Abstract
Steroidal saponins are a group of glycosides widely distributed among monocotyledonous families. They exert a wide spectrum of biological effects including cytotoxic and antitumor properties which are the most studied. This review is an update of our previous paper—Saponins as cytotoxic agents (Podolak et al. in Phytochem Rev 9:425–474, 2010) and covers studies that were since published (2010–2018). In this paper we refer to steroidal saponins presenting results of cytotoxicity studies, mechanisms of action and structure–activity relationships.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Steroidal saponins are an important group of glycosidic plant metabolites. They are mainly distributed among monocotyledonous families: Amarillidaceae (Agapanthus, Allium), Asparagaceae (Agave, Anemarrhena, Asparagus, Convallaria, Hosta, Nolina, Ophiopogon, Ornithogalum, Polygonatum, Ruscus, Sansevieria, Tupistra, Yucca), Costaceae (Costus), Dioscoreaceae (Dioscorea), Liliaceae (Fritillaria, Lilium), Melanthiaceae (Paris), Smilacaceae (Smilax). Although it is uncommon, steroidal saponins can also be found in some dicotyledonous angiosperms, such as: Fabaceae (Trigonella), Zygophyllaceae (Tribulus, Zygophyllum), Solanaceae (Solanum, Lycopersicon, Capsicum), Asteraceae (Vernonia), and Plantaginaceae (Digitalis) (Faizal and Geelen 2013; Rahman et al. 2017; Lanzotti 2005; Sobolewska et al. 2016; Tang et al. 2013; Wang et al. 2018). Moreover, these compounds have been identified in starfish and marine sponges (Ivanchina et al. 2011; Barnett et al. 1988; Regaldo et al. 2010).
Structurally, steroidal saponins are distinguished by the nature of the aglycone part. Sapogenins are polycyclic 27-C-compounds which can be divided into three distinct groups: spirostane, furostane, and open-chain (cholestane) compounds (Challinor and De Voss 2013). Some authors distinguish iso-spirostane-type saponins—possessing an equatorial oriented (hydroxy)methyl on F ring versus spirostane-type with an axial oriented C-27 group (Tian et al. 2017). Furthermore, spirosolane-type glycoalkaloids in which a nitrogen atom is incorporated in the steroid aglycone at the heterocyclic oxygen site (e.g. in solasodine) are sometimes included in the group of steroidal saponins. The sugar residue of steroidal saponins consists of one to three straight or branched sugar chains, which are composed usually of β-d-glucopyranosyl (Glc), α-l-rhamnopyranosyl (Rha), β-d-galactopyranosyl (Gal), β-l-arabinofuranosyl (Ara), β-d-xylopyranosyl (Xyl), β-d-fucopyranosyl (Fuc), β-d-mannopyranosyl (Man), or β-d-quinovopyranosyl (Qui) residues.
Since many years spirostanol sapogenins, such as e.g. disogenin or hecogenin, have been valued by pharmaceutical industry and used as substrates in the production of steroid hormones and drugs. Also, medicinal properties of saponin containing plants are well known. Some of most prominent examples include Ruscus aculeatus, which is used as vasoprotective agent, or Tribulus terrestris, found in many products dedicated to fertility stimulation in men (Masullo et al. 2016; Salgado et al. 2017). Steroidal saponins are a research target of many scientist groups. Numerous published reports have confirmed that these compounds exert a wide spectrum of pharmacological activities, including antimicrobial, anti-inflammatory, cardioprotective, cAMP phosphodiesterase inhibitory, or anti-adipogenic (Sohn et al. 2006; Huang et al. 2013; Tang et al. 2015; Ning et al. 2010; Nakamura et al. 1993; Poudel et al. 2014).
One of the activities that is especially widely explored is cytotoxic effect (Podolak et al. 2010; Böttger et al. 2012). The search for potential new chemotherapeutics within natural sources is obviously triggered by a growing need to provide effective treatment to counteract cancer. Results of studies on in vitro and in vivo cytotoxicity of steroidal saponins indicate that these compounds provide an interesting research target. In our previous review the results of experimental studies on cytotoxicity of saponins, both triterpene and steroidal, covering the period from 2005 to 2009 have been summarized (Podolak et al. 2010). Since then, a vast number of new experimental data have appeared in literature. This issue is however scarcely reviewed. Several papers that discussed biological activities of compounds found in a particular genus, like e.g. Allium or Smilax, (Sobolewska et al. 2016; Tian et al. 2017), referred also to their cytotoxic effects, but there are virtually none reviews focused entirely on this activity despite a growing number of reports with experimental data. Some more general aspects were tackled by Xu et al. (2016) who discussed anticancer saponins from Chinese plants. In a recent paper by Zhao et al. (2018), advances in antitumor potential of steroidal saponins have been focused on the mechanisms of action, and included examples of sapogenins and saponins, as well as some other compounds such as a cardiac glycoside—bufalin or cucurbitacins.
Taking into account a large number of experimental data referring to cytotoxicity of saponins, that have been published since our previous review, we decided to divide this update into two parts, each dedicated to the one of the distinct structural groups, that is triterpene and steroidal compounds.
Thus, in the current review, we present an update on the cytotoxic activity of steroidal saponins and sapogenins covering recent studies from the period of 2010 to 2018. Discussion of structure–activity data and mechanisms of action is also provided, together with a selection of most promising compounds with a potential for future development as anticancer chemotherapeutics.
The literature search was conducted in the following electronic databases: SCOPUS, EMBASE and MEDLINE/PubMed. The keywords used were: steroidal saponins, steroidal sapogenins, cancer, cytotoxicity.
Since 2010 year in vitro cytotoxicity studies have been performed on different human and animal cell cancer and normal lines, including:
-
Human cancer cell lines Breast: BT-549, MCF-7, MDA-MB-231, MDA-MB-435, MDA-MB-468, SK-BR-3; bone: 143-B, HOS; cervix: HeLa, Caski, KB, SiHa; colon: COLO, DLD-1, HT-29, HCT 116, HCT-15, CaCo-2, SW480, SW620, W480, LOVO; esophagus: KYSE 510; gingival: Ca9-22; glioblastoma: SF-268, SF-295, U251, U87MG; larynx: Hep2; leukemia: CCRF-CEM, HL-60, Jurkat, K562; liver: HLE, Hep3B, HepG2, HuH-7, C3A, BEL-7402, BEL-7403, BEL-7404, MHCC97-L, SMMC-7721, SMMC-7221, SNU-387, WRL; lung: 95D, A549, LAC, NCI-H1299, NCI-H446, NCI-H460, SK-MES-1; melanoma: A375, A375.S2, MM96L, SK-MEL, SK-MEL-2, WM-115; neuroblastoma: IMR-32, LA-N-2, NB-69; ovary: HO-8910PM, OVCAR-8, SK-OV-3; pancreas: BxPC-3, PANC-1; pharynx: 5-8F, CNE; prostate: DU145, PC-3; sarcoma: MG-63, Rh1; stomach: BGC-823, SGC-7901, SGC-7901/DDP [cisplatin (DDP)-resistant], HIF1α-knockdown BGC-823 (hypoxia-mimic sensitive), MGC-803; urinary bladder: ECV-304;
-
Animal cancer cell lines Breast: EMT6; glioblastoma: C6; leukemia: Baf3-WT; lung: LL2; colon: C26; melanoma: B16; sarcoma: WEHI-164, J-774;
-
Human normal cell lines Fibroblasts: HFF, NFF, Hs68; keratinocytes: HaCaT; kidney embryonic: HEK293; lung epithelial: MRS-5; vein endothelial: EA.hy926, HUVEC;
-
Animal normal cell lines Cardiomyoblasts: H9c2; epidermal: JB6 P+Cl-41; fibroblasts: 3T3; kidney epithelial: LLC-PK1; kidney fibroblasts: VERO.
The results of these studies have been summarized in Table 1.
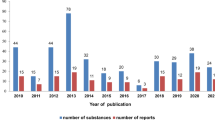
Based on data published in years 2010–2018 it may be concluded that out of 284 substances that are included in the current review, a vast majority, that is 96.8%, were pure single compounds, both saponins and sapogenins, either structurally novel or known previously. A graph representing a number of tested substances and a number of reports published in the time scope covered by this review (2010–2018) is shown in Fig. 1.
Cytotoxicity studies were performed on animal and human cell line models, with significant predominance of the latter, which accounted to 92.7% of all assays. The effects of steroidal saponins/sapogenins against human colon, breast and liver cancers have been most widely studied, accounting to 17.9%, 16.5% and 16% of all assays on human cell lines, respectively. A graph showing the share of experiments on specific types of tumors and normal cell lines in the total number of tests performed on human cell lines is shown in Fig. 2.
The largest number of substances was analysed against following cell lines: HepG2—human hepatocellular carcinoma, MCF-7—human breast adenocarcinoma, and A549—human lung adenocarcinoma cells, which constituted 27.8%, 27.4% and 23.5% of the pool of substances under study, respectively. Tests in which normal cell lines were included in the study accounted for only 4.4% of all assays conducted on human cell lines.
The most preferred method used was the MTT assay. In most cases (80.4% of all assays) IC50 values for analysed saponins were compared with a positive control. Well-known anticancer drugs such as doxorubicin, cisplatin and paclitaxel were most frequently used as reference substances. Other compounds with anticancer activity were chosen definitely less often and these include: actinomycin D, adriamycin, beta-l-(−)-dioxalane-cytidine (−)-OddC, camptothecin, elipticin, etoposide, 5-FU, mitamycin C, mitoxantrone, nimustine (ACNU), podophyllotoxin, staurosporine, tamoxifen, and troxacitabine. In one study resveratrol, which is not an approved anticancer drug, served as the control (Shen et al. 2012).
