Abstract
Atmospheric deposition is an important nutrient input to forests. The chemical composition of the rainfall is altered by the forest canopy due to interception and canopy exchange. Bulk deposition and stand deposition (throughfall plus stemflow) of Na+, Cl−, K+, Ca2+, Mg2+, PO 3−4 , SO 2−4 , H+, Mn2+, Al3+, Fe2+, NH +4 , NO −3 and Norg were measured in nine deciduous forest plots with different tree species diversity in central Germany. Interception deposition and canopy exchange rates were calculated with a canopy budget model. The investigated forest plots were pure beech (Fagus sylvatica L.) plots, three-species plots (Fagus sylvatica, Tilia cordata Mill. or T. platyphyllos Scop. and Fraxinus excelsior L.) and five-species plots (Fagus sylvatica, T. cordata or T. platyphyllos, Fraxinus excelsior, Acer platanoides L., A. pseudoplatanus L. or A. campestre L. and Carpinus betulus L.). The interception deposition of all ions was highest in pure beech plots and was negatively related to the Shannon index. The stand deposition of K+, Ca2+, Mg2+ and PO 3−4 was higher in mixed species plots than in pure beech plots due to higher canopy leaching rates in the mixed species plots. The acid input to the canopy and to the soil was higher in pure beech plots than in mixed species plots. The high canopy leaching rates of Mn2+ in pure beech plots indicated differences in soil properties between the plot types. Indeed, pH, effective cation exchange capacity and base saturation were lower in pure beech plots. This may have contributed to the lower leaching rates of K+, Ca2+ and Mg2+ compared to the mixed species plots. However, foliar analyses indicated differences in the ion status among the tree species, which may additionally have influenced canopy exchange. In conclusion, the nutrient input to the soil resulting from deposition and canopy leaching was higher in mixed species plots than in pure beech plots, whereas the acid input was highest in pure beech plots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atmospheric deposition is an important nutrient source in forests (e.g., Swank 1984; Lindberg et al. 1986). It is the total input of ions, gases and organic compounds to a canopy and can be divided into several fractions (Fig. 1). Precipitation deposition, which is the deposition of rain, snow and particles that are deposited due to gravitation, is independent of the receptor surface (Ulrich 1983a). In contrast, interception deposition, which is the deposition of fog and cloud droplets, aerosols and gases, depends on the filtering efficiency of the receptor (size, structure and chemical state) (Ulrich 1983a). In the forest canopy, the receptor surface consists of leaves, branches, stems and canopy lichens. The canopy can act as a source or a sink for the deposited chemical compounds. The stand deposition is the output of ions and organic compounds from the canopy and consists of throughfall and stemflow, which together represent the input to the forest floor. The presence of understory vegetation in the forest alters the stand deposition before it reaches the soil.
The deposition inputs and outputs of a forest canopy; adapted from Ulrich (1983a)
Stand precipitation is a major pathway in nutrient recycling, and annual nutrient return to the forest soil for potassium, sodium and sulphur is predominantly via stand precipitation, whereas little is due to litterfall (Parker 1983). However, deposited acid compounds and heavy metals can negatively influence the trees and the forest soil. Many authors have reported that this causes symptoms of forest decline in industrialized countries (e.g., Ulrich and Pankrath 1983; Georgii 1986; Johnson and Lindberg 1992; de Vries et al. 2001; Elling et al. 2007).
Several models have been developed to estimate interception deposition and canopy exchange on the basis of stand deposition and bulk or wet-only deposition measurements, e.g., the regression model of Lovett and Lindberg (1984) and the canopy budget models of Ulrich (1983a) and Beier et al. (1992). In the present study Ulrich’s canopy budget model (1983a, 1994) was used to estimate interception deposition and canopy exchange.
Besides abiotic factors, interception deposition depends on the leaf area, the physical and chemical properties of the leaf surface and the structural properties of the canopy (Erisman and Draaijers 2003; André et al. 2008). Interception deposition increases, for example, with increasing stand height and canopy roughness (Erisman and Draaijers 2003). The exchange processes in the canopy comprise uptake and leaching of chemical compounds and depend on the physiology and ion status of the trees and the ion permeability of the leaves (Draaijers et al. 1994; André et al. 2008). European beech (Fagus sylvatica L.) trees are known to have lower leaf ion concentrations than other deciduous trees, such as European hornbeam (Carpinus betulus L.) and small-leaved lime (Tilia cordata Mill.) (Krauß and Heinsdorf 2005). Hence, different tree species may affect interception and canopy exchange processes differently. Indeed, several studies have shown that stand deposition is significantly influenced by tree species composition (Potter et al. 1991; Draaijers et al. 1992; Lovett et al. 1996).
Because of changes in forest management, tree species diversity is increasing in German forests (Baumgarten and von Teuffel 2005; Knoke et al. 2005). However, how the alteration in tree species composition affects nutrient cycling in forests is not fully understood and has not yet been quantified. Hence, it is of interest to gain insight into the deposition and canopy exchange processes in mixed stands compared to single species ones (e.g., Nordén 1991; Berger et al. 2008).
We conducted an observational study in a temperate deciduous old-growth forest in central Germany to gain insight into the effects of tree species composition on deposition and canopy exchange processes. In contrast to observational studies, experiments in synthetic forest stands minimize the differences in soil properties and include all relevant treatments (monospecific plots of each tree species and all possible mixtures). However, planted synthetic stands often differ from natural forests in several respects, including trophic structure, tree age distribution and horizontal and vertical canopy structure (Leuschner et al. 2009). Furthermore, edge effects may interfere with species effects, and a quasi-steady state in soil development usually does not exist. Thus, experiments in planted synthetic stands should be combined with observational studies in existing forest stands differing in tree species diversity.
The present observational study examines the differences in the input of chemical compounds to the canopy with bulk and interception deposition, the canopy exchange processes, as well as the output of chemical compounds from the canopy with throughfall and stemflow along a tree species diversity gradient from monospecific beech plots to deciduous tree species-rich plots in central Germany.
Materials and methods
Study site
The study site (51°5′N, 10°30′E) is located in central Germany in the Hainich National Park. The typical vegetation types of the National Park are beech and deciduous mixed-beech forests. The forest has not been managed and has only been used for recreation since 1990. From the 1960s to 1990 the area was used for military training. For the last 40 years, only single trees have been extracted from the forest, and it has regenerated naturally. The area has been covered by deciduous forest for at least 200 years. Thus, it represents an old-growth forest with respect to stand continuity according to Wulf’s definition (2003) (Schmidt et al. 2009). The National Park is surrounded by deciduous forest, agricultural land and small villages. The nearest city (Erfurt) with more than 200,000 inhabitants is about 50 km to the southeast of the National Park.
