Abstract
Background and aims
Mixing effects during litter decomposition could occur between two or more different litter species because of the potential nutrient transfer. However, evidence of mixing effects is variable and the underlying mechanisms remain unclear. Using a three-year decomposition experiment, we aim to examine for the effects of litter mixing and position on decomposition rates and nitrogen (N) and phosphorus (P) dynamics.
Methods
We studied litter decomposition of Stipa krylovii (Sk) and Astragalus galactites (Ag), two dominant species with contrasting litter quality, in a typical steppe of northern China in both single decomposition and three mixing treatments. The three mixing treatments included thorough mixing (Sk-Ag), Ag over Sk (Ag/Sk), and Sk over Ag (Sk/Ag).
Results
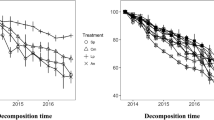
Both the Sk-Ag and the Sk/Ag mixture had negative mixing effects on the mass loss of the litter mixture, while the Ag/Sk mixture had a neutral mixing effect. The percent mass loss was higher when the litter species was placed at the top (25.0 and 51.9 % of mass remaining for Ag and Sk, respectively) than at the bottom (38.3 and 61.8 % of mass remaining for Ag and Sk, respectively). The Sk/Ag mixture had negative effects on the release of N while all three mixing treatments had positive effects on the release of P.
Conclusions
Our results indicate that: (1) mixing treatments can induce different mixing effects; (2) environmental factors likely play an important role in controlling the mixing effect; and (3) litter-mixtures have different non-additive effects on N and P, which may further increase the heterogeneity of N and P availability as the two litter species may fall differentially in terms of space and time.





Similar content being viewed by others
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Austin AT, Vitousek PM (2000) Precipitation, decomposition and litter decomposability of Metrosideros polymorpha in native forests on Hawai’i. J Ecol 88:129–138
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–558
Ball BA, Bradford MA, Hunter MD (2009) Nitrogen and phosphorus release from mixed litter layers is lower than predicted from single species decay. Ecosystems 12:87–100
Barantal S, Roy J, Fromin N, Schimann H, Hättenschwiler S (2011) Long-term presence of tree species but not chemical diversity affect litter mixture effects on decomposition in a neotropical rainforest. Oecologia 167:241–252
Berg B, Ekbohm G (1991) Litter mass-loss rates and decomposition patterns in some needle and leaf litter types - long-term decomposition in a Scots pine forest. VII. Can J Bot 69:1449–1456
Berg B, Laskowski R (2006) Litter decomposition: A guide to carbon and nutrient turnover. Elsevier, San Diego, pp 102–183
Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE (2002) Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 99:317–323
Daniel O, Anderson JM (1992) Microbial biomass and activity in contrasting soil materials after passage through the gut of the earthworm Lumbricus rubellus hoffmeister. Soil Biol Biochem 24:465–470
Dent DH, Bagchi R, Robinson D, Majalap-Lee N, Burslem DFRP (2006) Nutrient fluxes via litterfall and leaf litter decomposition vary across a gradient of soil nutrient supply in a lowland tropical rain forest. Plant Soil 288:197–215
Fyles JW, Fyles IH (1993) Interaction of Douglas-fir with red alder and salal foliage litter during decomposition. Can J For Res 23:358–361
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246
Gartner TB, Cardon ZG (2006) Site of leaf origin affects how mixed litter decomposes. Soil Biol Biochem 38:2307–2317
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–243
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition interrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218
Hector A, Beale AJ, Minns A, Otway SJ, Lawton JH (2000) Consequences of the reduction of plant diversity for litter decomposition: effects through litter quality and microenvironment. Oikos 90:357–371
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Hobbie SE, Shevtsova A, Chapin FS (1999) Plant responses to species removal and experimental warming in Alaskan tussock tundra. Oikos 84:417–434
Hoorens B, Aerts R, Stroetenga M (2003) Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 137:578–586
