (2020) Trehalose-6-phosphate phosphatase E modulates ABA-controlled root growth and stomatal movement in Arabidopsis. J Integr Plant Biol 62(10):1518–1534
Watkins JM, Hechler PJ, Muday GK (2014) Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol 164:1707–1717
Watkins JM, Chapman JM, Muday GK (2017) Abscisic acid-induced reactive oxygen species are modulated by flavonols to control stomata aperture. Plant Physiol 175:1807–1825
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Xu Z, Jiang Y, Jia B, Zhou G (2016) Elevated-CO2 response of stomata and its dependence on environmental factors. Front Plant Sci 7:657
Yao Y, Liu X, Li Z, Ma X, Rennenberg H, Wang X, Li H (2013) Drought-induced H2O2 accumulation in subsidiary cells is involved in regulatory signaling of stomatal closure in maize leaves. Planta 238:217–227
Yu G, Wang LG, Han Y, He QY (2012) Cluster profiler: an R package for comparing biological themes among gene clusters. Omics 16(5):284–287
Zhang T, Chen S, Harmon AC (2014a) Protein phosphorylation in stomatal movement. Plant Signal Behav 9(11):e972845
Zhang XL, Zhao XL, Li BZ, Xia JC, Miao YC (2014b) SRO1 regulates heavy metal mercury stress response in Arabidopsis thaliana. Chin Sci Bull 59(25):3134–3141
Zhang J, Wang N, Miao Y, Hauser F, McCammon JA, Rappel WJ, Schroeder JI (2018) Identification of SLAC1 anion channel residues required for CO2/bicarbonate sensing and regulation of stomatal movements. Proc Natl Acad Sci U S A 115(44):11129–11137
Zhang L, Li D, Yao Y, Zhang S (2020) H2O2, Ca2+, and K+ in subsidiary cells of maize leaves are involved in regulatory signaling of stomatal movement. Plant Physiol Biochem 152:243–251
Zheng M, Tao Y, Hussain S, Jiang QW, Peng SB, Huang JL, Cui KH, Nie LX (2016) Seed priming in dry direct-seeded rice: consequences for emergence, seedling growth and associated metabolic events under drought stress. Plant Growth Regul 78:167–178
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1604233) and Science and Technology Development Plan Project of Henan Province (192102110016).
Author information
Authors and Affiliations
Contributions
Baozhu Li, Chun-Peng Song, Xiaohong Zhu and Siyi Guo conceived and designed the experiment, Baozhu Li, Ruonan Fan, Yanting Fan, Shiquan Huang, Jiong Liu and Hui Zhang did the experiment, Guiling Sun, Ting Sun, Shenglong Bai analysis the transcriptome data; Baozhu Li and Pengtao Wang wrote the main manuscript text. Chun-Peng Song supervised the project and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Ricardo Aroca.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 414 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, B., Fan, R., Sun, G. et al. Flavonoids improve drought tolerance of maize seedlings by regulating the homeostasis of reactive oxygen species. Plant Soil 461, 389–405 (2021). https://doi.org/10.1007/s11104-020-04814-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04814-8