Abstract
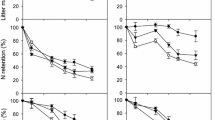
The large accumulation of organic matter in peatlands is primarily caused by slow rates of litter decomposition. We determined rates of decomposition of major peat-forming litters of vascular plants and mosses at five sites: a poor fen in New Hampshire and a bog hummock, a poor fen, a beaver pond margin and a beaver pond in Ontario. We used the litterbag technique, retrieving triplicate litterbags six or seven times over 3–5 years, and found that simple exponential decay and continuous-quality non-linear regression models could adequately characterize the decomposition in most cases. Within each site, the rate of decomposition at the surface was generally Typha latifolia leaves = Chamaedaphne calyculata leaves = Carex leaves > Chamaedaphne calyculata stems > hummock Sphagnum = lawn/hollow Sphagnum, with exponential decay constant (k) values generally ranging from 0.05 to 0.37 and continuous-quality model initial quality (q 0 ) values ranging from 1.0 (arbitrarily set for Typha leaves) to 0.7 (Sphagnum). In general, surface decay rates were slowest at the bog hummock site, which had the lowest water table, and in the beaver pond, which was inundated, and fastest at the fens. The continuous-quality model site decomposition parameter (u 0 ) ranged from 0.80 to 0.17. Analysis of original litter samples for carbon, nitrogen and proximate fractions revealed a relatively poor explanation of decomposition rates, as defined by k and q 0 , compared to most well-drained ecosystems. Three litters, roots of sedge and a shrub and Typha leaves, were placed at depths of 10, 30 and 60 cm at the sites. Decomposition rates decreased with depth at each site, with k means of 0.15, 0.08 and 0.05 y−1 at 10, 30 and 60 cm, respectively, and u 0 of 0.25, 0.13 and 0.07. These differences are primarily related to the position of the water table at each site and to a lesser extent the cooler temperatures in the lower layers of the peat. The distinction between bog and fen was less important than the position of the water table. These results show that we can characterize decomposition rates of surface litter in northern peatlands, but given the large primary productivity below-ground in these ecosystems, and the differential rates of decomposition with depth, subsurface input and decomposition of organic matter is an important and relatively uncertain attribute.





Similar content being viewed by others
REFERENCES
Aerts R. 1997. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–49
Aerts R, Verhoeven JTA, Whigham DF. 1999. Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology 80:2170–81
Ågren G, Bosatta E. 1996. Quality: a bridge between theory and experiment in soil organic matter studies. Oikos 76:522–8
Backéus I. 1990. Production and depth distribution of fine roots in a boreal open bog. Ann Bot Fenn 27:261–5
Baker TT, Lockaby BG, Conner WH, Meier CE, Stanturf JA, Burke MK. 2001. Leaf litter decomposition and nutrient dynamics in four southern forested floodplain communities. Soil Sci Soc Am J 65:1334–47
Banerjee RD, Sen SP. 1979. Antibiotic activity of bryophytes. Bryologist 82:141–53
Bartsch I, Moore TR. 1985. A preliminary investigation of primary production and decomposition in subarctic peatlands. Can J Bot 63:1241–8
Belyea LR. 1996. Separating the effects of litter quality and macroenvironment on decomposition rates in a patterned peatland. Oikos 77:529–39
Belyea LR, Malmer N. 2004. Carbon sequestration in peatland: patterns and mechanisms of response to climate change. Glob Change Biol 10:1043–52
Bubier J, Crill P, Mosedale A, Frolking S, Linder E. 2003a. Peatland responses to varying interannual moisture conditions as measured by automatic CO2 chambers. Glob Biogeochem Cycles 17(2). doi:10.1029/2002GB001946