Nine study plots (each 0.25 ha) with differing tree species diversity were selected within a radius of approximately 5 km in the north-eastern part of the Hainich National Park. The selected forest plots were located in a contiguous forest area. Each forest plot could be assigned to one of three tree species diversity levels (DL).
-
DL1:
the tree layer comprises at least 95% beech (Fagus sylvatica L.);
-
DL2:
the tree layer comprises at least 95% beech, lime (Tilia cordata Mill. or T. platyphyllos Scop.) and ash (Fraxinus excelsior L.);
-
DL3:
the tree layer comprises at least 95% beech, lime, ash, maple (Acer platanoides L., A. pseudoplatanus L. or A. campestre L.) and hornbeam (Carpinus betulus L.).
Three replicate plots were selected for each diversity level. The forest plots of DL1 are hereafter referred to as “pure beech plots”, and the forest plots of both DL2 and DL3, as “mixed species plots”. The tree species composition in the Hainich National Park is largely a result of previous forest management. Different ownership and management goals have resulted in a small-scale mosaic of forest stands differing in tree species diversity with pure beech stands growing in close proximity to species-rich forests with ash, lime, hornbeam, maple and beech. The practice of selective cutting (Plenterwald) or coppicing with standards (Mittelwald), which promote the development of species-rich stands, was very probably associated with a higher disturbance regime for the past 150 to 200 years than that experienced in the management of beech in age-class forests (Schmidt et al. 2009). The average age of the trees is 148, 85 and 100 years in the DL1, DL2 and DL3 plots, respectively (Schmidt et al. 2009).
In the studied forest stands, trees began to acquire leaves in early April and leaf-out was completed by the second half of May. Leaf shedding started at the beginning of September. The trees were leafless from December to beginning of April.
The mean annual precipitation of the observation period (2005 to 2007) measured at the nearest meteorological station “Weberstedt/Hainich” (51°10′N, 10°52′E; 270 m a.s.l.) was 652 mm (meteomedia GmbH). The long-term mean annual temperature is 7.5 ° C. The study plots are located at a mean altitude of 340 m a.s.l., have a mean slope of 3.1°, and all plots have north-eastern exposure. The bedrock of the Hainich National Park is Triassic limestone covered with a loess layer of differing thickness. The soil type is a Luvisol (FAO 1998). The soil texture of the loess layer is loamy to clayey silt and in the lower parts of the profile, silty clay. The soils of the pure beech plots had lower base saturation (BS), lower effective cation exchange capacity (CECe) and lower pH than the mixed species plots (Table 1) (Guckland et al. 2009).
The Shannon diversity index was used as a measure of diversity (Shannon and Weaver 1949). It is one of the most common measures for species diversity and depends not only on the number of species present in an ecosystem, but also on their relative abundance (Magurran 2004). In the study area, Tilia cordata and T. platyphyllos often hybridized. Hence, T. cordata and T. platyphyllos were considered as one species in the calculation of the Shannon index. The relative abundance of each species was based on the number of stems. Using the basal area as a measure for the relative abundance led to very similar values for the Shannon index. The average height of the tallest 20% of the trees was used (tree height: M. Jacob, pers. comm.) as the stand height. Canopy roughness was defined by the height difference between the mean height of the tallest 10% and smallest 10% of the trees. The leaf area index (LAI; leaf area in m2 m−2) of the study plots was based on leaf biomass (Jacob et al. 2010). Therefore, litter traps were placed next to the precipitation collectors (see “Rain water sampling and chemical analyses”) and emptied several times during autumnal leaf shedding. Leaves of all species were scanned and the leaf area was analyzed using WinFOLIA (Regent Instruments, Canada). Subsequently, all leaves were dried and weighed, and the specific leaf area (SLA) was calculated. The LAI was obtained by multiplying the stand leaf biomass of each species by the species-specific average of SLA (Jacob et al. 2010). The primary differences in the forest stand characteristics of the tree layer were found between the pure beech plots and the mixed species plots (Table 2). The characteristics of the mixed species plots of DL2 did not differ substantially from those of DL3.
Rain water sampling and chemical analyses
The following fractions of the rain water were sampled every two weeks: bulk precipitation, throughfall and stemflow. Bulk precipitation was sampled at four sites outside the forest, each with three continuously open precipitation collectors. At each site, the three collectors were placed in a triangle with a side length of 2 m. The sites were located about 50 m from the forest edge, and the distance to the selected forest plots ranged from 200 m to 1,200 m. For the chemical analyses, the water of the three precipitation collectors of each site was pooled, resulting in four replicate samples per date for the bulk precipitation. Throughfall was sampled with a total of 15 throughfall collectors on each forest plot. The collectors were located along three randomly selected 30 m long transects with five collectors along each transect. The collector positions along transects were defined randomly with a minimum distance of 4 m between collectors. For the chemical analyses, the water of the five throughfall collectors on each transect was pooled, resulting in three replicate samples per forest plot and date for the throughfall. The collectors used for bulk precipitation and throughfall sampling were placed at a height of 1 m and had a diameter of 10.5 cm. They were opaque to prevent the growth of algae. In the winter, snow was sampled with buckets. The diameter of the buckets was 25 cm, and the sampling design was the same as that used during the remainder of the year. The stemflow was sampled adjacent to the selected forest plots on a total of 50 trees representing the tree species and the diameter classes found on the forest plots. All stemflow samples were analyzed chemically. The stemflow volume per plot was calculated using species-specific regressions between the diameter at breast height (dbh) and stemflow volume per measuring period combined with stem number and dbh of the trees on the study plots. When there was no persistent correlation between dbh and stemflow for a certain species, an average value of the stemflow volume of all the measured trees of this species was calculated. The concentration of all investigated chemical compounds was multiplied by the water volume of each sample; this provided the quantity of each chemical compound per sample. For each tree species the median of this quantity was calculated and divided by the water volume per tree species, which determined the concentration of each chemical compound per tree species. This concentration was finally multiplied by the water volume per plot and upscaled to one hectare, which provided the stemflow quantity of each chemical compound per hectare (kg ha−1).
Bulk precipitation and throughfall were sampled manually every two weeks during a two-year period from July 2005 through June 2007. The stemflow was sampled manually every two weeks for a one-year period from July 2006 through June 2007. For further details on the water sampling see Krämer and Hölscher (2009).