In the majority of cases steroidal saponins were less active than the control substances. However, there were some noticeable examples of compounds which displayed cytotoxic effect higher than the reference drug. Three saponins isolated from Dracaena cambodiana dragon’s blood that are glycosides of diosgenin, pennogenin and spirost-5,25(27)-dien-1β,3β-diol, exerted stronger cytotoxic activity (IC50: 1.27 μM, 5.09 μM, 4.77 μM, respectively) against K-562 cells than paclitaxel (IC50: 5.98 μM), while a pennogenin glycoside showed higher cytotoxic effect on BEL-7402 than paclitaxel (IC50: 1.13 μM and 3.75 μM respectively) (Shen et al. 2014). Results obtained by Teponno et al. showed that a well known steroidal glycoside–dioscin–diosgenin 3-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-β-d-Glc (for the purpose of the study isolated from Dracaena viridiflora) had cytotoxic activity against Jurkat, Caco-2, SK-OV-3, and A549 cells (IC50: 1.70 ± 0.38 μg ml−1, 2.58 ± 0.21 μg ml−1, 1.90 ± 0.86 μg ml−1, and 0.42 ± 0.15 μg ml−1, respectively) comparable to doxorubicin used as positive control (IC50: 0.61 ± 0.04 μg ml−1, 2.32 ± 1.04 μg ml−1, 0.84 ± 0.08 μg ml−1, and 1.15 ± 0.84 μg ml−1, respectively) (Teponno et al. 2017). Another diosgenin derivative named SAP-1016 (diosgenin 3-O-β-d-Xyl-(1 → 3)-β-d-Glc-(1 → 4)-[α-l-Rha-(1 → 2)]-β-d-Glc) which was found in the fruits and roots of Balanites aegyptiaca showed potent antiproliferative activity against MCF-7 and HT-29 cancer cells (IC50: 2.4 ± 0.35 and 3.3 ± 0.19 μM, respectively) higher than dioscin (IC50: 3.1 ± 0.39 μM and 4.9 ± 0.32 μM, respectively) and cisplatin (IC50: 30.3 ± 0.33 μM and 40.2 ± 0.44 μM, respectively) (Beit-Yannai et al. 2011).
Structure–activity correlation
Despite a vast number of papers that cite the results of cytotoxic activity of steroidal saponins only a relatively small number include some reference to potential structure–activity elationships. These are usually not fully conclusive statements resulting from the observations made on a very limited number of compounds. In the time-span covered by this review, only a few studies have been specially designed to explore structure–activity correlations. These include the one by Pérez-Labrada et al. (2012a, b) who, for the purpose of their study, had synthesized twelve spirostanol glycosides differing mainly in C-ring functional groups, which influenced the lipophilicity and conformational flexibility of compounds (Pérez-Labrada et al. 2012a). These included methylene-, methoxyl-, α,β-unsaturated ketone and lactone. Two glycosylation pathways led to a series of 3,6-dipivaloylated β-d-glucosides (pivaloyl = 2,2-dimethylpropanoyl) and a series of β-chacotriosides (α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-β-d-Glc). The obtained compounds were analysed with respect to their cytotoxicity against the human myeloid leukemia cell line (HL-60) and benign blood cells. The results indicate that among the two glycosidic series, the one based on a β-chacotrioside moiety was more potent. This activity was however greatly correlated with the rigidity of the aglycone and its hydrophobic character. From among all tested saponins, chacotriosides either with a methylene group at C-12 or no substitution in C-ring showed the highest cytotoxic potential against malignant cell line. However, their selectivity as compared to 3,6-dipivaloylated spirostanyl glucosides was much lower.
In a subsequent study by the same research group on a larger variety of synthetic spirostanol glycosides, the partially pivaloylated β-d-glucosides of 5α-hydroxy-laxogenin were the most potent (Pérez-Labrada et al. 2012b). Comparison of the results obtained for different β-chacotriosides, has again confirmed that vast differences can be seen with a change in the aglycone part. Hecogenin derivative was highly cytotoxic against the tested HL-60 cell line (IC50 4.3 ± 1.0 μM) whereas 5α-hydroxy-laxogenin β-chacotrioside showed a complete loss of activity (IC50 > 100 μM).
Other studies in which any references to possible structure–activity relationships were made, generally indicate that both structural features of steroidal saponins, that is the nature of the aglycone and the sugar moiety, together determine their cytotoxicity.
Thirteen saponins isolated from the roots of Liriope muscari were analysed in this respect against a fairly wide panel of cancer cell lines (MDA-MB-435, 95D, HepG2, HeLa, MCF-7 and A549) (Wu et al. 2017b). The authors were able to distinguish three groups based on the structural features of the aglycone, namely the (25S)-ruscogenin, (25R)-ruscogenin, and neoruscogenin groups. This allowed to compare the potential contribution to the cytotoxic activity of the specific configuration at C-25, either 25R, 25S or 25,27-double bond. The obtained cytotoxicity results have shown that the impact of this structural feature is related to the nature of the sugar chain. In all saponins bearing β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-β-d-Xyl or β-d-Glc-(1 → 2)-[β-d-Xyl-(1 → 3)]-β-d-Glc sugar chains at C-1, the configuration at C-25 was of no consequence in all tested cell lines. Interestingly, a different sugar chain composed of β-d-Glc-(1 → 2)-[β-d-Ara-(1 → 3)]-β-d-Fuc, together with 25R configuration seemed to have a detrimental effect on the cytotoxicity, which was observed against all the tested cell lines. Similar regularity was seen when comparing compounds with yet another sugar chains, however not in case of the whole spectrum of tested cell lines.
In another study on ten saponins from Asparagus filicinus similar results with respect to C-25 configuration were obtained, suggesting that 25S spirostanol aglycone may be a more important structural feature (Wu et al. 2010). Another conclusion drawn from these studies refers to the sugar moiety, clearly indicating that its presence at C-23 significantly reduces the cytotoxic potential of these compounds.
Beit-Yannai et al. (2011) in their study on saponins from Balanites aegyptiaca have seen a pronounced difference in cytotoxicity against MCF-7 human breast cancer and HT-29 human colon cancer cells between two compounds differing in only one terminal sugar (dioscin vs SAP-884—diosgenin 3-O-β-d-Glc-(1 → 4)-[α-l-Rha-(1 → 2)]-β-d-Glc) led the authors to postulate that terminal l-rhamnose seems to be more beneficial than d-glucose (Beit-Yannai et al. 2011). Results of their study also confirmed previous observations with regard to the general aglycone type, that furostane derivatives have lesser cytotoxic effect as compared to spirostanes.
Also Wu et al., who analysed the activity of three new saponins from Paris polyphylla var. yunanensis against CNE cells, concluded that the presence of F ring in steroidal saponins may be the structural feature essential for their cytotoxicity (Wu et al. 2017a).
However, a study of Kang et al. showed contradictory results against human CCRF-CEM leukemia cells. From among twenty compounds (including saponins, sapogenins and sterols) isolated from P. polyphylla, only furostanols were active and their activity was highly potent. Both spirostanol saponins and sterols lacked any effect on this cell line (Kang et al. 2012).
In some papers included in this review the authors tried to draw conclusions referring solely to the composition and structure of the sugar moieties. This was possible when the isolated saponins differed only with respect to the sugar chain. However, the number of compounds was usually so small that it is hardly possible to consider these observations as contributing to more general statements which would be conclusive. For example, two pennogenyl saponins from Paris quadrifolia differing in the length and number of monosaccharides were compared on a single cell line, namely HeLa. Compound bearing a sugar chain at C-3 composed of two rhamnose unit was slightly more active than the one with single rhamnose unit (Stefanowicz-Hajduk et al. 2015).
Zolfaghari et al. (2013) in their study of four furostane glycosides from Allium vavilovii have suggested that xylose instead of galactose and glucose instead of rhamnose seem to enhance cytotoxicity against J-744 (murine macrophage) and WEHI-164 (murine fibrosarcoma) cell lines.
Mechanisms of action
Similarly to what have been published in our previous work (Podolak et al. 2010), most of the steroidal saponins, which are discussed in the present review, triggered cell death by apoptosis stimulation, mainly on its intrinsic pathway. Other effects observed while testing steroidal saponins impact on cancer cells included the stimulation of autophagy, phagocytosis or oncosis, the inhibition of metastatic properties of the tested cells or angiogenesis.