Bubier JL, Bhatia G, Moore TR, Roulet NT, Lafleur PM. 2003b. Spatial and temporal variability in growing season net ecosystem CO2 exchange at a large peatland, Ontario, Canada. Ecosystems 6:353–67. doi:10.1007/s1002–003–0125–0
Bubier JL, Moore TR, Bledzki LA. 2007. Effects of nutrient addition on vegetation and carbon cycling in an ombrotrophic bog. Glob Change Biol. 13:1168–1186
Bubier JL, Moore TR, Crosby G. 2006. Fine-scale vegetation distribution in a cool temperate bog. Can J Bot 84:910–23
Campbell C, Vitt DH, Halsey LA, Campbell ID, Thormann MN, Bayley SE. 2000. Net primary production and standing biomass in northern continental wetlands. Canadian forestry service information report NOR-X-369. Edmonton: Canadian Forestry Service
Clymo RS. 1965. Experiments on breakdown of Sphagnum in two bogs. J Ecol 53:737–57
Clymo RS, Turunen J, Tolonen K. 1998. Carbon accumulation in peatland. Oikos 81:368–88
Day FP. 1983. Effects of flooding on leaf litter decomposition in microcosms. Oecologia 56:180–84
Frolking S, Bubier JL, Moore TR, Ball T, Bellisario LM, Bhardwaj A, Carroll P, Crill PM, Lafleur PM, McCaughey JH, Roulet NT, Suyker AE, Verma SB, Waddington JM, Whiting GJ. 1998. The relationship between ecosystem productivity and photosynthetically active radiation for northern peatlands. Glob Biogeochem Cycles 12:115–26
Frolking S, Roulet NT, Moore TR, Richard PJH, Lavoie M, Muller SD. 2001. Modelling northern peatland decomposition and peat accumulation. Ecosystems 4:479–98
Gartner TB, Cardon ZG. 2004 Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–46
Gorham E. 1991. Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1:182–95
Heal OW, Latter PM, Howson G. 1978. A study of the rates of decomposition of organic matter. In: Heal OW Perkins DF, Eds. Production ecology of British Moors and Montane Grasslands. New York: Springer. pp. 136–59
Hobbie SE, Gough L. 2004. Litter decomposition in moist acidic and non-acidic tundra with different glacial histories. Oecologia 140:113–24
Hyvönen R, Ågren GI, Dalias P. 2005. Analysing temperature response of decomposition of organic matter. Glob Change Biol 11:770–8
Joffre R, Ågren GI, Gillon D, Bosatta E. 2001. Organic matter quality in ecological studies: theory meets experiment. Oikos 93:451–8
Johnson LC, Damman AWH. 1991. Species controlled Sphagnum decay on a south Swedish raised bog. Oikos 61:234–42
Johnson LC, Damman AWH. 1993. Decay and its regulation in Sphagnum peatlands. Adv Bryology 5:249–96
Laiho R, Finér L. 1996. Changes in root biomass after water-level drawdown on pine mires in southern Finland. Scan J For Res 11:251–60
Latter PM, Howson G, Howard DM, Scott WA. 1998. Long-term study of litter decomposition on a Pennine peat bog: which regression? Oecologia 113:94–103
Limpens J, Berendse F (2003) How litter quality affects mass loss and N loss from decomposing Sphagnum. Oikos 103:537–47
Lockaby BG, Wheat RS, Clawson RG. 1996. Influence of hydroperiod on litter conversion to soil organic matter in a floodplain forest. Soil Sci Soc Am J 60:1989–93
Melloh RA, Crill PM. 1996. Winter methane dynamics in a temperate peatland. Glob Biogeochem Cycles 10:247–54
Mitsch WJ, Gosselink JG. 2000. Wetlands. New York: Wiley
Moore TR, Basiliko N. 2006. Decomposition. In: Wieder RK, Vitt DH, Eds. Boreal peatland ecosystems, ecological studies, vol 188. Heidelberg: Springer. pp. 126–43
Moore TR, Bubier JL, Lafleur PM, Frolking S, Roulet NT. 2002. Plant biomass, production and CO2 exchange in an ombrotrophic bog. J Ecol 90:25–36