The water samples were filtered through a Whatman 589/1 filter paper with a pore size of 12–25 μm and stored at 3 °C until the chemical analyses were performed. The ions Na+, K+, Ca2+, Mg2+, PO 3−4 , SO 2−4 , H+, Mn2+, Al3+ and Fe2+ were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES, Spectro, Kleve, Germany). Cl− was measured potentiometrically, and the N compounds, photometrically, both with an automated continuous-flow analyzer (Skalar Analytic GmbH, Breda, The Netherlands). Carbon (C) compounds were measured by an automated C analyzer (Shimadzu TOC-5050, Duisburg, Germany).
Canopy budget model
This section briefly describes the canopy budget model that we used for calculating interception deposition, total deposition and canopy exchange. For more detailed explanations see Ulrich (1983a; 1994) and Bredemeier (1988). The total atmospheric deposition (TD) of an ecosystem is the sum of the precipitation deposition (PD) and the interception deposition (ID); the latter can be gaseous (IDgas) or particulate (IDpart). We used continuously open collectors outside the forest to measure precipitation deposition. Hence, we did not measure wet-only, but bulk deposition (BD) Eq. 1.
Bulk deposition is the sum of wet deposition and particles that are deposited due to gravitation. In addition, gaseous deposition may also be sampled with continuously open collectors, depending on the duration of surface wetness of the collectors (Cape and Leith 2002). The stand deposition (SD) is the sum of the deposition with throughfall (TF) and stemflow (SF), which were both measured in the field Eq. 2.
Leaves, branches and stems can act as sinks or sources of ions for the water passing through the canopy (throughfall) and along the stem (stemflow). This canopy exchange (CE) can be described by the difference between total deposition (TD) and stand deposition (SD) Eq. 3.
The difference can either be positive, which means that the canopy acts as a sink and the ions are taken up by the canopy, or negative, which means that the canopy acts as a source and the ions are leached from the canopy. Several processes contribute to the sink function of the forest canopy: assimilation (NH +4 , NO −3 ) (Boynton 1954; Matzner 1986), cation exchange in the leaf tissue (exchange of H+ for Ca2+ and Mg2+) (Ulrich 1983b; Roelofs et al. 1985), storage of particles (Al3+, heavy metals) and precipitation of dissolved ions (Al3+, heavy metals) (Godt 1986). Other processes contribute to the source function of the forest canopy: leaching of ions from senescent leaves mainly in autumn (Na+, Mg2+, Ca2+, Cl−, SO 2−4 ) (Ulrich 1983a), leaching of ions throughout the growing season due to metabolic processes (K+, Mn2+) (Ulrich 1983a), cation exchange in the leaf tissue (exchange of K+, Ca2+, Mg2+ for H+ or NH +4 ) (Ulrich 1983a; b; Stachurski and Zimka 2002), simultaneous leaching of cations (K+, Ca2+, Mg2+) and weak acids (e.g., weak organic anions, bicarbonate) (Draaijers and Erisman 1995; Chiwa et al. 2004) and dissolution of undissolved matter in deposited particles (Al3+, heavy metals) (Mayer 1983). It is often assumed that Na+, Cl−, SO 2−4 and NO −3 are neither leached from nor taken up by the forest canopy (Lindberg et al. 1986; Matzner 1986; Ulrich 1994; Draaijers and Erisman 1995). However, other authors state that there is canopy leaching of Na+, Cl− and SO 2−4 and canopy uptake of NO −3 (Staelens et al. 2008). It may be assumed that leaching is negligible for those ions whose stand deposition to bulk deposition ratio is constant throughout the year. In the present study this was the case for Na+, but not for Cl−, SO 2−4 and NO −3 . Consequently, we set the canopy exchange of Na+ to zero and used it as a tracer ion for the calculation of particulate interception deposition of several other ions. One major assumption of the canopy budget model is that the interception rate depends on the precipitation deposition (in our case bulk deposition) and not on the ions. Because Na+ is not exchanged in the canopy and does not exist in gaseous form, the particulate interception deposition can be calculated directly from stand deposition and bulk deposition according to Eq. 3. The ratio of particulate interception deposition to bulk deposition for Na+ was then used to calculate particulate interception deposition of the following ions: Cl−, K+, Ca2+, Mg2+, PO 3−4 , SO 2−4 , H+, Mn2+, Al3+, Fe2+, NH +4 and NO −3 . The assumption that particles containing these ions are deposited with the same efficiency as particles containing Na+ might not be true (Draaijers et al. 1997). However, the error introduced by this assumption is probably the same for all study plots since they are located in one coherent forest area. Thus, it is possible to compare interception deposition and canopy exchange between the study plots. The ions Cl−, SO 2−4 , H+, NH +4 and NO −3 may also be deposited as gases. Since it was not possible to estimate the gas deposition, total deposition and canopy uptake of these ions might be underestimated and canopy leaching, overestimated.
The canopy leaching of K+, Ca2+ and Mg2+ is assumed to be accompanied by the uptake of H+ and NH +4 or the leaching of weak acids. Thus, the canopy exchange of H+ and NH +4 can be calculated by subtracting the leaching of K+, Ca2+ and Mg2+ from the leaching of weak acids (Draaijers and Erisman 1995; Staelens et al. 2008). The quantity of weak acids in bulk deposition and stand deposition can be calculated from the cation-anion balance (Draaijers and Erisman 1995; Staelens et al. 2008). To separate the uptake of H+ and NH +4 , a relative uptake efficiency factor is normally used. However, this factor is tree-species specific (Staelens et al. 2008) and has not been determined for beech or any of the other tree species present on the study plots. Consequently, we did not use this approach. De Vries et al. (2001) suggested a method for calculating the canopy uptake of NO −3 . Since this approach is dependent on the uptake of NH +4 and again an efficiency factor for the uptake of NH +4 vs. NO −3 has to be used, we considered this calculation to be too unreliable.
The acid input to the forest canopies was calculated as the sum of the total deposition of the cations H+, Mn2+, Al3+, Fe2+ and NH +4 (Ulrich 1994). The acid buffering capacity of the canopies was calculated as the sum of the positive canopy exchange, that is, the uptake of the cations H+, Mn2+, Al3+, Fe2+ and NH +4 (Ulrich 1994).
Data analyses and statistics
The concentrations of the chemical compounds of bulk precipitation, throughfall and stemflow were checked for outliers. These outliers were identified by very high C, N, P or K contents. In total 40 out of 1,060 data points (i.e., less than 4%) were removed from the dataset before doing any calculations. Since we had three pooled samples per study plot and date and we never had to remove all of them, the deletion of single data points still allowed the calculation of annual budgets.
