Abstract
Aim
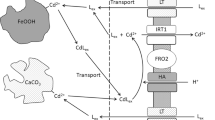
A mechanism of action for the performance of Fe chelates as soil-applied fertilizer has been hypothesized by Lindsay and Schwab (J Plant Nutr 5:821–840, 1982), in which the ligand participates in a cyclic process of delivering Fe at the root surface and mobilizing Fe from the soil. This “shuttle mechanism” seems appealing in view of fertilizer efficiency, but little is known about its performance. The chelate FeEDDHA is a commonly used Fe fertilizer on calcareous soils.
Methods
In this study, the performance of the shuttle mechanism has been examined for FeEDDHA chelates in soil interaction and pot trial experiments.
Results
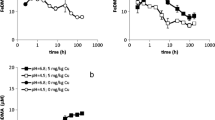
The specificity of EDDHA ligands for chelating Fe from soils of low Fe availability is limited. Experimental support for a shuttle mechanism in soil-plant systems with FeEDDHA was found: specific metal mobilization only occurred upon FeEDDHA-facilitated Fe uptake. The mobilized metals originated at least in part from the root surface instead of the soil.
Conclusion
The results from this study support the existence of a shuttle mechanism with FeEDDHA in soil application. If the efficiency of the shuttle mechanism is however largely controlled by metal availability in the bulk soil, it is heavily compromised by complexation of competing cations: Al, Mn and particularly Cu.







Similar content being viewed by others
Notes
o,o-H4EDDHA and o,p-H4EDDHA were kindly provided by AKZO-Nobel
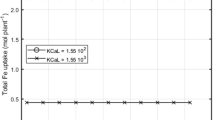
This is illustrated by the Co mobilization data from soil interaction experiment 1, presented as Supporting Information (SI-Figure 7).
Abbreviations
- o,o-FeEDDHA:
-
Iron (3+) ethylene diamine-N,N′-bis(2-hydroxy phenyl acetic acid) complex
- o,p-FeEDDHA:
-
Iron (3+) ethylene diamine-N-(2-hydroxy phenyl acetic acid)-N′-(4-hydroxy phenyl acetic acid) complex
- DOC:
-
Dissolved organic carbon
- DTPA:
-
Diethylene triamine penta acetic acid
- EDTA:
-
Ethylene diamine tetra acetic acid
- ICP-MS/AES:
-
Inductively coupled plasma mass spectrometry/atomic emission spectrometry
- SOC:
-
Soil organic carbon
- SOM:
-
Soil organic matter
- HFO:
-
Hydrous ferric oxide
References
Bailey NA, Cummins D, Mckenzie ED, Worthington JM (1981) Iron(III) compounds of phenolic ligands. The chrystal and molecular structure of the sexadentate ligand N, N′-ethylene-bis-(o-hydroxyphenylglycine). Inorg Chim Acta 50:111–120
Bannochie CJ, Martell AE (1989) Affinities of racemic and meso forms of N, N′-ethylenebis[2-(o-hydroxyphenyl)glycine] for divalent and trivalent metal ions. J Am Chem Soc 111:4735–4742
Bates SS, Tessier A, Campbell PGC, Buffle J (1982) Zinc adsorption and transport by Chlamydomonas-variabilis and Scenedesmus-subspicatus (Chlorophyceae) grown in semi-continuous culture. J Phycol 18:521–529
Bienfait HF, Vandenbriel W, Meslandmul NT (1985) Free space iron pools in roots—generation and mobilization. Plant Physiol 78:596–600
Bienfait HF, García-Mina J, Zamareno AM (2004) Distribution and secondary effects of EDDHA in some vegetable species. J Plant Nutr Soil Sci 50:1103–1110
Bihari S, Smith PA, Parsons S, Sadler PJ (2002) Stereoisomers of Mn(III) complexes of ethylenebis[(o-hydroxyphenyl)glycine]. Inorg Chim Acta 331:310–317