Moore TR, Trofymow AJ, Siltanen M, Prescott C, CIDET Working Group. 2005. Litter decomposition and C, N and P dynamics in upland forest and peatland sites, central Canada. Can J For Res 35:133–42
Painter TJ. 1991. Lindow Man, Tollund Man and other peat-bog bodies: the preservative and antimicrobial action of Sphanan, a reactive glycurnoglycan with tanning and sequestering properties. Carbohydr Polym 15:123–42
Rochefort L Vitt DH, Bayley SE. 1990. Growth, production, and decomposition dynamics of Sphagnum under natural and experimentally acidified conditions. Ecology 71:1986–2000
Roulet NT, Lafleur PM, Richard PJH, Moore TR, Humphreys E, Bubier JL. 2007. Comparison of a six year contemporary carbon balance and the carbon accumulation for the last 3,000 years for a northern peatland. Glob Change Biol 13:397–411
Saarinen T. 1996. Biomass and production of two vascular plants in a boreal mesotrophic fen. Can J Bot 74:934–8
Scanlon D, Moore T. 2000. CO2 production from peatland soil profiles: the influence of temperature, oxic/anoxic conditions and substrate. Soil Sci 165:153–60
Sjörs H. 1991. Phyto- and necromass above and below ground in a fen. Holarctic Ecol 14:208–18
Thormann MN, Bayley SE. 1997. Decomposition along a moderate-rich fen-marsh peatland gradient in boreal Alberta, Canada. Wetlands 17:123–37
Thormann MN, Bayley SE, Currah RS. 2001. Comparison of decomposition of belowground and aboveground plant litters in peatlands of boreal Alberta. Can J Bot 79:9–22
Trofymow JA, Moore TR, Titus B, Prescott C, Morrison I, Siltanen M, Smith S, Fyles J, Wein R, Camiré C, Duschene L, Kozak L, Kranabetter M, Visser S. 2002. Rates of litter decomposition over 6 years in Canadian forests: influence of litter quality and climate. Can J For Res 32:789–804
Turetsky MR. 2003. The role of bryophytes in carbon and nitrogen cycling. Bryologist 106:395–409
Verhoeven JTA, Liefveld WM. 1997. The ecological significance of organochemical compounds in Sphagnum. Acta Bot Neerl 46:117–30
Verhoeven JTA, Toth E. 1995. Decomposition of Carex and Sphagnum litter in fens: effect of litter quality and inhibition by living tissue homogenates. Soil Biol Biochem 27:271–5
Wallén B. 1986. Above and belowground dry mass of three main vascular plants on hummocks on a subarctic peat bog. Oikos 46:51–6
Wieder RK, Starr ST. 1998. Quantitative determination of organic fractions in highly organic, Sphagnum peat soils. Commun Soil Sci Plant Anal 29:847–57
Wylie GD. 1987. Decomposition and nutrient dynamics of litter of Quercus palustris and Nelumbo lutea in a wetland complex of Southeast Missouri, U.S.A. Arch für Hydrobiol 111:95–106
Yu ZC, Turetsky MR, Campbell ID, Vitt DH. 2001. Modelling long-term peatland dynamics. II Processes as inferred from litter and peat-core data. Ecol Model 144:159–73
ACKNOWLEDGEMENTS
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (to TM) and from the U.S. National Science Foundation (NSF grant DEB-0346625 (to JB)). We gratefully acknowledge the field and laboratory assistance of Mike Dalva, Andy Mosedale, Ruth Varner and several Mount Holyoke students. Professor Göran Ågren, of the Swedish University of Agricultural Sciences, Uppsala, suggested the use of the continuous-quality model and we are grateful for his advice in its implementation. Infrastructural facilities at Mer Bleue that made this work possible are supported by the Fluxnet Canada Research Network and at Sallie’s Fen by NSF LTREB Program grant DEB-0316326.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moore, T.R., Bubier, J.L. & Bledzki, L. Litter Decomposition in Temperate Peatland Ecosystems: The Effect of Substrate and Site. Ecosystems 10, 949–963 (2007). https://doi.org/10.1007/s10021-007-9064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-007-9064-5