The chemical analyses of the bulk precipitation samples revealed that the four sites outside the forest had a large variation in chemical composition among the sampling dates and the sites, but there were no consistent differences between the four sites. Hence, the sites were regarded as replicates for the bulk precipitation and mean values of the four replicate sites were calculated.
Mean values per sampling date were calculated for each forest plot using the three pooled throughfall samples per plot. The mean values of the three replicate forest plots at each diversity level were used for further calculations and statistical analyses because the forest plots are our true replicates. The mean values for each sampling date during the two year measurement period were summed up; this provided the values for the annual precipitation and throughfall fluxes. The mean value of the two annual fluxes was calculated to smooth annual fluctuations.
The stemflow data were also summed up to provide a one-year sum. To estimate the sum of the first year (July 2005 to June 2006), for which no stemflow data were available, the ratio of stemflow to throughfall for each plot and chemical compound of the second year (July 2006 to June 2007) was calculated and multiplied with the throughfall for each plot and chemical compound of the first year. Again, the mean value of the two annual fluxes was calculated to smooth annual fluctuations.
The two-year means of the throughfall and stemflow data were summed up to reveal the stand deposition. The two-year means of the bulk deposition (BD) and the stand deposition (SD) were used to calculate the interception deposition (ID), the total deposition (TD) and the canopy exchange (CE) of each plot and chemical compound as described above (see “Canopy budget model”).
The study objective was to evaluate differences in the interception deposition, total deposition, canopy exchange and stand deposition between the diversity levels and along the tree species diversity gradient. Therefore, the two-year means of the interception deposition, total deposition, canopy exchange and stand deposition of every chemical compound were used as dependent variables in an analysis of variance (ANOVA) with the diversity level as the explaining variable. Differences between the diversity levels were deemed to be significant if they exceeded the least significant difference, LSD, computed for every pair of diversity levels (p < 0.05). In addition, interception deposition, total deposition, canopy exchange and stand deposition on each plot and of each chemical compound were used in a multiple linear regression model with the Shannon index and the leaf area index (LAI) as explaining variables. The ANOVA with diversity level as explaining variable and the multiple linear regression model with Shannon index and LAI as explaining variables are different approaches toward elucidating the influence of the tree species composition on deposition and canopy exchange.
All statistical analyses were done with R version 2.7.2 (R Development Core Team 2007).
Results
Forest stand characteristics
The investigated forest plots differed in their stand characteristics (Table 2). Pure beech plots (i.e., lowest Shannon index) were taller and had rougher canopies than mixed species plots. Shannon index, stand height and canopy roughness correlated significantly with one another, whereas LAI only correlated with stand height (Table 3).
Since most of the stand characteristics were linearly correlated, the effects of single stand characteristics on the canopy deposition and exchange processes could not be isolated. We used the Shannon index as a measure for the correlated stand characteristics and the LAI as an additional stand characteristic in a multiple linear regression model to explain differences in interception deposition, canopy exchange and stand deposition.
Variations in ion fluxes
During the course of the year the ratio of stand deposition to bulk deposition was constant for Na+, whereas this ratio was much larger in summer and autumn than during the rest of the year for the ions K+, Ca2+, Mg2+ and to a lesser extent also for Cl−, PO 3−4 , SO 2−4 and NO −3 . This indicates that Na+ was not exchanged in the canopy, whereas the other ions were leached from the canopy during summer and autumn, which resulted in seasonal variations.
The quantity of stemflow was very low compared to the quantity of bulk precipitation and throughfall (0.4% to 4.9% of bulk precipitation) (Krämer and Hölscher 2009); hence, the ion fluxes were generally also very low. The stemflow fluxes of the ions were on average 3.7% of the throughfall fluxes, with H+ having the lowest percentage (0.2%) and Al3+ having the highest (17.6%). Stemflow fluxes did not differ between the diversity levels.
Deposition and canopy exchange
Total and interception deposition of all ions significantly decreased with increasing diversity level (ANOVA) and were negatively related to the Shannon index but not related to the LAI (multiple linear regression model; adjusted R 2 = 0.86, Shannon index: p < 0.001, LAI: p = 0.10). The interception deposition was calculated in the same manner for all ions. Hence, the differences in interception deposition between the diversity levels were the same for all ions. Since the total deposition is the sum of bulk deposition and interception deposition and the bulk deposition is the same for all diversity levels, the differences between the diversity levels for total deposition were the same as those for interception deposition.
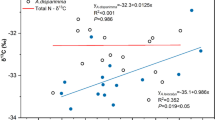
There was no indication of meaningful Na+ canopy exchange in the study plots. Most of the other ions were leached from the canopy (Table 4). Only NH +4 was taken up in all investigated forest plots, and H+ ions were taken up in the mixed species plots and leached in the pure beech plots. The results of the ANOVA showed that the canopy exchange rates of most of the ions were different in pure beech than in mixed species plots, only the exchange rates of Al3+, NH +4 , and NO −3 were comparable in all investigated forest plots. The canopy leaching of Cl−, K+, Ca2+, Mg2+, PO 3−4 , SO 2−4 , and Fe2+ increased with increasing diversity level, whereas the leaching of Mn2+ was largest in the pure beech plots. The multiple linear regression model revealed that the exchange rates of Mg2+, PO 3−4 , SO 2−4 , Al3+, Fe2+, NH +4 and NO −3 were not related to the Shannon index (Table 5). The leaching of Cl−, K+ and Ca2+ was positively related to it, and the leaching of Mn2+ was negatively related to the Shannon index (Table 5 and Fig. 2). No canopy exchange rate of any ion was related to the LAI.
According to the canopy exchange rates, the stand deposition of Na+, Cl−, K+, Ca2+, Mg2+, PO 3−4 , Fe2+ and Norg was larger in mixed species plots than in pure beech plots, whereas the stand deposition of H+, Mn2+ and NH +4 was largest in pure beech plots, and did not differ among the diversity levels for SO 2−4 , Al3+, NO −3 and Nt. Stand deposition was positively related to the Shannon index for K+ and Ca2+ and negatively related to it for Na+, H+, Mn2+, NH +4 , NO −3 and Nt (Table 5). The stand deposition of Cl−, Mg2+, PO 3−4 , SO 2−4 , Al3+, Fe2+ and Norg was not related to the Shannon index. Only the stand deposition of NO −3 was positively related to the LAI.
The acid input to the canopies of the study plots decreased with increasing diversity level (Fig. 3) and was negatively related to the Shannon index but not to the LAI (adjusted R 2 = 0.86, Shannon index: p < 0.001, LAI: p = 0.10). However, the acid buffering capacity of the canopies did not differ significantly between the diversity levels and was neither related to the Shannon index nor to the LAI (adjusted R 2 =−0.19, Shannon index: p = 0.44, LAI: p = 0.79).