Boxma R (1972) Bicarbonate as the most important soil factor in lime-induced chlorosis in the Netherlands. Plant Soil 37:233–243
Chaney RL (1984) Diagnostic practices to identify iron deficiency in higher plants. J Plant Nutr 7:47–67
Chaney RL, Brown JC, Tiffin LO (1972) Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol 50:208–213
Fest EPMJ, Temminghoff EJM, Griffioen J, Riemsdijk WHV (2005) Proton buffering and metal leaching in sandy soils. Environ Sci Technol 39:7901–7908
Frost AE, Freedman HH, Westerback SJ, Martell AE (1958) Chelating tendencies of N, N′-ethylenebis-[2-(o-hydroxyphenyl)]-glycine. J Am Chem Soc 80:530–536
García-Marco S, Martínez N, Yunta F, Hernández-Apaolaza L, Lucena JJ (2006) Effectiveness of ethylenediamine-N(o-hydroxyphenylacetic)-N ′(p-hydroxy-phenylacetic) acid (o, p-EDDHA) to supply iron to plants. Plant Soil 279:31–40
Hassler CS, Slaveykova VI, Wilkinson KJ (2004) Discriminating between intra- and extracellular metals using chemical extractions. Limnol Oceanogr Methods 2:237–247
Hernández-Apaolaza L, Lucena JJ (2001) Fe(III)-EDDHA and -EDDHMA sorption on Ca-montmorillonite, ferrihydrite, and peat. J Agric Food Chem 49:5258–5264
Hernández-Apaolaza L, Lucena JJ (2011) Influence of the soil/solution ratio, interaction time, and extractant on the evaluation of iron chelate sorption/desorption by soils. J Agric Food Chem 59:2493–2500
Hernández-Apaolaza L, García-Marco S, Nadal P, Lucena JJ, Sierra MA, Gómez-Gallego M, Ramírez-López P, Escudero R (2006) Structure and fertilizer properties of byproducts formed in the synthesis of EDDHA. J Agric Food Chem 54:4355–4363
Houba VJG, Van Der Lee JJG, Novozamsky I (1997) Soil and plant analysis. Wageningen University, Wageningen
Houba VJG, Temminghoff EJM, Gaikhorst GA, Van Vark W (2000) Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun Soil Sci Plant Anal 31:1299–1396
Kalis EJJ, Temminghoff EJM, Weng LP, Van Riemsdijk WH (2006) Effects of humic acid and competing cations on metal uptake by Lolium perenne. Environ Toxicol Chem 25:702–711
Kalis EJJ, Temminghoff EJM, Visser A, Van Riemsdijk WH (2007) Metal uptake by Lolium perenne in contaminated soils using a four-step approach. Environ Toxicol Chem 26:335–345
Lindsay WL (1979) Chemical equilibria in soils. John Wiley and Sons, New York
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Lindsay WL, Schwab AP (1982) The chemistry of iron in soils and its availability to plants. J Plant Nutr 5:821–840
Lucena JJ (2003) Fe chelates for remediation of Fe chlorosis in strategy I plants. J Plant Nutr 26:1969–1984
Marschner H, Römheld V, Kissel M (1986) Different strategies in higher-plants in mobilization and uptake of iron. J Plant Nutr 9:695–713
Mortvedt JJ (1991) Correcting iron deficiencies in annual and perennial plants: present technologies and future prospects. Plant Soil 130:273–279
Nowack B, Sigg L (1997) Dissolution of Fe(III)(hydr)oxides by metal-EDTA complexes. Geochim Cosmochim Acta 61:951–963
Orera I, Rodríguez-Castrillón JA, Moldovan M, García-Alonso JI, Abadía A, Abadía J, Álvarez-Fernández A (2010) Using a dual-stable isotope tracer method to study the uptake, xylem transport and distribution of Fe and its chelating agent from stereoisomers of an Fe(III)-chelate used as fertilizer in Fe-deficient Strategy I plants. Metallomics 2:646–657