Hoorens B, Coomes D, Aerts R (2010) Neighbour identity hardly affects litter-mixture effects on decomposition rates of New Zealand forest species. Oecologia 162:479–489
Jonsson M, Wardle DA (2008) Context dependency of litter-mixing effects on decomposition and nutrient release across a long-term chronosequence. Oikos 117:1674–1682
Liu P, Huang J, Han X, Sun OJ, Zhou Z (2006) Differential responses of litter decomposition to increased soil nutrients and water between two contrasting grassland plant species of Inner Mongolia, China. Appl Soil Ecol 34:266–275
Liu P, Huang J, Sun OJ, Han X (2010) Litter decomposition and nutrient release as affected by soil nitrogen availability and litter quality in a semiarid grassland ecosystem. Oecologia 162:771–780
Madritch MD, Cardinale BJ (2007) Impacts of tree species diversity on litter decomposition in northern temperate forests of Wisconsin, USA: a multi-site experiment along a latitudinal gradient. Plant Soil 292:147–159
McArthur JV, Aho JM, Rader RB, Mills GL (1994) Interspecific leaf interactions during decomposition in aquatic and floodplain ecosystems. J North Am Bentholl Soc 13:57–67
McGroddy ME, Silver WL, de Oliveira RC Jr (2004) The effect of phosphorus availability on decomposition dynamics in a seasonal lowland Amazonian forest. Ecosystems 7:172–179
McTiernan KB, Ineson P, Coward PA (1997) Respiration and nutrient release from tree leaf litter mixtures. Oikos 78:527–538
Moore TR, Bubier JL, Bledzki L (2007) Litter decomposition in temperate peatland ecosystems: the effect of substrate and site. Ecosystems 10:949–963
Nelson D, Sommers L (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour MA, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. Part 3, Chemical methods. Soil Science Society of America, American Society of Agronomy, Madison, pp 961–1010
Nilsson MC, Wardle DA, Dahlberg A (1999) Effects of plant litter species composition and diversity on the boreal forest plant-soil system. Oikos 86:16–26
Prescott C (2005) Decomposition and mineralization of nutrients from litter and humus. In: BassiriRad H (ed) Nutrient acquisition by plants. Springer, Berlin, pp 15–41
Swift M, Heal O, Anderson J (1979) Decomposition in terrestrial ecosystems. University of California Press, Los Angeles
Throop HL, Archer SR (2009) Resolving the dryland decomposition conundrum: Some new perspectives on potential drivers. In: Lüttge U, Beyschlag W, Büdel B, Francis D (eds) Progress in botany. Springer, Berlin, pp 171–194
Vivanco L, Austin AT (2006) Intrinsic effects of species on leaf litter and root decomposition: a comparison of temperate grasses from North and South America. Oecologia 150:97–107
Wang L, D’Odorico P, Manzoni S, Porporato A, Macko S (2009) Soil carbon and nitrogen dynamics in southern African savannas: the effect of vegetation-induced patch-scale heterogeneities and large scale rainfall gradients. Clim Change 94:63–76
Wardle DA, Bonner KI, Nicholson KS (1997) Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 79:247–258
Wardle DA, Nilsson MC, Zackrisson O, Gallet C (2003) Determinants of litter mixing effects in a Swedish boreal forest. Soil Biol Biochem 35:827–835
Yahdjian L, Sala O, Austin A (2006) Differential controls of water input on litter decomposition and nitrogen dynamics in the Patagonian Steppe. Ecosystems 9:128–141
Yang H, Wu M, Liu W, Zhang Z, Zhang N, Wan S (2011) Community structure and composition in response to climate change in a temperate steppe. Glob Change Biol 17:452–465
Acknowledgements
The authors would like to thank Ang Li and Jianyang Xia for their constructive comments on an earlier version of this manuscript. We are also extremely grateful for two anonymous reviewers and the section editor Tim Moore for their constructive comments. This study was financially supported by the National Basic Research Program of China (2009CB421102) and the National Natural Science Foundation of China (41073056). We also thank the Duolun Restoration Ecology Research Station for permission to access the study site and for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tim Moore.
Rights and permissions
About this article
Cite this article
Tan, Y., Chen, J., Yan, L. et al. Mass loss and nutrient dynamics during litter decomposition under three mixing treatments in a typical steppe in Inner Mongolia. Plant Soil 366, 107–118 (2013). https://doi.org/10.1007/s11104-012-1401-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1401-6