Discussion
Comparison of deposition with other data
In Germany, the fluxes of Na+ and Cl− in precipitation decrease with increasing distance from the North Sea. This decrease is more pronounced in stand deposition than in bulk deposition, which indicates that interception of seaborne particles decreases rapidly with increasing distance from the shore (Bredemeier 1988). The molar ratio of Na+ to Cl− is 0.86 in sea water. In the studied forest plots it was 1.08 for bulk deposition and 0.56 to 0.74 for stand deposition. This agrees with other studies (Bredemeier 1988; Nordén 1991). Since Na+ was not meaningfully exchanged in the studied canopies (see next paragraph) and may be considered as solely derived from atmospheric sea salt deposition (Ulrich 1983a), the deviations of the measured ratios from the ratio in sea water have to be due to relatively more Cl− in the stand precipitation than in sea water. In addition, the ratio decreased with increasing diversity level. This can be explained by increasing canopy leaching rates for Cl− with increasing diversity level (see “Differences in interception deposition and canopy exchange between the diversity levels”).
The use of Na+ as a tracer ion for calculating particulate interception deposition of other ions requires that Na+ is not exchanged in the canopy. However, canopy leaching of Na+ during the short period of leaf emergence was reported (Staelens et al. 2007). If ions are exchanged in the canopy, seasonal differences in the ratio of stand deposition to bulk deposition can be observed. For Na+, this ratio differed among sampling dates but did not differ among seasons or plots. In contrast, the stand deposition to bulk deposition ratio differed seasonally for the other ions that are often assumed to undergo no reactions in the canopy: Cl−, SO 2−4 and NO −3 . Thus, we concluded that Na+ was not exchanged and, hence, can be used as a tracer ion in the canopy budget model. For the other ions, canopy exchange was assumed.
Compared with the total deposition of other German beech forests in the year 2002, the total deposition reported in the present study is approximately the same for K+ and 80% and 90% lower for Mg2+ and Ca2+, respectively (Meesenburg et al. 2009). The bulk and stand deposition of PO 3−4 were 80% to 95% higher in the investigated forest plots than in deciduous mixed forests in Southern Sweden (Nordén 1991). In contrast, the bulk and stand deposition of SO 2−4 were 80% to 90% lower in the investigated forest plots than in German beech forests in the 1980s (Bredemeier 1988). This agrees with the reduced sulphur deposition in Germany during the last several decades (Meesenburg et al. 1995; Ulrich et al. 2006). Even compared with more recent data of similar German beech forests, the sulphur deposition of the investigated forest plots is more than 90% lower (Meesenburg et al. 2009). This could be explained by the remote location of our forest plots. The stand and total depositions of H+ and Mn2+ were 80% to 90% lower, and those of NH +4 50% to 60% lower in our study than reported in Nordén (1991), Matzner and Meiwes (1994) and Meesenburg et al. (2009), which indicates a reduction of acid emissions and the remote location of the study site. The stand deposition of NO −3 was approximately the same as reported in Matzner and Meiwes (1994), and the total deposition, about 80% lower than reported in Meesenburg et al. (2009). Only small quantities of the total N deposited by throughfall were in organic form (8% to 10%), whereas Gaige et al. (2007) reported that organic N made up more than 80% of Nt in throughfall.
In summary, total and stand deposition of SO 2−4 , H+, Mn2+ and NH +4 reported in this paper were much lower than those reported in the above-mentioned studies, whereas total and stand deposition of K+, Ca2+ and Mg2+ were approximately the same as or also much lower than those reported in the above-mentioned studies. This agrees with the fact that the quantity of bulk precipitation of the investigated forest plots was either the same as or up to 35% lower than that reported in the other studies (Bredemeier 1988; Nordén 1991; Matzner and Meiwes 1994; Meesenburg et al. 2009), which is known to influence the deposition quantity of several ions (Croisé et al. 2005). In addition, not only the reduced emission of sulphur and to a lesser extent nitrogen during the last years, but also the remote location of the study area contributed to the low deposition of sulphur and nitrogen. The Federal Environmental Agency of Germany (UBA 2006) reported values of 4 μg NO2 per m3 and 0.9 μg SO2 per m3 for June 2006 at a relatively unpolluted location about 70 km away from the study site.
Differences in interception deposition and canopy exchange between the diversity levels
The stand deposition of the investigated chemical compounds differed between the diversity levels, with the exceptions of SO 2−4 , Al3+, NO −3 and Nt. Hence, the tree species composition may have influenced the nutrient input to the forest soil by altering the nutrient composition of rainfall while it was passing through the canopy (Potter et al. 1991; Draaijers et al. 1992; Lovett et al. 1996). Therefore, we will take a closer look at the processes in the canopy that may explain the observed differences in stand deposition among the forest plots: interception deposition and canopy exchange.
Interception deposition depends on the aerodynamic properties of the receiving surface (Erisman and Draaijers 2003). Properties determined by the canopy as a whole—such as canopy roughness, canopy length, canopy cover and LAI—influence the interception deposition, but also properties of individual canopy elements—such as the efficiency of leaves in capturing or absorbing gases and particles, or the surface wetness (Erisman and Draaijers 2003)—contribute to this. The pure beech plots were the tallest and had the roughest canopies of the study plots. This may be explained by the former management of the study plots and the tree species present. The beech plots were oldest and natural regeneration may have led to large height differences, which, in turn, result in their pronounced canopy roughness. The interception deposition of all ions was largest in the pure beech plots and negatively related to the Shannon index. The LAI did not explain any variation in the interception deposition. Since, with the exception of the LAI, the stand characteristics were correlated, it is not possible to isolate which factor contributed most to the differences in interception deposition among the study plots.
In summary, we can state that the interception deposition decreased along the investigated tree species diversity gradient from monospecific beech plots to tree species-rich plots. Ion deposition differed significantly between pure beech plots and mixed species plots, but did not differ significantly between the mixed species plots (DL2 and DL3). The same pattern was found for the stand characteristics (except for LAI); this may explain the differences in ion deposition along the investigated tree species diversity gradient. However, the differences in stand characteristics are probably not typical for other forests, which may for example have more pronounced canopy roughness in mixed than in single species stands.
The ions Cl−, SO 2−4 , H+, NH +4 and NO −3 may also originate from gas deposition. Because it was not possible to estimate the gas deposition, the total deposition and the canopy uptake of these ions might be underestimated and the canopy leaching, overestimated. For example, the canopy uptake of NH +4 is likely to be underestimated, whereas the canopy leaching of NO −3 , overestimated. Since the gaseous deposition is influenced by the stand characteristics, the actual differences in canopy exchange between the diversity levels might differ somewhat from the calculated differences. Hence, the interception and total deposition of these ions and also the calculated canopy exchange have to be interpreted with care.