Patch MG, Simolo KP, Carrano CJ (1982) The cobalt(III), chromium(III), copper(II), and manganese(III) complexes of ethylenebis((ortho-hydroxyphenyl)glycine): models for metallotransferrins. Inorg Chem 21:2972–2977
Pérez-Sanz A, Lucena JJ (1995) Synthetic iron oxides as sources of Fe in a hydroponic culture of sunflower. In: Abadia J (ed) Iron nutrition in soils and plants. Kluwer Academic Publishers, Dordrecht, pp 241–246
Quevauviller P, Lachica M, Barahona E, Rauret G, Ure A, Gomez A, Muntau H (1996) Interlaboratory comparison of EDTA and DTPA procedures prior to certification of extractable trace elements in calcareous soil. Sci Total Environ 178:127–132
Rajan KS, Mainer S, Rajan NL, Davis JM (1981) Studies on the chelation of aluminum for neurobiological application. J Inorg Biochem 14:339–350
Reed DW, Lyons CG Jr, Mceachern GR (1988) Field evaluation of inorganic and chelated iron fertilizers as foliar sprays and soil application. J Plant Nutr 11:1369–1378
Riley PE, Pecoraro VL, Carrano CJ, Raymond KN (1983) Siderophilin metal coordination. 3. Crystal structures of the cobalt(III), gallium(III), and copper(II) complexes of ethylenebis[(o-hydroxyphenyl)glycine]. Inorg Chem 22:3096–3103
Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397:694–697
Rojas CL, Romera FJ, Alcántara E, Pérez-Vicente R, Sariego C, García-Alonso JI, Boned J, Marti G (2008) Efficacy of Fe(o, o-EDDHA) and Fe(o, p-EDDHA) isomers in supplying Fe to strategy i plants differs in nutrient solution and calcareous soil. J Agric Food Chem 56:10774–10778
Rombolà AD, Tagliavini M (2006) Iron nutrition of fruit tree crops. In: Barton LL, Abadia A (eds) Iron nutrition in plants and rhizospheric microorganisms. Springer, Dordrecht, pp 61–83
Schenkeveld WDC, Reichwein AM, Temminghoff EJM, Van Riemsdijk WH (2007) The behaviour of EDDHA isomers in soils as influenced by soil properties. Plant Soil 290:85–102
Schenkeveld WDC, Dijcker R, Reichwein AM, Temminghoff EJM, Van Riemsdijk WH (2008) The effectiveness of soil-applied FeEDDHA treatments in preventing iron chlorosis in soybean as a function of the o, o-FeEDDHA content. Plant Soil 303:161–176
Schenkeveld WDC, Reichwein AM, Bugter MHJ, Temminghoff EJM, Van Riemsdijk WH (2010a) Performance of soil-applied FeEDDHA isomers in delivering Fe to soybean plants in relation to the moment of application. J Agric Food Chem 58:12833–12839
Schenkeveld WDC, Temminghoff EJM, Reichwein AM, Van Riemsdijk WH (2010b) FeEDDHA-facilitated Fe uptake in relation to the behaviour of FeEDDHA components in the soil-plant system as a function of time and dosage. Plant Soil 332:69–85
Schenkeveld WDC, Weng LP, Temminghoff EJM, Reichwein AM, Van Riemsdijk WH (2010c) Evaluation of the potential impact from Cu competition on the performance of o,o-FeEDDHA in soil application. In: Iron fertilization with FeEDDHA - the fate and effectiveness of FeEDDHA chelates in soil-plant systems (PhD thesis). Wageningen University, Wageningen, pp 139–160
Schenkeveld WDC, Hoffland E, Reichwein AM, Temminghoff EJM, Van Riemsdijk WH (2012a) The biodegradability of EDDHA chelates under calcareous soil conditions. Geoderma 173:282–288
Schenkeveld WDC, Reichwein AM, Temminghoff EJM, Van Riemsdijk WH (2012b) Effect of soil parameters on the kinetics of the displacement of Fe from FeEDDHA chelates by Cu. J Phys Chem A 116:6582–6589
