Abstract
Objective
Childhood hydrocephalus patients treated by ventriculo-peritoneal (v.-p.) shunting are sometimes referred years after this therapy for evaluation of suspicious pituitary enlargement. Since pituitary size has been shown to depend on cerebrospinal fluid (CSF) pressure, we assume this phenomenon to be caused by shunt overdrainage. Therefore, we studied pituitary size and morphology in shunted hydrocephalus patients with radiological signs of high CSF drainage.
Patients and methods
Retrospective study of pituitary size and morphology in 15 shunted patients with non-tumoral hydrocephalus and 7 shunted hydrocephalus patients due to childhood brain tumor compared to a population mean. In five brain tumor patients also pre- and postsurgical comparisons were performed.
Results
Pituitary mid-sagittal size and pituitary volume were significantly higher in both hydrocephalus groups, compared to the population mean (midsagittal size t = 5.91; p < 0.001; pituitary volume, t = 3.03; p = 0.006). In patients available for pre- and postoperative comparison, there was also a significant increase in pituitary size and volume postoperatively (mean preoperative midsagittal height 2.54 ± 1.0 mm vs. 6.6 ± 0.7 mm post-surgery; mean pre-operative pituitary volume 120.5 ± 69.2 mm3 vs. 368.9 ± 57.9 mm3 post-surgery).
Conclusion
Our results confirmed a significant increase in pituitary size and volume, mimicking pituitary pathology, after v.-p. shunt insertion. This phenomenon can be explained by the Monro–Kellie doctrine, stating that intracranial depletion of CSF—as caused by v.p. shunting—leads to compensatory intracranial hyperemia, especially in the venous system, with the consequence of engorged venous sinuses, most likely responsible for enlargement of the pituitary gland.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The U.S. American neurosurgeon Harvey Cushing is well known for his meticulous research and treatment in the field of pituitary pathologies. It is less well known, that he also made important contributions to the treatment of congenital and childhood-acquired hydrocephalus, a condition known since antiquity and, until some decades ago, associated with a dismal prognosis. He was the first to note, that cerebrospinal fluid (CSF) was produced by the choroid plexus [1] and proposed a variety of methods for temporary and permanent CSF drainage in his young hydrocephalic patients [2] However, up to the 1950s, all developed shunt systems were hampered by a high failure rate, mostly due to insufficient implant materials [3]. With the combined invention of artificial valves and silicone for shunt catheters around 1960, a worldwide therapeutic breakthrough in hydrocephalus treatment was achieved [3]. Many patients with insufficient CSF drainage were all of a sudden enabled to survive this formally lethal condition and lead independent lives, albeit at the cost of a myriad of complications such as shunt infection or disconnection [4]. Moreover, especially before the advent of programmable valves, many of the implanted shunt systems led to CSF overdrainage, a condition that can remain clinically asymptomatic or become apparent by orthostatic headache, pain or stiffness of the neck, nausea, diplopia, or, at worst, the development of subdural hematomas [5].
Working in a large neurosurgical department, we made the experience on several occasions that patients with hydrocephalus treated by ventriculo-peritoneal (v.-p.) shunting in childhood or young adulthood were referred years later to our department for neurosurgical evaluation of suspected pituitary pathology such as pituitary adenoma. However, we found this tentative diagnosis, usually raised first by the radiologists, who evaluated MRI images of these shunted patients, not to be accompanied by any other clinical, biochemical or radiological signs (apart from pituitary enlargement) supporting the diagnosis of pituitary neoplasia. We, therefore, assumed the diagnosis of adenoma or other pituitary pathology to be a diagnostic pitfall, supported by the observation that the pituitary gland has been shown to change in size in different states of CSF pressure [6]. In order to look into this matter more systematically, we conducted a retrospective, explorative study of pituitary size and morphology in patients with hydrocephalus and radiological markers of high CSF drainage treated after birth or in childhood by v.-p. shunting.
Method
We performed an exploratory search of the magnetic resonance imaging (MRI) report database of the Institute of Radiology and Neuroradiology of the University Hospital Essen for keywords relating to shunt insertion and radiological markers of high CSF drainage such as v.-p. shunt, slit ventricles, and/or (pachy)meningeal Gadolinium enhancement [7] for a 10 years’ time period from 2012 to 2022. Of the retrieved radiology reports, the majority related to multiple investigations of a limited number of patients. The respective imaging investigations of the individual patients were screened and those with limited interpretation capacity of the pituitary gland were discarded. Measurement of pituitary gland size was performed on mid-sagittal and coronal images, using always the greatest extension in the respective plane. Pituitary volume was estimated using the formula: V = antero-posterior dimension × craniocaudal dimension × transverse dimension × 0.52 as described in [8]. Clinically relevant data of the patients were extracted from chart records.