Canopy exchange of ions can be due to passive diffusion between the water layer covering the leaves and the apoplast or due to ion exchange at cuticular exchange sites (Draaijers et al. 1994). Several factors affect these processes, some of which probably do not differ among the studied forest plots (foliar wax degradation, quantity and duration of precipitation and abiotic stresses), whereas others do. These factors are the wettability of foliage, which is found to differ considerably among tree species, tree physiology and possibly the age distribution of leaves (Draaijers et al. 1994). Hence, differences in the canopy exchange between the diversity levels may be due to differences in the above-mentioned factors among the tree species. In addition, the differences in canopy exchange may also arise from differences in soil properties, which are likely to affect the foliar ion status and canopy exchange (Nordén 1991).
Similar to the stand deposition, the canopy exchange rates of the investigated forest plots differed among the diversity levels (exceptions being Al3+, NH +4 and NO −3 ); for the ions Cl−, K+, Ca2+, H+ and Mn2+ canopy exchange rates were also related to the Shannon index. Most of the investigated ions were leached from the canopies; only NH +4 and in mixed species plots H+ ions were taken up by the canopy. Na+ was assumed to be neither leached from nor taken up by the trees. The leaching of Cl−, K+, Ca2+, Mg2+, PO 3−4 and SO 2−4 increased, whereas the leaching of the soil acidifying ions Mn2+ and H+ decreased with increasing diversity level. The pronounced leaching of Mn2+ in the pure beech plots is an indication that the soil properties of the plots were not the same. The mobility and plant availability of Mn2+ is greater under acidic soil conditions (Tyler 1976). If the concentration of soluble Mn2+ increases in the soil water, the tree roots probably take up more Mn2+, leading to higher leaf contents and canopy leaching rates. The soils of pure beech plots were indeed more acidic than those of the mixed species plots (Guckland et al. 2009), and the quantity of Mn2+ in both the soil (Guckland et al. 2009) and the soil solution (unpublished data) was highest in pure beech plots.
The different soil properties may also explain the differences in leaching of K+, Ca2+ and Mg2+. The soils of pure beech plots have lower effective cation exchange capacity and base saturation than those of mixed species plots (see Table 1) (Guckland et al. 2009). Hence, the trees can take up more K+, Ca2+ and Mg2+ in mixed species plots than in pure beech plots, and this, in turn, results in higher leaf concentrations of these ions (Jacob 2009) and in higher leaching rates (Nordén 1991). Similarly, the higher leaching rates of PO 3−4 in the mixed species than in the pure beech plots may be explained by the different soil properties. Indeed, the mixed species plots have larger soil phosphorus pools (Talkner et al. 2009). The canopy leaching of PO 3−4 made up 25% (DL1) to 67% (DL3) of the stand deposition. Due to negligible amounts of phosphorus in the ambient air and precipitation, canopy leaching can contribute up to 90% of PO 3−4 in stand deposition (Parker 1983).
Differences in soil properties probably influenced the previous management practices, which led to pure beech stands in areas with low BS, CECe and pH. This in turn resulted in a further decrease in pH and possibly also in BS and CECe in the pure beech stands due to the soil acidifying properties of beech (Nordén 1994; Finzi et al. 1998; Neirynck et al. 2000; Hagen-Thorn et al. 2004) and the lower decay rates of beech litter compared to ash and lime litter (Melillo et al. 1982). The results of Guckland et al. (2009) suggest that species-related differences in the intensity of the cation cycling between soil and tree contributed to the observed differences in soil acidification and BS among the studied forest plots. Hence, the tree species may have contributed to the differences in soil properties.
Differences in the physiology and ion status among the tree species may account for the observed differences in canopy exchange rates. Krauß and Heinsdorf (2005) showed that tree species differ in their leaf ion concentrations, independently of the soil properties. The investigated beech trees tended to have lower concentrations of K+, Ca2+, Mg2+ and PO 3−4 -P in their green leaves than the other deciduous tree species studied (Jacob 2009). This may have contributed to the lower canopy leaching rates of these ions in the pure beech plots. It is important to mention that the concentrations of Ca2+, Mg2+ and Nt in the green leaves of the investigated beech trees did not differ between the diversity levels, and that the concentration of K+ was lowest in DL2 plots and that of PO 3−4 -P highest in pure beech plots (Jacob 2009). Hence, the differences in soil properties are not reflected by the ion concentrations in green leaves in beeches. This is a confirmation of the species-specific differences in ion concentrations found by Krauß and Heinsdorf (2005) and emphasizes that the soil properties do not solely influence the ion status of trees. In addition, in a common garden test with 14 tree species including T. cordata, A. pseudoplatanus, A. platanoides, F. sylvatica and C. betulus, tree species influenced soil properties directly through variation in the quantity and chemistry of their litter and indirectly through the effect of their litter on detritivores (Reich et al. 2005). These effects led to rapid (within three decades) and widespread changes of soil properties beneath the different tree species (Reich et al. 2005).
The calculated acid input to the canopy was highest in pure beech plots, whereas the acid buffering capacity of the canopy did not differ between the diversity levels. Hence, the higher base saturation of the soils and the additional tree species in the mixed species plots did not lead to a higher canopy buffering capacity than in the pure beech plots. It is important to bear in mind that H+ buffering in the canopy removes acidity from stand precipitation, but does not decrease the total H+ input into the soil. This is because buffering in the canopy occurs by cation exchange from inner leaf tissue surfaces and these cation exchange buffer sites are recharged by cations taken up by the roots through exchange with protons in the rhizosphere of the soil (Ulrich 1983b).
In summary, we can state that the leaching of the cations K+, Ca2+, Mg2+ and the anions Cl−, PO 3−4 and SO 2−4 increased along the investigated tree species diversity gradient from monospecific beech plots to tree species-rich plots. The leaching of these ions not only differed between pure beech and mixed species plots but also between the mixed species plots (DL2 and DL3). In contrast, the canopy leaching of the soil acidifying ions Mn2+ and H+ decreased along the investigated tree species diversity gradient from monospecific beech plots to tree species-rich plots (with H+ being taken up in mixed species plots). In this case, the difference was most pronounced between pure beech plots and mixed species plots, while mixed species plots did not differ. Pure beech and mixed species plots differed significantly in soil properties, whilst the mixed species plots (DL2 and DL3) had similar soil properties. Thus, the canopy exchange of Mn2+ and H+ may mainly have been influenced by the differences in soil properties, whereas the leaching of K+, Ca2+, Mg2+, Cl−, PO 3−4 and SO 2−4 may also have been influenced by the physiology and ion status of the tree species present in the investigated forest plots.