Results of in vitro studies
Apoptosis stimulation
Lin et al. (2018) described the effect of protodioscin on human cervical cancer cells, trying to determine the molecular mechanism of the compound. The authors observed that protodioscin inhibited the viability of cervical cancer cells by stimulating apoptotic process in the cells, expressed by the up-regulation of caspases 8, 3 and 9, but also down-regulation of Bcl-2 expression. Moreover, protodioscin stimulated ROS and ER stress pathway in the examined cells and increased p38 and JNK levels. The authors suggest that protodioscin stimulated ER-stress dependent apoptosis in the human cervical cancer cells and the observed effect could be additionally mediated by the activation of JNK and p38 pathways (Lin et al. 2018). Terrestrosin D (hecogenin 3-O-β-d-Gal-(1 → 2)-[β-d-Xyl-(1 → 3)]-β-d-Glc-(1 → 4)-β-d-Gal), isolated from T. terrestris, significantly decreased the viability of androgen-independent (DU-145, PC-3, PC-3M) and androgen-dependent (LNCaP, 22RV1) human prostate cancer cells, in dose-dependent manner (Wei et al. 2014). Moreover, the compound induced PC-3 cell cycle arrest in G1 phase and stimulated caspase-independent apoptosis in the cells. Wang et al. indicated that macrostemonoside A (tigogenin 3-O-β-d-Glc-(1 → 2)-[β-d-Glc-(1 → 3)]-β-d-Glc-(1 → 4)-β-d-Gal) stimulated apoptosis in colorectal cancer SW480 cells, manifesting as caspase activation, increase in proapoptotic and decrease of antiapoptotic Bcl-2 family proteins expression. Moreover, the compound induced reactive oxygen species (ROS) production in the examined cells (Wang et al. 2013c). Two studies concern the activity of saponins isolated from P. polyphylla. In the first one, four pennogenyl saponins PS1–PS4 were examined on a panel of human cancer and normal cell lines. The results indicated that only saponins PS1 (pennogenin 3-O-β-d-Glc-(1 → 3)-[α-l-Rha-(1 → 2)]-β-d-Glc) and PS2 (pennogenin 3-O-α-l-Rha-(1 → 4)-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-β-d-Glc) markedly inhibited cell viability in HepG2, MCF-7 and PC-3 cells. The two compounds also induced apoptosis and caused cell cycle arrest in HepG2 cells affecting multiple targets, including mitochondrial caspase-dependent and independent pathway, cyclin-dependent kinase 1 activation or PI3K/Akt signalling (Long et al. 2015). In another study P. polyphylla steroidal saponins decreased the viability of human lung cancer A549 cells through both apoptosis and autophagy, with the activation of caspase-8 and 3 and PARP cleavage for the former, and up-regulation of Beclin1 and conversion from LC3 I to LC3 II for the latter process, respectively (He et al. 2014). For the same cell line, A549, an apoptosis inducement was described as the effect of a treatment with novel steroidal saponin cholestanol glucoside CG. The compound had cytotoxic effect also in PC-3 and HepG2 cells, but A549 cell line was most susceptible, with the observed ROS generation inducement and the loss of mitochondrial membrane permeability (Valayil et al. 2016). Similar effect of ROS accumulation was also described for aspafilioside B (sarsasapogenin 3-O-β-d-Xyl-(1 → 4)-[α-l-Ara-(1 → 6)]-β-d-Glc), isolated from Asparagus filicinus. The compound additionaly inhibited both viability and proliferation of HepG2 cells, by arresting the cells in G2 phase and stimulating apoptosis. The underlying mechanism included up-regulation of H-Ras and N-Ras proteins, c-Raf phosphorylation and the activation of ERK and p38. Interesting proapoptotic mechanism was recently proposed for a sapogenin–diosgenin by Chen et al. (2018). The compound was found to inhibit TAZ, one of the transcription co-activators in Hippo signalling pathway, which may play a role as an oncogenic factor in the cells. Diosgenin also inhibited the growth and migration of human liver cancer cells (Chen et al. 2018). Its widely known glycoside–dioscin exerted rare mechanism of proapoptotic activity by triggering both intrinsic (loss of mitochondrial membrane potential, activation of tBid and Bak proteins) and extrinsic (up-regulation of death ligands and receptors) apoptosis pathways in human leukemia cells. Additionally, the compound induced the differentiation of promyelocytes to granulocytes and monocytes (Chan et al. 2018).
Oncosis stimulation
Oncosis is a non-apoptotic cell death mode, manifested as marked cell swelling, coagulation of the cytoplasm and alterations in cell cytoskeleton elements, noted within a short time after the application of the tested substance. The only report describing oncosis stimulation for steroidal saponins was published by Sun et al. (2011) for solamargine (solasodine 3-O-α-l-Rha-(1 → 2)-[α-l-Rha-(1 → 4)]-β-d-Glc)—a steroidal alkaloid glycoside in human K562 leukemia and KB squamous carcinoma cells. The authors suggested that compound initiated cell membrane blebbing, the increase in cytoplasm volume and also disrupted microtubules and actin filaments within the tested cells (Sun et al. 2011).
Angiogenesis inhibition
Terrestrosin D isolated from T. terrestris effectively inhibited viability of HUVEC cells and also induced cell cycle arrest and apoptosis in the cells, which suggests its antiangiogenic potential in vitro (Wei et al. 2014). Similar observations were made for ASC (diosgenin 3-O-[2-O-acetyl-α-l-Rha-(1 → 2)]-[β-d-Xyl-(1 → 4)]-β-d-Glc), a steroidal saponin from Ophiopogon japonicus, which markedly inhibited the proliferation of HUVEC cells and induced G2/M phase arrest in the cells by decreasing the expression of cdc2 and cyclin B1. The compound also significantly inhibited the invasive potential of the examined cells in transwell migration and tube formation assays. Moreover, ASC was found to be a strong inhibitor of Src/Akt/mTOR-dependent metalloproteinases pathway, which may explain its antiangiogenic properties (Zeng et al. 2015). Antiangiogenic properties were also described for another compound from the O. japonicus, ophiopogonin T (26-O-β-d-Glc (25R)-furost-5-ene-1β,3β,22β,26-tetraol 1-O-β-d-Xyl-(1 → 3)-[α-l-Rha-(1 → 2)]-β-d-Fuc), which inhibited tube formation of HUVEC cells (Lee et al. 2016).
Metastasis inhibition
Ophiopogonin D (25(R)-ruscogenin 1-O-α-l-Rha-(1 → 2)-[β-d-Xyl-(1 → 3)]-β-d-Fuc) isolated from O. japonicus significantly decreased not only the proliferation of MDA-MB-435 melanoma cells, but also decreased the cell invasion properties, probably through the inhibition of the MMP-9 matrix metalloproteinase expression and suppression of the p38/MAPK pathway. The compound inhibited also the adhesion of melanoma cells to human umbilical vascular endothelial cells and fibronectin (Zhang et al. 2015). An interesting explanation for the antiinvasive potential was proposed for dioscin in the experiment on murine B16 melanoma cells. The compound significantly affected the transcription and translation of connexin 43 via retinoid acid signalling pathway and at the same time enhanced the transporting function of connexin 43. Additionally, dioscin increased the secretion of pro-inflammatory interleukines 6 and 1β and TNFα, but also the increase in phagocytic activity of tumor-associated magrophages was observed (Kou et al. 2017).
Multidrug resistance decreasing
Interesting study was described by Wang et al. on the potential of steroidal saponin from Trillium tschonoskii in reversing multidrug resistance (MDR) in hepatocellular carcinoma cells (Wang et al. 2013a). The compound not only reversed MDR in the cells but also enhanced the chemosensitization of the cells to doxorubicin, demonstrated as the significant decrease in IC50 value for the anticancer drug. Moreover, the compound suppressed the P-glucoprotein expression in the drug resistant cells, which led to the accumulation of doxorubicin inside the cells, and also blocked the expression of some genes coding multidrug resistance (Wang et al. 2013a).
Results of in vivo studies
Only a small number of papers describe the in vivo effects of steroidal saponins. In one of them, after 35 days of intraperitoneal administration of 10, 50 or 100 mg kg−1 daily of macrostemonoside A to BALB/c nude mice (with SW480 cells injected s.c.), a significant decrease in tumor volume and weight was noted (Wang et al. 2013c). Similar effect was described by Wei et al. (2014) for terrestrosin D, a steroidal saponin isolated from T. terrestris. The compound at the doses of 25 or 50 mg kg−1 was administered 3 times a week for 4 weeks to BALB/c nude mice bearing PC-3 prostate cancer cells and reduced the tumor growth when compared to the control animals. Moreover, no toxic effect was noted during the treatment. Another steroidal saponin, aspafilioside B, significantly inhibited tumor growth in nude mice bearing HepG2 human hepatocellular carcinoma cells, when administered in 5 and 10 mg kg−1 doses. Further analysis indicated the increase in the expression of H-Ras and N-Ras signalling proteins in the tumor cells obtained from aspafilioside B treated animals. Moreover, no side effects were observed during treatment in terms of haematological or histopathological parameters. In a similar study, dioscin revealed significant anti-metastatic effects, activating the expression of a gap junction protein connexin 43 both in metastatic lung nodes and in situ tumor animal models (Kou et al. 2017). An interesting experiment was described by Chen et al. (2016) on the effect of dioscin aglycone–diosgenin on benign prostate hyperplasia in rats (Chen et al. 2016). After 3 weeks of administration the compound at the doses of 50 and 100 mg kg−1 significantly decreased prostate index and PSA level but also improved the pathological changes of the prostate in the treated animals. Moreover, diosgenin down-regulated the expression of Bcl-2 and up-regulated that of Bax and p53 in the treated animals, which suggests the efficacy of the compound in the treatment of prostate enlargement. Interesting antiangiogenic properties of ASC, isolated from O. japonicus, were described in matrigel plug in vivo assay. The compound significantly inhibited the formation of new blood vessels and decreased the number of the cells with the expression of PECAM-1, cell adhesion molecule, but also the number of MMP-2, MMP-9 and VEGF positive cells (Zeng et al. 2015).
Compounds with a potential as future anti-cancer therapeutic agents
Several reports indicate that some saponins/sapogenins can be considered as potential candidates for cancer treatment. In many studies on human cancer cell lines of different origin they displayed significant in vitro and in vivo activities through different signaling pathways associated with cell cycle. What is most important, apart from direct cytotoxic effect these compounds revealed also other activities, for example anti-inflammatory, that may be of importance in order to obtain the multidirectional therapeutic effect in cancer treatment. The authors of the present review have chosen five compounds: diosgenin, dioscin, polyphyllin I, paris saponin II, and timosaponin III, which, in our opinion have some interesting features, that make them especially promising for future development as anticancer agents. All selected saponins, except timosaponin AIII, share a common structural feature that is the same sapogenin–diosgenin as well as the presence of one branched sugar chain. It is noteworthy that this sapogenin itself can be considered as a potential lead compound for future development. Below, a short summary of the most interesting data referring to complex mechanisms of action is provided. Moreover, the results of the studies referring to their mechanisms of action at the molecular level, that were published in years 2010–2018 are summarized in details in Table S2 (Tab. S2)—see supplementary material. The structures of selected compounds are presented on Fig. 3.
Diosgenin (3β,25R)-spirost-5-en-3-ol, was discovered for the first time in Dioscorea tokoro in 1935 (Chen et al. 2015). Since then it has been found in numerous plants of several genera: Dioscorea, Costus, Smilax, Paris, Alteris, Allium, Helicteres, Trillium, and Trigonella (Sethi et al. 2018; Sobolewska et al. 2016; Deshpande and Bhalsing 2014–2015). Diosgenin exerts different pharmacological activities: hypolipemic, neuroprotective, gastro- and hepatoprotective (Jesus et al. 2016; Sethi et al. 2018). Of current interest are its anti-proliferative properties as well as anti-inflammatory effects.