In five brain tumor patients, in whom repeated imaging investigations before and after shunt insertion were available, we measured the size of the pituitary before, shortly after and at least 1 year after shunt insertion. In those patients who received their v.-p. shunt shortly after birth, mostly only individual follow-up MRIs were achieved in the database which were used for measurement.
Statistics
All statistical analyses were conducted with SPSS 27. Descriptive statistics are presented as mean, standard error of mean (SEM) and range. For comparative analyses, the normal distribution of data was controlled for with the Shapiro–Wilk test. Independent t-test were used to compare the patients’ pituitary measures to an averaged age-matched population mean, which we calculated from [8] by averaging the pituitary measurements available for different age ranges based on the number of patients in the respective age ranges. Non-normally distributed data were compared with Mann–Whitney U tests.
Results
Patient characteristics
A total of 20 patients could be identified by our radiology report search. Two further patients fulfilling those criteria were identified during a routine visit of the neurosurgical outpatient department (both referred for suspected pituitary adenoma), resulting in a total of 22 patients (15 female, 7 male; mean age 22.3 ± 2.3 years) available for further investigation. They were divided into two patient groups: The first comprised 8 female and 7 male non-tumoral hydrocephalus patients (called non-tumor hydrocephalus group; NTHG). The diagnoses leading to shunt insertion were postmenigitic (n = 1) or posthemorrhagic hydrocephalus after premature birth (n = 5), hydrocephalus due to meningomyelocele with or without Chiari malformation (n = 5), other connatal malformations with hydrocephalus (n = 3) and suspected aqueductal stenosis with ventricular enlargement in one patient. Their age at shunt insertion was about 0 years (= shunt insertion in the first weeks after birth) except for two patients, who received their shunts at age of 8 and 21 years. Only one of the NTHG patients was implanted with an adjustable shunt valve, the other patients had received either medium pressure (n = 10) or high pressure (n = 1) valves. In three patients the valve type was not clearly identifiable on lateral radiographs. Theses valves had been implanted, however, before the era of programmable valves.
The second patient group was made up of a total of 7 (6 female, 1 male) patients with occlusive hydrocephalus due to childhood brain tumor (4 pilocytic astrocytomas and 3 medulloblastomas), here called tumor hydrocephalus group (THG). Their mean age of shunt insertion was 9.3 years (range 2–15 years). In all of these patients, non-adjustable medium pressure valves had been implanted. In five of the childhood cancer survivors (CCS) in the THG, endocrinological work-ups had been performed as part of childhood oncology surveillance protocols, in the remaining two endocrinological assessment was missing. Only one CCS patient developed post treatment hypopituitarism during the follow-up period, necessitating thyreotrophic and somatotrophic hormone replacement. In one of the NTHG patients, endocrinological investigation was prompted by significant pituitary enlargement following shunt insertion with unremarkable results. Apart of the CCS patient with partial hypopituitarism, none of the other patients in the THG received any hormone replacement or had clinical evidence of hormonal dysfunction.
Four of the investigated patients had clinical signs of CFS overdrainage, which manifested in all cases as orthostatic headache.
Pituitary morphology, size and volume
Across all investigated patients, pituitary surface was convex in 16 (10 in the NTHG and 6 in the THG) and planar in six (5 in the NTHG and 1 in the THG). In none of the patients, indirect signs of pituitary adenoma such as a deviation of the pituitary stalk or bony enlargement/arrosion of bony sella and adjacent bone structures were present. In the NTHG, midsagittal pituitary height was 8.9 ± 1.7 mm (range 6.3–12.6 mm) and pituitary volume 460.3 ± 133.4 mm3 (range 260.8–644.0 mm3). In the THG patients, postsurgical pituitary midsagittal height and volume amounted to 8.2 ± 0.9 mm, range 6.1–13.2 mm and 411.5 ± 55.2 mm3, range 231.8–679.5 mm3, respectively. Pituitary size and volume did not statistically differ between the NTHG and the THG (all Z ≤ − 1.48, all p ≥ 0.138) (see Table 1).