Conclusion
The results of this observational study showed that atmospheric deposition was influenced by the stand composition (i.e., Shannon index). However, it was not possible to isolate the role of single stand characteristics such as stand height and canopy roughness, since they were closely correlated. One can assume that this is normally the case in most natural forest ecosystems. Canopy exchange processes were influenced by differences in ion status between the tree species as well as soil properties. Soil properties that influence canopy exchange processes are pH, cation exchange capacity and base saturation. Trees influence these properties and beech trees in particular can lower pH, base saturation, and cation exchange capacity. Hence, in addition to their direct influence on deposition and canopy exchange processes, the tree species also have an indirect effect by altering the above-mentioned soil properties, which, in turn, affects the uptake of ions by the trees and the subsequent canopy exchange.
References
André F, Jonard M, Ponette Q (2008) Spatial and temporal patterns of throughfall chemistry within a temperate mixed oak-beech stand. Sci Total Environ 397:215–228
Baumgarten M, von Teuffel K (2005) Nachhaltige Waldwirtschaft in Deutschland. In: von Teuffel K et al (eds) Waldumbau. Springer, Berlin, pp 1–10
Beier C, Gundersen P, Rasmussen L (1992) A new method for estimation of dry deposition of particles based on throughfall measurements. Atmos Environ 26a:1553–1559
Berger TW, Untersteiner H, Schume H, Jost G (2008) Throughfall fluxes in a secondary spruce (Picea abies), a beech (Fagus sylvatica) and a mixed spruce-beech stand. For Ecol Manage 255:605–618
Boynton D (1954) Nutrition by foliar application. Annu Rev Plant Physiol 5:31–54
Bredemeier M (1988) Forest canopy transformation of atmospheric deposition. Water Air Soil Pollut 40:121–138
Cape JN, Leith ID (2002) The contribution of dry deposited ammonia and sulphur dioxide to the composition of precipitation from continuously open gauges. Atmos Environ 36:5983–5992
Chiwa M, Crossley A, Sheppard LJ, Sakugawa H, Cape JN (2004) Throughfall chemistry and canopy interactions in a Sitka spruce plantation sprayed with six different simulated polluted mist treatments. Environ Pollut 127:57–64
Croisé L, Ulrich E, Duplat P, Jaquet O (2005) Two independent methods for mapping bulk deposition in France. Atmos Environ 39:3923–3941
de Vries W et al (2001) Intensive monitoring of forest ecosystems in Europe. Technical report 2000, UN/ECE, EC, Forest Intensive Monitoring Coordinate Institute, Heerenveen
Draaijers GPJ, Erisman JW (1995) A canopy budget model to assess atmospheric deposition from throughfall measurements. Water Air Soil Pollut 85:2253–2258
Draaijers GPJ, van Ek R, Meijers R (1992) Research on the impact of forest stand structure on atmospheric deposition. Environ Pollut 75:243–249
Draaijers GPJ, Erisman JW, van Leeuwen NFM, Römer FG, te Winkel BH, Vermeulen AT, Wyers GP, Hansen K (1994) A comparison of methods to estimate canopy exchange at the Speulder Forest. National Institute of Public Health and Environmental Protection, Bilthoven, Report No. 722108004
Draaijers GPJ, Erisman JW, van Leeuwen NFM, Römer FG, te Winkel BH, Veltkamp AC, Vermeulen AT, Wyers GP (1997) The impact of canopy exchange on differences observed between atmospheric deposition and throughfall fluxes. Atmos Environ 31:387–397
Elling W, Heber U, Polle A, Beese F (2007) Schädigung von Waldökosystemen. Elsevier, München
Erisman JW, Draaijers G (2003) Deposition to forests in Europe: Most important factors influencing dry deposition and models used for generalization. Environ Pollut 124:379–388
FAO (1998) FAO, ISRIC, ISSS World reference base for soil resources. World Soil Resources Reports 84, Rome
Finzi AC, Canham CD, van Breemen N (1998) Canopy tree-soil interactions within temperate forests: Species effects on pH and cations. Ecol Appl 8:447–454
Gaige E, Dail D, Hollinger D, Davidson E, Fernandez I, Sievering H, White A, Halteman W (2007) Changes in canopy processes following whole-forest canopy nitrogen fertilization of a mature spruce-hemlock forest. Ecosystems 10:1133–1147
Georgii HW (1986) Atmospheric pollutants in forest areas. D Reidel Publishing Company, Dordrecht
Godt J (1986) Untersuchung von Prozessen im Kronenraum von Waldökosystemen. Berichte des Forschungszentrums Waldökosysteme/Waldsterben, Band 17, Göttingen
Guckland A, Jacob M, Flessa H, Thomas FM, Leuschner C (2009) Acidity, nutrient stocks and organic matter content in soils of a temperate deciduous forest with different abundance of European beech (Fagus sylvatica L.). J Plant Nutr Soil Sci 172:500–511
Hagen-Thorn A, Callesen I, Armolaitis K, Nihlgård B (2004) The impact of six European tree species on the chemistry of mineral topsoil in forest plantations on former agricultural land. For Ecol Manage 195:373–384
Jacob M (2009) Productivity and nutrient relations of trees in deciduous forests with contrasting tree species diversity. University of Goettingen, Dissertation
Jacob M, Leuschner C, Thomas FM (2010) Productivity of temperate broad-leaved forest stands differing in tree species diversity. Ann For Sci 67:503
Johnson DW, Lindberg SE (1992) Atmospheric deposition and forest nutrient cycling. Ecol Stud 91, Springer, New York
Knoke T, Stimm B, Ammer C, Moog M (2005) Mixed forests reconsidered: A forest economics contribution on an ecological concept. For Ecol Manage 213:102–116
Krämer I, Hölscher D (2009) Rainfall partitioning along a tree diversity gradient in a deciduous old-growth forest in Central Germany. Ecohydrol 2:102–114
Krauß HH, Heinsdorf D (2005) Ernährungsstufen für wichtige Wirtschaftsbaumarten. Beitr Forstwirtsch Landsch ökol 39:172–179
Leuschner C, Jungkunst HF, Fleck S (2009) Functional role of forest diversity: pros and cons of synthetic stands and across-site comparisons in established forests. Basic Appl Ecol 10:1–9
Lindberg SE, Lovett GM, Richter DD, Johnson DW (1986) Atmospheric deposition and canopy interactions of major ions in a forest. Science 231:141–145