Multiple molecular targets of this sapogenin are noteworthy. It is able to modulate various oncogenic processes (cancer cells proliferation, migration, apoptosis), inhibit angiogenesis, reverse multi-drug resistance in cancer cells and sensitize cancer cells to chemotherapy (Stehi et al. 2018; Chen et al. 2015). Diosgenin was suggested to be a good candidate for lung cancer therapy as an inhibitor of hTERT gene expression (Rahmati-Yamchi et al. 2013). Its activity against lung cancer cell line A549 was time- and dose-dependent, with the best effect after 72 h. The compound revealed also antimetastatic potential, which was observed for example on breast cancer cell line MDA-MB-231 (He et al. 2014). A significant suppression of cell migration was seen at the concentration as low as 5 µM, after only 24 h of incubation, without affecting cell proliferation. Moreover, except from downregulation of STAT3 signaling pathway and the inhibition of human hepatocellular carcinoma cells proliferation, diosgenin also potentiated paclitaxel and doxorubicin apoptotic effects (Li et al. 2010). This synergistic effect may be of special importance for further studies of this compound. Diosgenin also downregulated the peroxidation reaction and enhanced the indigenous antioxidant defense system in female rats with NMU-induced mammary cancer (Jagadeesan et al. 2012). As cancer is often related to the hyperactivity of free radicals, this activity profile completes and expands the direct impact of diosgenin on cancer cells.
Dioscin Diosgenin 3-O-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-β-d-Glc, is a spirostanol saponin found mostly in Dioscorea species; and also in other genera such as Allium, Polygonatum, and Smilax (Sobolewska et al. 2016; Rani et al. 2012; Xu et al. 2016; Wang et al. 2001; Tian et al. 2017). Dioscorea nipponica and Dioscorea zingiberensis are especially good sources of dioscin and provide raw material for the synthesis of steroid hormone drugs. Many pharmacological studies described antimicrobial, lipid-lowering, hepatoprotective, and anti-allergic activities of dioscin (Cho et al. 2013; Kwon et al. 2003; Tao et al. 2018). A large number of experimental data have confirmed not only its direct cytotoxicity towards cancer cells but also anti-inflammatory and immunoregulatory activities that may contribute to the widely reported anti-tumor effect (Tao et al. 2018; Wu et al. 2015).
Numerous studies were focused on the possible mechanism of antitumor activity of dioscin (Tab. S2). The compound was found to inhibit cancer cell viability via different pathways: G2/M cell arrest, induction of apoptosis and autophagy, downregulation of anti-apoptotic proteins, induction DNA damage mediated by ROS (Xu et al. 2016). Dioscin treatment increased cellular apoptosis in ovarian cancer SK-OV-3 cells in a dose-dependent manner. At the concentrations of 2.5 or 5 µM it significantly decreased PI3K and phosphorylated (p)-AKT, VEGFR2 protein expression compared with the non-treated control group, and induced expression of p-p38 protein (Guo and Ding 2018). Dioscin induced apoptosis in SGC-7901 cells in a dose-dependent manner (Hu et al. 2011). It was more active than hCPT (IC50 of 1.2 μg ml−1 and IC50 of 25.2 μg ml−1, respectively).
Paris saponin II (PSII, formosanin C) Diosgenin 3-O-α-l-Rha-(1 → 4)-α-l-Rha-(1 → 4)-[α-l-Rha-(1 → 2)]-β-d-Glc is one of the main active components of Paridis rhizoma obtained from P. polyphylla var. yunnanensis and P. polyphylla var. chinensis. This saponin was reported also in other Paris sp., as well as in Cestrum, Allium, Ypsilandra, and Dioscorea species (Xia et al. 2016; Ribeiro et al. 2016a, b; Sobolewska et al. 2006). With respect to the mechanisms underlying its cytotoxic activity it was found that paris saponin II induced apoptosis via activation of caspase 2, S-phase arrest, and suppressed expression of metalloproteinases MMP-1, -2, and -9 (Li et al. 2014; Man et al. 2011). Intraperitoneal administration of formosanin C at 15 and 25 mg kg−1 in a xenograft mouse model of ovarian cancer led to a 46% and 70% tumor growth inhibition, respectively (Xiao et al. 2012). It is noteworthy that a combination of PSII and curcumin exerted synergic anti-cancer activity on different lung cancer cells, revealed as the increase in the cellular uptake and the bioavailability of both compounds (Man et al. 2018). Additionally, formosanin C showed immunomodulatory activity when given intraperitoneally to mice. The compound activated natural killer cells and induced interferon production (Wu et al. 1990), what can be considered as another aspect of multitargeted anticancer treatment.
Polyphyllin I (PPI) Diosgenin 3-O-α-l-Rha-(1 → 2)-[β-l-Ara-(1 → 4)]-β-d-Glc, is a spirostanol saponin isolated from the rhizomes of P. polyphylla. Polyphyllin I significantly suppressed in vitro proliferation of A549, NCI-H460 and SK-MES-1 cell lines with significantly low values of IC50 1.24, 2.40, and 2.33 μg ml−1, respectively and the tumor growth of A549 cells in the nude mice (Kong et al. 2010). PPI inhibited also the vasculogenic mimicry formation in both hepatocellular carcinoma cell lines (HCC) and xenografts of HCC (Xiao et al. 2018). The activity of PPI against osteosarcoma was examined both in vitro and in vivo, with interesting results. The compound was found to suppress in vitro growth of osteosarcoma 143-B and HOS cells, as well as the primary cells from a osteosarcoma patient and, what is more important, inhibited in vivo intratibial primary tumor growth in xenograft orthotopic mouse model. Moreover, it induced cell apoptosis, cell cycle arrest and inhibited the invasion and migration of osteosarcoma cells (Chang et al. 2017). Other interesting effects were obtained in the studies on concomitant administration of PPI with other compounds, including currently used chemotherapeutics. The combination of polyphyllin I and paris saponin II showed synergistic anti-tumor activity on HepG2 cells. Both compounds inhibited liver cancer growth through the induction of apoptosis, G1 phase arrest and inhibition of the cellular migration (Liu et al. 2016a). It was shown that the combined treatment of PPI and erlotinib resulted in the strengthened drug response and prolonged survival of lung cancer patients (Lou et al. 2017).
Timosaponin AIII (TAIII) Sarsasapogenin 3-O-β-d-Glc-(1 → 2)-β-d-Gal, was isolated by Kawasaki et al. in 1963 (Kawasaki and Yamauchi 1963; Kawasaki et al. 1963). It is the main active ingredient of the rhizomes of Anemarrhena asphodeloides. The compound exerts a wide range of pharmacological effects including anti-inflammatory, antiplatelet, antithrombotic, anti-diabetic, anti-depressant, improving learning and memory deficits activities (Han et al. 2018; Cong et al. 2016). In recent years, it was found that timosaponin AIII is a promising compound that inhibits the growth of a variety of tumor cells. In different studies it was reported that TAIII may induce autophagy in cancer cells followed by apoptotic cell death, cell cycle arrest in the G0/G1 and G2/M phases, and suppresses HGF-induced invasiveness of cancer cells (Sy et al. 2008; Huang et al. 2015).
Summary
A large number of experimental data that are published each year on antitumor potential of steroidal saponins and their interesting results indicate that these natural compounds are considered to be valuable research targets in the process of development of novel chemotherapeutics for human cancers. Similarly to previous years, the majority of experiments were performed in in vitro conditions with a relatively small number of compounds to enter in vivo studies. Assays performed on human cancer-derived cell lines were definitely predominant over animal cell models. Interestingly several cell lines were used most widely and the pool of experimental data is therefore more conclusive. These include: HepG2—human hepatocellular carcinoma, MCF-7—human breast adenocarcinoma, and A549—human lung adenocarcinoma. Based on the studies summarized in this review (see Tab. 1), it can be seen that the analyzed steroidal saponins/sapogenins revealed a differentiated cytotoxic effect. It is worth noting, however, that tests in which normal cell lines were included in the study accounted for only about 4% of all assays conducted on human cell lines. In addition, simultaneous studies on the cytotoxic activity of a given compound on cancer cells and normal cells derived from the same organ or tissue were extremely rare. Thus, it is difficult to draw more general conclusions with regard to selectivity of steroidal saponins towards cancer cells. Similarly, studies relevant to structure–activity relationships are lacking. It is noteworthy that some species containing steroidal saponins have been more frequently evaluated as sources of cytotoxic compounds in comparison to other, three of them were especially extensively analysed: A. asphodeloides, P. polyphylla var. yunanensis, and O. japonicus.
Several compounds, such as diosgenin, dioscin, paris saponin II, polyphyllin I, and timosaponin AIII seem to be specially promising as candidates for future antitumor agents. Not only their activity has been confirmed by numerous studies, but also these compounds are easily accessible for isolation being present in substantial amounts in several plant species. All of them have revealed multidirectional mechanisms of cytotoxicity as well as other effects, e.g. anti-inflammatory, that may contribute to the overall antitumor activity. Moreover, they were effective not only in in vitro assays, but also in animal models and in most cases a significant reduction in tumor size and angiogenesis was seen, especially with respect to prostate, breast, and lung cancers, of which non-small lung cancer seems to be most susceptible. Further studies on steroidal saponins with respect to their anti-cancer potential are certainly needed and worth continuing with more attention paid to compound selectivity and synergistic effects of combinations with currently applied chemotherapeutics.