Moreover, in five patients in the THG, pre- and postoperative MRIs could be analyzed. In this small subgroup, pituitary surface was concave before brain tumor treatment and shunt implantation, but planar or convex in the follow-up MRIs. Pituitary measurements preoperatively were 2.54 ± 1.0 mm, range 1.4–4.2 mm (pre-operative midsagittal height) and 120.5 ± 69.2 mm3, range 82.3–230.7 mm3 (pre-operative pituitary volume) as compared to 6.6 ± 0.7 mm, range 5.8–7.7 mm (post-operative midsagittal height) and 368.9 ± 57.9 mm3, range 329.5–465.7 mm3 (post-operative pituitary volume) 1 year after surgery. This difference was statistically significant (Z = − 2.02, p = 0.043) (see Table 2).
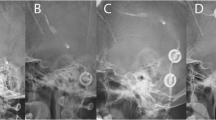
The two remaining CSS were not included in this comparison, as only postoperative MRIs after shunt insertion were available. Figure 1 shows the course of pituitary size in a THG patient before shunting, immediately postoperatively and after 1 and 4 years.
Time course of pituitary size in a child with a medulloblastoma: a preoperatively: note the partial empty sella due to increased intracranial pressure caused by obstructive hydrocephalus. b One day after tumor removal: note the mild increase in pituitary size despite the suboptimal scan quality due to movement artefacts. c 10 months after shunt insertion: note the development of convex pituitary surface. d 3.5 years after shunt insertion: note the further change in the height of the pituitary and slight change in sellar floor morphology
Since pituitary size and volume did not statistically differ between the NTHG and the THG, the comparison with the published age-matched healthy population mean was performed with the entire patient cohort (mean midsagittal height 8.5 ± 0.3 mm, range 6.1–13.2 mm, mean pituitary volume 444.8 ± 29.0 mm3, range 231.8–679.5 mm3). As expected, pituitary height (t = 5.91; p < 0.001) and volume (t = 3.03; p = 0.006) in the shunted hydrocephalus patients differed (highly) significantly to the averaged population mean [8]. Mean pituitary height and volume of those patients with clinical signs of CSF overdrainage did not differ significantly from those without (all Z ≤ − 1.56, all p ≥ 0.119). Figure 2 shows the change of pituitary morphology in a NTHG patient, while Fig. 3 illustrates the changed sellar floor morphology and pituitary hyperemia.
Change in pituitary morphology before and after shunt insertion in a patient with triventricular hydrocephalus of unknown etiology. a–c Note the change in ventricular and pituitary size before and more than 10 years after shunt insertion. On image b you can see the slight deformation of the optic chiasm caused by pituitary enlargement, while image c also shows a change in sellar floor morphology as compared to a
CT scan of the same patient as in Fig. 2. Note the pituitary hyperemia as illustrated by the slightly hyperdense aspect of the gland on CT scan (red arrow) and the engorgement of the cavernous sinus (blue arrow)
Discussion
Our exploratory analysis confirmed our hypothesis of significant increase in pituitary size and volume, mimicking pituitary pathology, after v.-p. shunt insertion in children and adolescents with radiological signs of shunt overdrainage in comparison to the published population mean. In a small subgroup of brain tumor patients shunted for obstructive hydrocephalus, we could also demonstrate a change in pituitary morphology changing from a concave surface before treatment to planar or convex thereafter.
Up to now, pituitary hyperplasia occurring in the wake of v.-p. shunt insertion has been described infrequently in the literature. One Finnish group reported an increased pituitary size in children and adolescents with shunted hydrocephalus as compared to age and sex-matched normal controls which was associated with enhanced gonadotropin secretion [9]. However, based on this association and the observation of accelerated pubertal development in patients with shunted hydrocephalus [10], they postulated pituitary hyperplasia to be caused by “central stimulation, possibly hypothalamic in origin”, but did not provide any further explanations for this effect. On the contrary, other studies reported precocious puberty and amenorrhea to be the consequence of increased intracranial pressure in chronic untreated hydrocephalus and that CSF shunting led to a normalization of gonadotroph axis function (for an overview see [11]). A further argument speaking against increased gonadotropin secretion to be the cause of increased pituitary size in shunted patients can be found in the results published by van Beek et al. in 2000 [12], showing that no significant change occurred in any pituitary size or shape parameter following gondadotropin releasing hormone analogue therapy.