Lovett GM, Lindberg SE (1984) Dry deposition and canopy exchange in a mixed oak forest as determined by analysis of throughfall. J Appl Ecol 21:1013–1027
Lovett GM, Nolan SS, Driscoll CT, Fahey TJ (1996) Factors regulating throughfall flux in a New Hampshire forested landscape. Can J For Res 26:2134–2144
Magurran AE (2004) Measuring biological diversity. Blackwell Science, Oxford
Matzner E (1986) Deposition/Canopy-interactions in two forest ecosystems of northwest Germany. In: Georgii HW (ed) Atmospheric Pollutants in Forest Areas. D Reidel Publishing Company, Dordrecht, pp 247–262
Matzner E, Meiwes KJ (1994) Atmospheric pollutants—Long-term development of element fluxes with bulk precipitation and throughfall in two German forests. J Environ Qual 23:162–166
Mayer R (1983) Interaction of forest canopies with atmospheric constituents: Aluminum and heavy metals. In: Ulrich B, Pankrath J (eds) Effects of accumulation of air pollutants in forest ecosystems. D Reidel Publishing Company, Dordrecht, pp 47–55
Meesenburg H, Meiwes KJ, Rademacher P (1995) Long term trends in atmospheric deposition and seepage output in northwest German forest ecosystems. Water Air Soil Pollut 85:611–616
Meesenburg H, Eichhorn J, Meiwes KJ (2009) Atmospheric deposition and canopy interaction. In: Brumme R, Khanna PK (eds) Functioning and management of European beech ecosystems. Springer, New York
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Neirynck J, Mirtcheva S, Sioen G, Lust N (2000) Impact of Tilia platyphyllos Scop., Fraxinus excelsior L., Acer pseudoplatanus L., Quercus robur L. and Fagus sylvatica L. on earthworm biomass and physico-chemical properties of a loamy topsoil. For Ecol Manage 133:275–286
Nordén U (1991) Acid deposition and throughfall fluxes of elements as related to tree species in deciduous forests of South Sweden. Water Air Soil Pollut 60:209–230
Nordén U (1994) Influence of tree species on acidification and mineral pools in deciduous forest soils of south Sweden. Water Air Soil Pollut 76:363–381
Parker GG (1983) Throughfall and stemflow in the forest nutrient cycle. Adv Ecol Res 13:57–133
Potter CS, Ragsdale HL, Swank WT (1991) Atmospheric deposition and foliar leaching in a regenerating southern appalachian forest canopy. J Ecol 79:97–115
R Development Core Team (2007) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Reich PB, Oleksyn J, Modrzynski J, Mrozinski P, Hobbie SE, Eissenstat DM, Chorover J, Chadwick OA, Hale CM, Tjoelker MG (2005) Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol Lett 8:811–818
Roelofs JGM, Kempers AJ, Houdijk ALFM, Jansen J (1985) The effect of air-borne ammonium sulphate on Pinus nigra var. maritima in the Netherlands. Plant Soil 84:45–56
Schmidt I, Leuschner C, Mölder A, Schmidt W (2009) Structure and composition of the seed bank in monospecific and tree species-rich temperate broad-leaved forests. For Ecol Manage 257:695–702
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Stachurski A, Zimka JR (2002) Atmospheric deposition and ionic interactions within a beech canopy in the Karkonosze Mountains. Environ Pollut 118:75–87
Staelens J, de Schrijver A, Verheyen K (2007) Seasonal variation in throughfall and stemflow chemistry beneath a European beech (Fagus sylvatica L.) tree in relation to canopy phenology. Can J For Res 37:1359–1372
Staelens J, Houle D, de Schrijver A, Neirynck J, Verheyen K (2008) Calculating dry deposition and canopy exchange with the canopy budget model: Review of assumptions and application to two deciduous forests. Water Air Soil Pollut 191:149–169
Swank WT (1984) Atmospheric contributions to forest nutrient cycling. Water Resour Bull 20:313–321
Talkner U, Jansen M, Beese FO (2009) Soil phosphorus status and turnover in central-European beech forest ecosystems with differing tree species diversity. Eur J Soil Sci 60:338–346
Tyler G (1976) Soil factors controlling metal ion absorption in the wood anemone Anemone nemorosa. Oikos 27:71–80
UBA (Umweltbundesamt) (2006) Konzentrationen von SO2, NO2, O3 und PM10 aus dem Luftmessnetz des Umweltbundesamtes. UBA, Dessau-Roßlau. http://www.umweltbundesamt.de/luft/luftmessnetze/mb2006/mb0606.pdf, last visited: 02.03.2010
Ulrich B (1983a) Interaction of forest canopies with atmospheric constituents: SO2, Alkali and earth alkali cations and chloride. In: Pankrath J (ed) B Ulrich. Effects of accumulation of air pollutants in forest ecosystems. D Reidel Publishing Company, Dordrecht, pp 33–45
Ulrich B (1983b) A concept of forest ecosystem stability and of acid deposition as driving force for destabilization. In: Ulrich B, Pankrath J (eds) Effects of accumulation of air pollutants in forest ecosystems. D Reidel Publishing Company, Dordrecht, pp 1–29
Ulrich B (1994) Nutrient and acid-base budget of central European forest ecosystems. In: Godbold DL, Hüttermann A (eds) Effects of acid rain on forest processes. Wiley-Liss, pp 1–50
Ulrich B, Pankrath J (1983) Effects of accumulation of air pollutants in forest ecosystems. D Reidel Publishing Company, Dordrecht
Ulrich KU, Paul L, Meybohm A (2006) Response of drinking-water reservoir ecosystems to decreased acidic atmospheric deposition in SE Germany: Trends of chemical reversal. Environ Pollut 141:42–53
Wulf M (2003) Preference of plant species for woodlands with differing habitat continuities. Flora 198:444–460
Acknowledgement
This study was funded by the German Research Council (DFG) within the Research Training Group 1086 (‘The role of biodiversity for biogeochemical cycles and biotic interactions in temperate deciduous forests’). We thank our colleagues from the Research Training Group for providing us with stand characteristic data, Birgit Köhler for reviewing the manuscript, two anonymous reviewers for their valuable comments and suggestions and Ingrid Rosenfelder for improving the language of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Erik A. Hobbie.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Talkner, U., Krämer, I., Hölscher, D. et al. Deposition and canopy exchange processes in central-German beech forests differing in tree species diversity. Plant Soil 336, 405–420 (2010). https://doi.org/10.1007/s11104-010-0491-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0491-2