References
Abdel-Sattar E, Farag MA, Mahrous EA (2015) Chemical constituents from Solanum glabratum Dunal var. sepicula. Rec Nat Prod 9(1):94–104
Acharya D, Mitaine-Offer A-C, Kaushik N et al (2010) Steroidal saponins from Chlorophytum orchidastrum. J Nat Prod 73(1):7–11
Barnett D, Dean PW, Hart RJ et al (1988) Determination of contents of steroidal saponins in starfish tissues and study of their biosynthesis. Comp Biochem Phys Part B Comp Biochem 90(1):141–145
Beit-Yannai E, Ben-Shabat S, Goldschmidt N et al (2011) Antiproliferative activity of steroidal saponins from Balanites aegyptiaca—an in vitro study. Phytochem Lett 4(1):43–47
Bhuvanalakshmi G, Basappa Rangappa KS et al (2017) Breast cancer stem-like cells are inhibited by diosgenin, a steroidal saponin, by the attenuation of the Wnt β-catenin signaling via the Wnt antagonist secreted frizzled related protein-4. Front Pharmacol 8:124
Böttger S, Hofmann K, Melzig MF (2012) Saponins can perturb biologic membranes and reduce the surface tension of aqueous solutions: a correlation? Bioorg Med Chem 20(9):2822–2828
Challinor VL, De Voss JJ (2013) Open-chain steroidal glycosides, a diverse class of plant saponins. Nat Prod Res 30(3):429–454
Challinor VL, Parsons PG, De Voss JJ (2012) Steroidal saponins from the roots of Smilax sp.: structure and bioactivity. Steroids 77(5):504–511
Chan SH, Liang PH, Guh JH (2018) An integrated approach to elucidate signaling pathways of dioscin-induced apoptosis energy metabolism and differentiation in acute myeloid leukemia. Naunyn Schmiedebergs Arch Pharmacol 391(6):587–602
Chang J, Li Y, Wang X et al (2017) Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/β-catenin pathway in vitro and in vivo. Sci Rep 7:7605
Chen P-S, Shih Y-W, Huang H-C et al (2011a) Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by ceducing matrix metalloproteinases expression. PLoS 6(5):e20164
Chen P-Y, Chen C-H, Kuo C-C et al (2011b) Cytotoxic steroidal saponins from Agave sisalana. Planta Med 77(9):929–933
Chen Y, Tang Y-M, Yu S-L et al (2015) Advances in the pharmacological activities and mechanisms of diosgenin. Chin J Nat Med 13(8):578–587
Chen J, Zhang H-F, Xiong C-M et al (2016) Inhibitory effect of diosgenin on experimentally induced benign prostatic hyperplasia in rats. J Huazhong Univ Sci Technolog Med Sci 36(6):806–810
Chen Z, Xu J, Wu Y et al (2018) Diosgenin inhibited the expression of TAZ in hepatocellular carcinoma. Biochem Biophys Res Commun 503(3):1181–1185
Cho J, Choi H, Lee J et al (2013) The antifungal activity and membrane-disruptive action of dioscin extracted from Dioscorea nipponica. Biochim Biophys Acta 1828:1153–1158
Chu C, Cui T, Li S et al (2018) Structure and activity of a new sapogenin from Chlorophytum laxum R. Br. Chem Res Chin Univ 34(5):732–735
Cong Y, Wang L, Peng R et al (2016) Timosaponin AIII induces antiplatelet and antithrombotic activity via Gq-mediated signaling by thromboxane A2 receptor. Sci Rep 6:38757
Deshpande HA, Bhalsing SR (2014–2015) Plant derived novel biomedical: diosgenin. Int J Pharmacogos Phytochem Res 6(4):780–784
Dong R, Guo J, Zhang Z et al (2018) Polyphyllin I inhibits gastric cancer cell proliferation by downregulating the expressin of fibroblast activation protein alpha (FAP) and hepatocyte growth factor (HGF) in cancer-associated fibroblasts. Biochem Biophys Res Commun 497(4):1129–1134
Duan C-L, Li Y-J, Li P et al (2010) Spirostanol saponins from the fibrous roots of Ophiopogon japonicus (Thunb.) Ker-Gawl. Helv Chim Acta 93(2):227–232
Duan C-L, Li Y-J, Wang F-Y et al (2018) New steroidal glycosides from the fibrous roots of Ophiopogon japonicus. J Asian Nat Prod Res 20(8):744–751
Eskander J, Sakka OK, Harakat D et al (2013) Steroidal saponins from the leaves of Yucca de-smetiana and their in vitro antitumor activity: structure activity relationships through a molecular modeling approach. Med Chem Res 22(10):4877–4885
Esposito D, Munafo JP Jr, Lucibello T et al (2013) Steroidal glycosides from the bulbs of Easter lily (Lilium longiflorum Thunb.) promote dermal fibroblast migration in vitro. J Ethnopharmacol 148(2):433–440
Faizal A, Geelen D (2013) Saponins and their role in biological processes in plants. Phytochem Rev 12:877–893
Fouedjou RT, Teponno RB, Quassinti L et al (2014) Steroidal saponins from the leaves of Cordyline fruticosa (L.) A. Chev. and their cytotoxic and antimicrobial activity. Phytochem Lett 7:62–68
Gajdus J, Kaczyński Z, Kawiak A et al (2014) Isolation and identification of cytotoxic compounds from the rhizomes of Paris quadrifolia L. Pharmacogn Mag 10(38):S324–S333
Gergely JE, Dorsey AE, Dimri GP et al (2018) Timosaponin A-III inhibits oncogenic phenotype via regulation of PcG protein BMI1 in breast cancer cells. Mol Carcinog 57(7):831–841
Ghaly NS, Nabil M, Miyase T et al (2014) Steroidal saponins from the roots of Dracaena marginata tam. Pharm Lett 6(2):132–141
Gu L, Feng J, Xu H et al (2013) Polyphyllin I inhibits proliferation of metastasis of ovarian cancer cell line HO-8910PM in vitro. J Trad Chin Med 33(3):325–333
Guo X, Ding X (2018) Dioscin suppresses the viability of ovarian cancer cells by regulating the VEGFR2 and PI3K/AKTR/MAPK signaling pathways. Oncol Lett 15(6):9537–9542
Guo J, Xu C, Xue R (2015) Cytotoxic activities of chemical constituents from rhizomes of Anemarrhena asphodeloides and their analogues. Arch Pharm Res 38:598–603
Hamid AA, Aiyelaagbe OO, Negi AS et al (2016) Bioguided isolation and antiproliferative activity of constituents from Smilax korthalsii A.D.C. leaves. J Chin Chem Soc 63:562–571
Han W, Hou G, Liu L (2015) Polyphyllin I (PPI) increased the sensitivity of hepatocellular carcinoma HepG2 cells to chemotherapy. Int J Clin Exp Med 8(11):20664–20669
Han F-Y, Song X-Y, Chen J-J et al (2018) Timosaponin AIII: a novel potential anti-tumor compound from Anemarrhena asphodeloides. Steroids 140:125–130
He Z, Chen H, Li G et al (2014) Diosgenin inhibits the migration of human breast cancer MDA-MB-231 cells by suppressing Vav2 activity. Phytomedicine 21:871–876
Hien TTT, Tuan HA, Huong DP et al (2018) Two new steroidal saponins from Solanum procumbens. Nat Prod Commun 13(10):1271–1274
Hsieh M-J, Tsai T-L, Hsieh Y-S et al (2013) Dioscin-induced autophagy mitigates cell apoptosis through modulation of PI3K/Akt and ERK and JNK signaling pathways in human lung cancer cell lines. Arch Toxicol 87(11):1927–1937
Huang W, Zou K (2015) Cytotoxicity of the saponin TTB2 on Ewing sarcoma cells. Exp Ther Med 10:625–628
Huang H-C, Lin M-K, Hwang S-Y et al (2013) Two anti-inflammatory steroidal saponins from Dracaena angustifolia Roxb. Molecules 18(8):8752
Huang H-L, Chiang W-L, Hsiao P-C et al (2015) Timosaponin AIII mediates caspase activation and induces apoptosis through JNK1/2 pathway in human promyelocytic leukemia cells. Tumor Biol 36:3489–3497
Ivanchina NV, Kicha AA, Stonik VA (2011) Steroid glycosides from marine organisms. Steroids 76:425–454
Jabrane A, Ben Jannet H, Miyamoto T (2011) Spirostane and cholestane glycosides from the bulbs of Allium nigrum L. Food Chem 125(2):447–455
Jagadeesan J, Nandakumar N, Rengarajan T et al (2012) Diosgenin, a steroidal saponin, exhibits anticancer activity by attenuating lipid peroxidation via enhancing antioxidant defense system during NMU induced breast carcinoma. J Environ Pathol Toxicol Oncol 31(2):121–129
Jesus M, Martins AP, Gallardo E et al (2016) Diosgenin: recent highlights on pharmacology and analytical methodology. J Anal Methods Chem 2016:4156293
Jiang S, Fan J, Wang Q et al (2016) Diosgenin induces ROS-dependent autophagy and cytotoxicity via mTOR signaling pathway in chronic myeloid leukemia cells. Phytomedicine 23:243–252
Jing S-S, Wang Y, Li X-J et al (2017) Chemical constituents from Paris thibetica and their antitumor activities. Chin Trad Herb Drugs 48(6):1093–1098
Jung O, Lee J, Lee YJ et al (2016) Timosaponin AIII inhibits migration and invasion of A549 human non-small-cell lung cancer cells via attenuations of MMP-2 and MMP-9 by inhibitions of ERK1/2, Src/FAK and β-catenin signaling pathways. Bioorg Med Chem Lett 26(16):3963–3967
Kang Y-J, Chung H-J, Nam J-W et al (2011) Cytotoxic and antineoplastic activity of timosaponin A-III for human colon cancer cells. J Nat Prod 74(4):701–706