In another case study, highlighting the potential effects of chronic CSF overdrainage on skull base structures, the authors postulated that their patient’s pituitary was not truly enlarged, but that such an impression was given as the consequence of overdrainage-induced shrinkage of the sella turcica and subsequent upward extrusion of the pituitary gland [13]. Yet our results show, that a true and significant increase of pituitary size and volume occurs after v.-p. shunt insertion, while acknowledging from own unpublished observations that bony changes of the sella may additionally occur in patients with long-standing shunt overdrainage (for examples confer to Figs. 1 and 2).
In adults, pituitary hyperplasia has frequently been described in spontaneous intracranial hypotension (SIH), a condition characterized by a loss of CSF due to leakage through the dural membrane, i.e., at the level of the cervicothoracic spine, and classically accompanied by severe orthostatic headache [14, 15]. According to a recent study, this phenomenon of pituitary enlargement constitutes the most frequent early radiological sign of SIH, present in 97.6% of 42 investigated patients 1–6 days after symptom onset [16]. On the other end of the spectrum, a flattened pituitary (empty sella or partial empty sella) is recognized as one diagnostic marker in patients with benign intracranial hypertension, a condition of increased CSF production and consecutively elevated ICP, which can lead to chronic headaches and visual loss [17].
The altered size of the pituitary in relation to CSF pressure is best explained by the so-called Monro–Kellie doctrine, a hypothesis stating that the sum of the volumes of the brain, CSF and intracranial blood content is always constant due to their encasement by the rigid skull [18, 19]. An increase in one of the volumes should, thus be followed by a decrease in one or both of the remaining two. Since the brain volume remains nearly constant in states of decreased CSF volume, a compensatory intracranial hyperemia occurs, primarily in the venous system, as reflected by engorgement of venous sinuses and diffuse venous meningeal hyperemia [19]. In terms of the pituitary, this means that any drop in CSF—be it caused by shunting or spontaneous loss as in SIH—should incur an increase of blood volume in the ample, widely anastomosed arterial and sinusoidal venous blood supply of this gland [20] with the consequence of pituitary enlargement. On the other hand, an increase of ICP mediated by disturbance of CSF resorption or outflow, as in malresorptive or occlusive hydrocephalus, should lead to a decrease in the blood supply of the pituitary, making the soft, endocrine tissue more vulnerable for compression.
In sum, we showed a significant pituitary enlargement after v.-p. shunt insertion in children and adolescents in comparison to the age-related population mean. Together with the findings published in the literature we believe this enlargement to be primarily mediated by the drop in intracranial pressure after shunting, as exemplified by the radiological marker of slit ventricles. However, since this was a retrospective analysis with some patients lost to follow-up, endocrinological assessment to definitely rule out end organ failure or increased gonadotropin secretion as alternative explanations for the observed pituitary enlargement was not available in all patients. There was, however, no clinical evidence of severe untreated hypothyroidism in any of the investigated patients, that would have explained the pituitary hyperplasia that occurred in temporal relation with shunt insertion.
Next to the retrospective design and the predefined inclusion criterion of presence of slit ventricles as radiological markers of shunt overdrainage, an additional drawback of the present study is the relatively small sample size, which necessitated the calculation of normative estimates to explore pituitary parameter differences in comparison to the normal population and limits the generalizability of the present results. Future studies with larger patient samples and a prospective design, allowing for pre-post shunt comparisons and a stratification by clinical (i.e., age of shunt insertion, duration of treatment) and technical parameters (such as shunt system and valve pressure) are needed to confirm and further differentiate the present results. Moreover, it would be very interesting to investigate, whether neuroradiological aspects of shunt overdrainage such as pituitary morphology will regress after adjustment of shunt valve pressure. Already now, we can say that physicians involved in the diagnosis and therapy of patients with childhood hydrocephalus should know the potential consequences of this treatment on the pituitary gland and be aware of the entity of shunt-induced pituitary enlargement, not to be confused with pituitary adenoma.
Data availability
The anonymized dataset is available from the corresponding author at due request.