Schwertmann U (1964) The differentiation of iron oxides in soil by extraction with ammonium-oxalate solution. J Plant Nutr Soil Sci 105:194–202
Siebner-Freibach H, Hadar Y, Chen Y (2003) Siderophores sorbed on Ca-montmorillonite as an iron source for plants. Plant Soil 251:115–124
Strasser O, Kohl K, Römheld V (1999) Overestimation of apoplastic Fe in roots of soil grown plants. Plant Soil 210:179–187
Tipping E, Rieuwerts J, Pan G, Ashmore MR, Lofts S, Hill MTR, Farago ME, Thornton I (2003) The solid-solution partitioning of heavy metals (Cu, Zn, Cd, Pb) in upland soils of England and Wales. Environ Pollut 125:213–225
Walinga I, Kithome M, Novozamsky I, Houba VJG, Lee JJVD (1992) Spectrophotometric determination of organic carbon in soil. Commun Soil Sci Plant Anal 23:1935–1944
Acknowledgments
The authors wish to express their sincere appreciation and gratitude to the following: AkzoNobel for financing this project which was initiated by P. Weijters and M. Bugter, Rob Dijcker and Marina Hernandez-Lopez for conducting a part of the experimental work and P. Nobels for his help with the ICP-measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
SI-Figure 1
Percentage of a) racemic o,o-EDDHA; b) meso o,o-EDDHA; and c) o,p-EDDHA added, chelated to Fe, Cu, Mn and Al in solution upon interaction with Xeraco L soil as a function of time. Error bars indicate standard deviations. (DOC 162 kb)
SI-Figure 2
Percentage of a) racemic o,o-EDDHA; b) meso o,o-EDDHA; and c) o,p-EDDHA added, chelated to Fe, Cu, Mn and Al in solution upon interaction with Nadec soil as a function of time. Error bars indicate standard deviations. (DOC 260 kb)
SI-Figure 3
Percentage of a) racemic o,o-EDDHA; b) meso o,o-EDDHA; and c) o,p-EDDHA added, chelated to Fe, Cu, Mn and Al in solution upon interaction with Hofuf soil as a function of time. Error bars indicate standard deviations. (DOC 163 kb)
SI-Figure 4
Metal-EDDHA concentrations in solution after 24 hours of interaction between EDDHA isomers and Xeraco L soil as a function of the concentration of a) racemic o,o-EDDHA; b) meso o,o-EDDHA; and c) o,p-EDDHA added. Error bars indicate standard deviations. (DOC 258 kb)
SI-Figure 5
Metal-EDDHA concentrations in solution after 24 hours of interaction between EDDHA isomers and Nadec soil as a function of the concentration of a) racemic o,o-EDDHA; b) meso o,o-EDDHA; and c) o,p-EDDHA added. Error bars indicate standard deviations. (DOC 260 kb)
SI-Figure 6
Metal-EDDHA concentrations in solution after 24 hours of interaction between EDDHA isomers and Hofuf soil as a function of the concentration of a) racemic o,o-EDDHA; b) meso o,o-EDDHA; and c) o,p-EDDHA added. Error bars indicate standard deviations. (DOC 152 kb)
SI-Figure 7
Percentage of EDDHA isomers added, chelated to Co in solution upon interaction with a) Santomera soil; b) Xeraco L soil; c) Nadec soil; and d) Hofuf soil as a function of time. Error bars indicate standard deviations. (DOC 170 kb)
Rights and permissions
About this article
Cite this article
Schenkeveld, W.D.C., Reichwein, A.M., Temminghoff, E.J.M. et al. Considerations on the shuttle mechanism of FeEDDHA chelates at the soil-root interface in case of Fe deficiency. Plant Soil 379, 373–387 (2014). https://doi.org/10.1007/s11104-014-2057-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2057-1