Kang L-P, Liu Y-X, Eichhorn T et al (2012) Polyhydroxylated steroidal glycosides from Paris polyphylla. J Nat Prod 75(6):1201–1205
Kawasaki T, Yamauchi T (1963) Saponins of timo (Anemarrhenae rhizoma). II. Structure of timosaponin A-III. Chem Pharm Bull 11(10):1221–1224
Kawasaki T, Yamauchi T, Itakura N (1963) Saponins of timo (Anemarrhenae rhizoma). I Yakugaku Zasshi 83:892–896
Kicha AA, Ivanchina NV, Huong TTT et al (2010) Two new asterosaponins, archasterosides A and B, from the Vietnamese starfish Archaster typicus and their anticancer properties. Bioorg Med Chem Lett 20(12):3826–3830
Kim DS, Jeon BK, Lee YE et al (2012) Diosgenin induces apoptosis in HepG2 cells through generation of reactive oxygen species and mitochondrial pathway. Evid Based Complement Alternat Med 2012:981675
Kim CS, Oh JY, Choi SU et al (2013) Chemical constituents from the roots of Cynanchum paniculatum and their cytotoxic activity. Carbohydr Res 381:1–5
Kim KM, Im A-R, Kim SH et al (2016) Timosaponin AIII inhibits melanoma cell migration by suppressing COX-2 and in vivo tumor metastasis. Cancer Sci 107:181–188
Kirmizibezkmez H, Masullo M, Festa M et al (2014) Steroidal glycosides with antiproliferative activities from Digitalis trojana. Phytother Res 28:534–538
Kong M, Fan J, Dong A et al (2010) Effects of polyphyllin I on growth inhibition of human non-small lung cancer cells and in xenograft. Acta Biochim Biophys 42:827–833
Kou Y, Ji L, Wang H et al (2017) Connexin 43 upregulation by dioscin inhibits melanoma progression via suppressing malignancy and inducing M1 polarization. Int J Cancer 141(8):1690–1703
Kougan GB, Miyamoto T, Tanaka C et al (2010) Steroidal saponins from two species of Dracaena. J Nat Prod 73(7):1266–1270
Kwon C-S, Sohn HY, Kim SH et al (2003) Anti-obesity effect of Dioscorea nipponica Makino with lipase-inhibitory activity in rodents. Biosci Biotechnol Biochem 67(7):1451–1456
Lanzotti V (2005) Bioactive saponins from Allium and Aster plants. Phytochem Rev 4(2–3):95–100
Lee SR, Han J-Y, Kang HR et al (2016) A new steroidal saponin from the tubers of Ophiopogon japonicus and its protective effect against cisplatin-induced renal cell toxicity. J Braz Chem Soc 27(4):706–711
Lepage C, Liagre B, Cook-Moreau J et al (2010) Cyclooxygenase-2 and 5-lipoxygenase pathways in diosgenin-induced apoptosis in HT-29 and HCT-116 colon cancer cells. Int J Oncol 36:1183–1191
Lepage C, Léger DY, Bertrand J et al (2011) Diosgenin induces death receptor-5 through activation of p38 pathway and promotes TRAIL-induced apoptosis in colon cancer cells. Cancer Lett 301:193–202
Li F, Fernandez PP, Rajendran P et al (2010) Diosgenin, a steroidal saponin, inhibits STAT3 signaling pathway leading to suppression of proliferation and chemosensitization of human hepatocellular carcinoma cells. Cancer Lett 292(2):197–207
Li N, Zhang L, Zeng K et al (2013) Cytotoxic steroidal saponins from Ophiopogon japonicus. Steroids 78(1):1–7
Li Y, Man S, Li J et al (2014) The antitumor effect of formosanin C on HepG2 cell as revealed by 1H-NMR based metabolic profiling. Chem Biol Interact 220:193–199
Li Y, Wang X, He H et al (2015) Steroidal saponins from the roots and rhizomes of Tupistra chinensis. Molecules 20(8):13659
Li L, Wu J, Zheng F et al (2016) Inhibition of EZH2 via activation of SAPK/JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J Exp Clin Canc Res 5:112–124
Li G-B, Fu R-Q, Shen H-M et al (2017) Polyphyllin I induces mitophagic and apoptotic cell death in human breast cancer cells by increasing mitochondrial PINK1 levels. Oncotarget 8(6):10359–10374
Liang F, He J-W, Zhu G-H et al (2016) New steroidal saponins with cytotoxic activities from Smilax trinervula. Phytochem Lett 16:294–298
Lim W-Ch, Kim H, Kim Y-J et al (2017) Dioscin suppresses TGF-β1-induced epithelial-mesenchymal transition and suppresses A549 lung cancer migration and invasion. Bioorg Med Chem Lett 27(15):3342–3348
Lin C-L, Lee C-H, Chen C-M et al (2018) Protodioscin induces apoptosis through ROS-mediated endoplasmic reticulum stress via the JNK/p38 activation pathways in human cervical cancer cells. Cell Physiol Biochem 46:322–334
Liu C-X, Guo Z-Y, Xue Y-H et al (2012a) Five new furostanol saponins from the rhizomes of Tupistra chinensis. Fitoterapia 83(2):323–328
Liu C-X, Guo Z-Y, Xue Y-H et al (2012b) Tupisteroide A–C, three new polyhydroxylated steroidal constituents from the roots of Tupistra chinensis. Magn Res Chem 50(4):320–324
Liu X, Zhang H, Niu X-F et al (2012c) Steroidal saponins from Smilacina japonica. Fitoterapia 83(4):812–816
Liu J, Man S, Liu Z et al (2016a) A synergistic antitumor effect of polyphyllin I and formosanin C on hepatocarcinoma cells. Bioorg Med Chem Lett 26:4970–4975
Liu W, Ning R, Chen R-N et al (2016b) Aspafilioside B induces G2/M cell cycle arrest and apoptosis by up-regulating H-Ras and N-Ras via ERK and p38 MAPK signaling pathways in human hepatoma HepG2 cells. Mol Carcinog 55(5):440–457
Liu J, Zhang Y, Chen L et al (2017a) Polyphyllin I induces G2/M phase arrest and apoptosis in U251 human glioma cells via mitochondrial dysfunction and the JNK signaling pathway. Acta Biochim Biophys Sin 49(6):1–8
Liu X, Liang J, Pan L-L et al (2017b) Six new furostanol glycosides from Smilax glauco-china and their cytotoxic activity. J Asian Nat Prod Res 19(8):754–765
Liu X, Sun Z, Deng J et al (2018a) Polyphyllin I inhibits invasion and epithelial-mesenchymal transition via CIP2A/PP2A/ERK signaling in prostate cancer. Int J Oncol 53(3):1279–1288
Liu Y, Wang M, Liu K et al (2018b) New steroidal saponins from the rhizomes of Paris vietnamensis and their cytotoxicity. Molecules 23(3):588
Long FY, Chen YS, Zhang L et al (2015) Pennogenyl saponins induce cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. J Ethnopharmacol 162:112–120
Lou W, Chen Y, Zhu K et al (2017) Polyphyllin I overcomes EMT-associated resistance to erlotinib in lung cancer cells via IL-6/STAT3 pathway inhibition. Biol Pharm Bull 40:1306–1313
Lu Y, Chen C-X, Ni W et al (2010) Spirostanol tetraglycosides from Ypsilandra thibetica. Steroids 75(12):982–987
Lu Y, Luo J, Kong L (2011) Steroidal alkaloid saponins and steroidal saponins from Solanum surattense. Phytochemistry 72(7):668–673
Luo Q, Yang D, Qi Q et al (2018) Role of the death receptor and endoplasmic reticulum stress signaling pathways in polyphyllin I-regulated apoptosis of human hepatocellular carcinoma HepG2 cells. Biomed Res Int 2018:5241941
Lv L, Zheng L, Dong D et al (2013) Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: a potential new drug for treatment of glioblastoma multiforme. Food Chem Toxicol 59:657–669
Mai NT, Cuc NT, Anh HLT et al (2017) Steroidal saponins from Datura metel. Steroids 121:1–9
Man S, Gao W, Zhang Y et al (2011) Formosanin C-inhibited pulmonary metastasis through repression of matrx metalloproteinases on mouse lung adenocarcinoma. Cancer Biol Ther 11(6):592–598
Man S, Zhang L, Cui J et al (2018) Curcumin enhances the anticancer effect of paris saponin II in lung cancer cells. Cell Prolif 51(4):e12458
Manase MJ, Mitaine-Offer A-C, Pertuit D et al (2012) Solanum incanum and S. heteracanthum as sources of biologically active steroid glycosides: confirmation of their synonymy. Fitoterapia 3(6):1115–1119
Mao Z-J, Tang Q-J, Zhang Z-A et al (2012) Anti-proliferation and anti-invasion effects of diosgenin on gastric cancer BGC-823 cells with HIF-1α shRNAs. Int J Mol Sci 13:6521–6533
Marelia CB, Sharp AE, Shemwell TA et al (2018) Anemarrhena asphodeloides Bunge and its constituent timosaponin-AIII induce cell cycle arrest and apoptosis in pancreatic cancer cells. FEBS Open Bio 8:1155–1166
Masullo M, Pizza C, Piacente S (2016) Ruscus genus: a rich source of bioactive steroidal saponins. Planta Med 82:1513–1524
Mirunalini S, Arulmozhui V, Shahira R (2011) Effect of diosgenin—a plant steroid on lipid peroxidation and antioxidant status in human laryngeal carcinoma cells (Hep2). Int J Pharm Pharm Sci 3(4):94–100
Mosad RR, Ali MH, Ibrahim MT et al (2017) New cytotoxic steroidal saponins from Cestrum parqui. Phytochem Lett 22:167–173
Nakamura O, Mimaki Y, Sashida Y et al (1993) Agapanthussaponins A-D, new potent cAMP phosphodiesterase inhibitors from the underground parts of Agapanthus inaperatus. Chem Pharm Bull 41(10):1784–1789