References
Demerdash A, Singh R, Loukas M, Tubbs RS (2016) A historical glimpse into treating childhood hydrocephalus. Child’s Nerv Syst 32(3):405–407
Chesler DA, Pendleton C, Ahn ES, Quinones-Hinojosa A (2013) Harvey Cushing’s early management of hydrocephalus: an historical picture of the conundrum of hydrocephalus until modern shunts after WWII. Clin Neurol Neurosurg 115(6):699–701
Aschoff A, Kremer P, Hashemi B, Kunze S (1999) The scientific history of hydrocephalus and its treatment. Neurosurg Rev 22(2–3):67–93 discussion 94–95
Symss NP, Oi S (2015) Is there an ideal shunt? A panoramic view of 110 years in CSF diversions and shunt systems used for the treatment of hydrocephalus: from historical events to current trends. Childs Nerv Syst 31(2):191–202
Mokri B (2001) Spontaneous intracranial hypotension. Curr Pain Headache Rep 5(3):284–291
D’Antona L, Asif H, Craven CL, McHugh JA, Vassiliou A, Thorne L, Matharu MS, Watkins LD, Bremner F, Toma AK (2021) Brain MRI and ophthalmic biomarkers of intracranial pressure. Neurology 96(22):e2714-2723
Mokri B (2000) Cerebrospinal fluid volume depletion and its emerging clinical/imaging syndromes. Neurosurg Focus 9(1):e6
Yadav P, Singhal S, Chauhan S, Harit S (2017) MRI evaluation of size and shape of normal pituitary gland: age and sex related changes. JCDR 11(12):TC01–TC04
Löppönen T, Pääkkö E, Laitinen J, Saukkonen AL, Serlo W, Tapanainen P, Ruokonen A, Pirttiniemi P, Poikela A, Knip M (1997) Pituitary size and function in children and adolescents with shunted hydrocephalus. Clin Endocrinol (Oxf) 46(6):691–699
Löppönen T, Saukkonen AL, Serlo W, Tapanainen P, Ruokonen A, Knip M (1996) Accelerated pubertal development in patients with shunted hydrocephalus. Arch Dis Child 74(6):490–496
Abdolvahabi RM, Mitchell JA, Diaz FG, McAllister JP 2nd (2000) A brief review of the effects of chronic hydrocephalus on the gonadotropin releasing hormone system: implications for amenorrhea and precocious puberty. Neurol Res 22(1):123–126
Van Beek JT, Sharafuddin MJ, Kao SC, Luisiri A, Garibaldi LR (2000) Prospective assessment of pituitary size and shape on MR imaging after suppressive hormonal therapy in central precocious puberty. Pediatr Radiol 30(7):444–446
Yoon MK, Parsa AT, Horton JC (2013) Skull thickening, paranasal sinus expansion, and sella turcica shrinkage from chronic intracranial hypotension. J Neurosurg Pediatr 11(6):667–672
Alvarez-Linera J, Escribano J, Benito-León J, Porta-Etessam J, Rovira A (2000) Pituitary enlargement in patients with intracranial hypotension syndrome. Neurology 55(12):1895–1897
Mokri B (2001) Spontaneous intracranial hypotension. Curr Neurol Neurosci Rep 1(2):109–117
Chen ST, Wu JW, Wang YF, Lirng JF, Hseu SS, Wang SJ (2022) The time sequence of brain MRI findings in spontaneous intracranial hypotension. Cephalalgia 42(1):12–19
Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V (2015) Brain imaging in idiopathic intracranial hypertension. J Neuroophthalmol 35(4):400–411
Macintyre I (2014) A hotbed of medical innovation: George Kellie (1770–1829), his colleagues at Leith and the Monro-Kellie doctrine. J Med Biogr 22(2):93–100
Mokri B (2001) The Monro–Kellie hypothesis: applications in CSF volume depletion. Neurology 56(12):1746–1748
Spinelli CP, Iwanaga J, Hur MS, Dumont AS, Tubbs RS (2022) Discovery of a trans-sellar vascular supply for the pituitary gland. Anat Cell Biol 55(2):124–129
Acknowledgements
The authors acknowledge the administrative assistance of Mrs. Janine Szybowicz, study nurse, in the present study.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AG and YL collected and evaluated the clinical and imaging data, WXC performed the statistical analysis. The first version of the manuscript was drafted by AG and IK-A. All authors discussed the dataset and first manuscript version from their interdisciplinary perspective and participated in the writing of the final manuscript and the revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
N/A. Not a study on humans, but retrospective evaluation of clinical and imaging data, anonymized at the source.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grzywotz, A., Li, Y., Unger, N. et al. Pituitary enlargement in patients with cerebrospinal fluid drainage due to ventricular shunt insertion: know the condition and do not mistake for adenoma. Pituitary 26, 164–170 (2023). https://doi.org/10.1007/s11102-022-01296-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-022-01296-y