Nie C, Zhou J, Qin X et al (2016) Diosgenin–induced autophagy and apoptosis in a human prostate cancer cell line. Mol Med Rep 14:4349–4359
Ning Z, Li Y, Zhang R (2010) Effects of methyl protodioscin on [Ca2+]i and ATPase activity in cardiomyocytes and analysis of mechanisms. Zhongguo Zhong Yao Za Zhi 35(1):80–83
Pan Z-H, Li Y, Liu J-L et al (2012) A cytotoxic cardenolide and a saponin from the rhizomes of Tupistra chinensis. Fitoterapia 83(8):1489–1493
Park JE, Woo KW, Choi SU et al (2014) Two new cytotoxic spirostane-steroidal saponins from the roots of Bletilla striata. Helv Chim Acta 97(1):56–63
Pérez-Labrada K, Brouard I, Estévez S et al (2012a) Effect of C-ring modifications on the cytotoxicity of spirostan saponins and related glycosides. Bioorg Med Chem 20(14):4522–4531
Pérez-Labrada K, Brouard I, Estévez S et al (2012b) New insights into the structure–cytotoxicity relationship of spirostan saponins and related glycosides. Bioorg Med Chem 20(8):2690–2700
Podolak I, Galanty A, Sobolewska D (2010) Saponins as cytotoxic agents: a review. Phytochem Rev 9:425–474
Poudel B, Lim S-W, Ki H-H et al (2014) Dioscin inhibits adipogenesis through the AMPK/MAPK pathway in 3T3-L1 cells and modulates fat accumulation in obese mice. Int J Mol Med 34:1401–1408
Qin X-J, Yu M-Y, Ni W et al (2016) Steroidal saponins from stems and leaves of Paris polyphylla var. yunnanensis. Phytochemistry 121:20–29
Qin X-J, Si Y-A, Chen Y et al (2017) Cytotoxic steroidal saponins from Trillium kamtschaticum. Bioorg Med Chem Lett 27:2267–2273
Qin X-J, Ni W, Chen C-X et al (2018) Seeing the light: shifting from wild rhizomes to extraction of active ingredients from above-ground parts of Paris polyphylla var. yunnanensis. J Ethnopharmacol 224:134–139
Rahman SU, Ismail M, Khurram M et al (2017) Bioactive steroids and saponins of the genus Trillium. Molecules 22:2156
Rahmati-Yamchi M, Ghareghomi S, Haddadchi G et al (2013) Diosgenin inhibits hTERT gene expression in the A549 lung cancer cell line. Asian Pac J Cancer Prev 14(11):6945–6948
Rani AS, Sulakshana G, Patnaik S (2012) Costus speciosus, an antidiabetic plant—review. FS J Pharm Res 1(3):52–53
Raslan MA, Melek FR, Said AA et al (2017) New cytotoxic dihydrochalcone and steroidal saponins from the aerial parts of Sansevieria cylindrica Bojer ex Hook. Phytochem Lett 22:39–43
Regaldo EL, Tasdemir D, Kaiser M et al (2010) Antiprotozoal steroidal saponins from the marine sponge Pandaros acanthifolium. J Nat Prod 73(8):1404–1410
Rezgui A, Mitaine-Offer A-C, Paululat T et al (2014) Cytotoxic steroidal glycosides form Allium flavum. Fitoterapia 93:121–125
Ribeiro PR, Araújo AJ, Costa-Lotufo LV et al (2016a) Spirostanol glucosides from the leaves of Cestrum laevigatum L. Steroids 106:35–40
Ribeiro PR, Braz-Filho R, Araújo AJ et al (2016b) New epimeric spirostanol and furostanol-type steroidal saponins from Cestrum laevigatum L. J Braz Chem Soc 27(12):2170–2180
Said A, Aboutabl EA, Melek FR et al (2015) Steroidal saponins and homoisoflavanone from the aerial parts of Sansevieria cylindrica Bojer ex Hook. Phytochem Lett 12:113–118
Salgado RM, Marques-Silva MH, Gonçalves E et al (2017) Effect of oral administration of Tribulus terrestris extract on semen quality and body fat index of infertile men. Andrologia 49(5):e12655
Selim S, Al Jaouni S (2015) Anticancer and apoptotic effects on cell proliferation of diosgenin isolated from Costus speciosus (Koen.) Sm. BMC Complem Altern Med 15(1):301–307
Sethi G, Shanmugam MK, Warrier S et al (2018) Pro-apoptotic and anti-cancer properties of diosgenin: a comprehensive and critical review. Nutrients 10:456–645
Shen S, Li G, Huang J et al (2012) Steroidal saponins from Fritillaria pallidiflora Shrenk. Fitoterapia 83:785–794
Shen H-Y, Zuo W-J, Wang H et al (2014) Steroidal saponins from dragon’s blood of Dracaena cambodiana. Fitoterapia 94:94–101
Shi Y-M, Yang L, Geng Y-D et al (2015) Polyphyllin I induced-apoptosis is enhanced by inhibition of autophagy in human hepatocellular carcinoma cells. Phytomedicine 22(13):1139–1149
Shi F, Lu L, Li Y et al (2018) Abstract 2871: diosgenin inhibits the proliferation of MCF-7 breast cancer cells through the demethylation of miR-145 gene. Cancer Res 78(13 Suppl):2871
Shu J, Zhu G, Huang G et al (2017) New steroidal saponins with l-arabinose moiety from the rhizomes of Smilax scobinicaulis. Phytochem Lett 21:194–199
Shwe HH, Aye M, Sein MM et al (2010) Cytotoxic steroidal saponins from the rhizomes of Tacca integrifolia. Chem Biodiver 7(3):610–622
Sobolewska D, Janeczko Z, Kisiel W et al (2006) Steroidal saponins from the underground parts of Allium ursinum L. and their cytostatic and antimicrobial activity. Acta Pol Pharm Drug Res 63(3):219–223
Sobolewska D, Michalska K, Podolak I et al (2016) Steroidal saponins from the genus Allium. Phytochem Rev 15:1–35
Sohn H-Y, Kum E-J, Ryu H-Y et al (2006) Antifungal activity of fistulosides, steroidal saponins from Allium fistulosum L. J Life Sci 16(2):310–314
Stefanowicz-Hajduk J, Kawiak A, Gajdus J et al (2011) Cytotoxic activity of Paris quadrifolia extract and isolated saponin fractions against human tumor cell lines. Acta Biol Cracov Series Bot 53(2):127–131
Stefanowicz-Hajduk J, Bartoszewski R, Bartoszewska S et al (2015) Pennogenyl saponins from Paris quadrifolia L. induce extrinsic and intrinsic pathway of apoptosis in human cervical cancer HeLa cells. PLoS ONE 10(8):e0135993
Sun L, Zhao Y, Yuan H et al (2011) Solamargine, a steroidal alkaloid glycoside, induces oncosis in human K562 leukemia and squamous cell carcinoma KB cells. Cancer Chemother Pharmacol 67(4):813–821
Sun Y, Qiu H, Ou M et al (2016) Taccaoside induces apoptosis in hepatocellular carcinoma cell lines. J Int Med Res 44(6):395–1402
Sy LK, Yan SC, Lok CN et al (2008) Timosaponin A-III induces preceding mitochondria-mediated in HeLa cancer cells. Cancer Res 68(24):10229–10237
Tabopda TK, Mitaine-Offer A-C, Tanaka C et al (2014) Steroidal saponins from Dioscorea preusii. Fitoterapia 97:198–203
Tabopda TK, Mitaine-Offer A-C, Paululat T et al (2016) Steroidal saponins from Chlorophytum deistelianum. Phytochemistry 126:34–40
Tang Y, Li N, Duan J-A et al (2013) Structure, bioactivity, and chemical synthesis of OSW-1 and other steroidal glycosides in the genus Ornithogalum. Chem Rev 113(7):5480–5514
Tang Y, He X, Ye M et al (2015) Cardioprotective effect of total saponins from three medicinal species of Dioscorea against isoprenaline-induced myocardial ischemia. J Ethnopharmacol 175:451–455
Tao X, Xu L, Yin L et al (2017) Dioscin induces prostate cancer cell apoptosis through activation of estrogen receptor-β. Cell Death Dis 8:e2989
Tao X, Yin L, Peng J (2018) Dioscin: a diverse acting natural compound with therapeutic potential in metabolic diseases, cancer, inflammation and infections. Pharmacol Res 137:259–269
Tapondjou LA, Jenett-Siems K, Böttger S et al (2013) Steroidal saponins from the flowers of Dioscorea bulbifera var. sativa. Phytochemistry 95:341–350
Teponno RB, Tanaka C, Jie B et al (2016) Trifasciatosides A-J, steroidal saponins from Sansevieria trifasciata. Chem Pharm Bull 64(9):1347–1355
Teponno RB, Dzoyem JP, Nono RN et al (2017) Cytotoxicity of secondary metabolites from Dracaena viridiflora Engl & Krause and their semisynthetic analogues. Rec Nat Prod 11(5):421–430
Tian L-W, Zhang Z, Long H-L et al (2017) Steroidal saponins from the genus Smilax and their biological activities. Nat Prod Bioprospect 7:283–298
Timité G, Mitaine-Offer A-C, Miyamoto T et al (2013) Structure and cytotoxicity of steroidal glycosides from Allium schoenoprasum. Phytochemistry 88:61–66
Tong Q-Y, Qing Y, Shu D et al (2011) Deltonin, a steroidal saponin, inhibits colon cancer cell growth in vitro and tumor growth in vivo via induction of apoptosis and antiangiogenesis. Cell Physiol Biochem 27(3–4):233–242
Tong Q-Y, He Y, Zhao Q-B et al (2012) Cytotoxicity and apoptosis-inducing effect of steroidal saponins from Dioscorea zingiberensis Wright against cancer cells. Steroids 77(12):1219–1227
Tong Q, Qing Y, Wu Y et al (2014) Dioscin inhibits colon tumor growth and tumor angiogenesis through regulating VEGFR2 and AKT/MAPK signaling pathways. Toxicol Appl Pharmacol 281(2):166–173
Valayil JM, Kuriakose GC, Jayabaskaran C (2016) Isolation, purification and characterization of a novel steroidal saponin cholestenol glucoside from Lasiodiplodia theobromae that induces apoptosis in A549 cells. Anticancer Agents Med Chem 16(7):865–874
Wang W, Meng H (2015) Cytotoxic, anti-inflammatory and hemostatic spirostane-steroidal saponins from the ethanol extract of the roots of Bletilla striata. Fitoterapia 101:12–18
Wang Z, Zhou J, Ju Y et al (2001) Effects of two saponins extracted from the Polygonatum zanlanscianense pamp on the human leukemia (HL-60) cells. Biol Pharm Bull 24(2):159–162
Wang K-W, Zhang H, Shen L-Q et al (2011) Novel steroidal saponins from Liriope graminifolia (Linn) Baker with anti-tumor activities. Carbohydr Res 346(2):253–258
Wang H, Zhai Z, Li N et al (2013a) Steroidal saponin of Trillium tschonoskii. Reverses multidrug resistance of hepatocellular carcinoma. Phytomedicine 20(11):985–991
Wang N, Feng Y, Zhu M et al (2013b) A novel mechanism of XIAP degradation induced by timosaponin AIII in hepatocellular carcinoma. Biochim Biophys Acta 1833:2890–2899
Wang Y, Tang Q, Jiang S et al (2013c) Anti-colorectal cancer activity of macrostemonoside A mediated by reactive oxygen species. Biochem Biophys Res Commun 441:825–830
Wang L, Jiang X-L, Zhang W-M et al (2017a) Homo-aro-cholestane, furostane and spirostane saponins from the tubers of Ophiopogon japonicus. Phytochemistry 136:125–132
Wang Y, Xu L, Lou L-L et al (2017b) Timosaponin AIII induces and autophagy in human melanoma A375-S2 cells. Arch Pharm Res 40(1):69–78
Wang J, Song H, Wu X et al (2018) Steroidal saponins from Vernonia amygdalina Del. and their biological activity. Molecules 23:579
Watanabe S, Suzuki T, Hara F et al (2017) Polyphyllin D, a steroidal saponin in Paris polyphylla, induces apoptosois and necroptosis cel death of neuroblastoma cells. Pediatr Surg Int 33:713–719
Wei Y, Xu Y, Han X et al (2013) Anti-cancer effects of dioscin on three kinds of human lung cancer cell lines through inducing DNA damage and activating mitochondrial signal pathway. Food Chem Toxicol 59:118–128
Wei S, Fukuhara H, Chen G et al (2014) Terrestrosin D, a steroidal saponin from Tribulus terrestris L. inhibits growth and angiogenesis of human prostate cancer in vitro and in vivo. Pathobiology 81:123–132
Wen Y-S, Ni W, Qin X-J et al (2015) Steroidal saponins with cytotoxic activity from the rhizomes of Paris polyphylla var. yunnanensis. Phytochem Lett 12:31–34
Wu RT, Chiang HC, Fu WC et al (1990) Formosanin-C, an immunomodulator with antitumor activity. Int J Immunopharmacol 12(7):777–786
Wu J-J, Cheng K-W, Zuo X-F et al (2010) Steroidal saponins and ecdysterone from Asparagus filicinus and their cytotoxic activities. Steroids 75(10):734–739
Wu X, Wang L, Wang G-C et al (2012a) New steroidal saponins and sterol glycosides from Paris polyphylla var. yunnanensis. Planta Med 78(15):1667–1675
Wu X, Wang L, Wang H et al (2012b) Steroidal saponins from Paris polyphylla var. yunnanensis. Phytochemistry 81:133–143
Wu S, Xu H, Peng J et al (2015) Potent anti-inflammatory effect of dioscin mediated by suppression of TNF-α-induced VCAM-1, ICAM-1 and EL expression via the NF-κB pathway. Biochimie 110:62–72
Wu X, Chen N-H, Zhang Y-B et al (2017a) A new steroid saponin from the rhizomes of Paris polyphylla var. yunanensis. Chem Nat Comp 53(1):93–98
Wu Y, Wang X-M, Bi S-X et al (2017b) Novel cytotoxic steroidal saponins from the roots of Liriope muscari (Decne.) L.H. Bailey. RSC Adv 7(23):13696–13706
Wu Y, Bi S-X, Huang Z et al (2018) Novel steroidal saponins with cytotoxic activities from the roots of Ophiopogon japonicus (L. f.) Ker-Gawl. RSC Adv 8:2498–2505
Xia L, Ouyang P-Y, Gao W et al (2016) Rapid and sensitive determination of the major steroidal saponins of Ypsilandra thibetica Franch by ultra high-performance liquid chromatography coupled with quadrupole mass sectrometry. J Chromatogr Sci 54(6):1010–1015
Xiang S, Zou P, Wu J et al (2018) Crosstalk of NF-κB/P65 and lncRNA HOTAIR-mediated repression of MUC1 expression contribute to synergistic inhibition of castration-resistant prostate cancer by polyphyllin 1-enzalutamide combination treatment. Cell Physiol Biochem 47(2):759–773
Xiao X, Zou J, Bui-Nguyen TM et al (2012) Paris saponin II of Rhizoma Paridis—a novel inducer of apoptosis in human ovarian cancer cells. BioSci Trends 6(4):201–211
Xiao T, Zhong W, Zhao J et al (2018) Polyphyllin I suppresses the formation of vasculogenic mimicry via Twist1/VE-cadherin pathway. Cell Death Dis 9:906–919
Xu X-H, Li T, Fong CMV et al (2016) Saponins from Chinese medicines as anticancer agents. Molecules 21(10):1326
Yang M, Zou J, Zhu H et al (2015) Paris saponin II inhibits human ovarian cancer cell-induced angiogenesis by modulating NF-κB signaling. Oncol Rep 33:2190–2198
Yang B-Y, Zhang J, Liu Y et al (2016a) Steroidal saponins from the rhizomes of Anemarrhena asphodeloides. Molecules 21(8):1075
Yang J, Wang P, Wu W et al (2016b) Steroidal saponins in oat bran. J Agric Food Chem 64(7):1549–1556
Yang B-Y, Bi X-Y, Liu Y et al (2017) Four new glycosides from the rhizome of Anemarrhena asphodeloides. Molecules 22(11):1995
Yang B, Xu B, Zhao H et al (2018) Dioscin protects against coronary heart disease by reducing oxidative stress and inflammation via Sirt1/Nrf2 and p38 MAPK pathways. Mol Med Rep 18(1):973–980
Yu H, Liu Y, Niu C et al (2018a) Diosgenin increased DDX3 expression in hepatocellular carcinoma. Am J Transl Res 10(11):3590–3599
Yu S, Wang L, Cao Z et al (2018b) Anticancer effect of polyphyllin I in colorectal cancer cells through ROS-dependent autophagy and G2/M arrest mechanism. Nat Prod Res 32(12):1489–1492
Zaki AA, Ali Z, Wang Y-H et al (2017) Cytotoxic steroidal saponins from Panicum turgidum Forssk. Steroids 125:14–19
Zeng K-W, Song F-J, Li N et al (2015) ASC, a bioactive steroidal saponin from Ophitopogin japonicas, inhibits angiogenesis through interruption of src tyrosine kinase-dependent matrix metalloproteinase pathway. BCPT 116(2):115–123
Zhang C, Feng S, Zhang L et al (2013) A new cytotoxic steroidal saponin from the rhizomes and roots of Smilax scobinicaulis. Nat Prod Res 27(14):1255–1260
Zhang Y, Han Y, Zhai K et al (2015) Ophiopogonin D suppresses MDA-MB-435 cell adhesion and invasion by inhibiting matrix metalloproteinase-9. Mol Med Rep 12(1):1493–1498
Zhang L, Man S, Wang Y et al (2016) Paris saponin II induced apoptosis via activation of autophagy in human lung cancer cells. Chem Biol Interact 253:125–133
Zhang Y-W, Zhao Y-F, Wang Y-R et al (2017) Steroidal saponins with cytotoxic activities from the rhizomes of Anemarrhena asphodeloides Bge. Phytochem Lett 20:102–105
Zhang S, Pang H, Sun M et al (2018a) Timosaponin AIII inhibits the growth of human leukaemia cells HL-60 by down-regulation of PI3K/AKT and Wnt/β-catenin pathways. Biotechnol Biotec Eq 32(1):150–155
Zhang Y, Huang P, Liu X et al (2018b) Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J Pharmacol Sci 137:305–312
Zhang Y-S, Ma Y-L, Thakur K et al (2018c) Molecular mechanism and inhibitory targets of dioscin in HepG2 cells. Food Chem Toxicol 120:143–154
Zhao X, Tao X, Peng J (2016) Dioscin induces apoptosis in human cervical carcinoma HeLa and SiHa cells through ROS-mediated DNA damage and the mitochondrial signaling pathway. Molecules 21(6):730
Zhao Y-Z, Zhang Y-Y, Han H et al (2018) Advances in the antitumor activities and mechanisms of action of steroidal saponins. Chin J Nat Med 16(10):732–748
Zhiyu W, Yue C, Neng W et al (2012) Dioscin induces cancer cell apoptosis through elevated oxidative stress mediated by downregulation of peroxiredoxins. Cancer Biol Ther 13(3):138–147
Zolfaghari B, Sadeghi M, Troiano R et al (2013) Vavilosides A1/A2–B1/B2, new furostane glycosides from the bulbs of Allium vavilovii with cytotoxic activity. Bioorg Med Chem 21(7):1905–1910
Zuo S-Q, Liu Y-N, Yang Y (2018) Aspidsaponins A–D, four new steroidal saponins from the rhizomes of Aspidistra elatior Blume and their cytotoxicity. Phytochem Lett 25:126–131
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sobolewska, D., Galanty, A., Grabowska, K. et al. Saponins as cytotoxic agents: an update (2010–2018). Part I—steroidal saponins. Phytochem Rev 19, 139–189 (2020). https://doi.org/10.1007/s11101-020-09661-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-020-09661-0