Abstract
Saponins make up an important group of natural glycosidic compounds which are distinguished by triterpene or steroidal aglycone. Although widely distributed in terrestrial flora, especially higher plants, they can also be found in some marine organisms. Cytotoxic activity is one of the most frequently reported from a wide array of pharmacological activities known for these metabolites. The current review is an update of our previous paper—Saponins as cytotoxic agents (Podolak et al. Phytochem Rev 9:425–474, 2010), and covers studies that were since published (2010–2021). This part refers to triterpene saponins and complements the first, which was devoted solely to steroidal saponins (Sobolewska et al. Phytochem Rev 19:139–189, 2020). Cytotoxic activities in vitro and in vivo are presented with a main focus on structure-activity relationships and molecular mechanisms of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Saponins are natural products of glycosidic nature, well known for their surface activity and foaming properties, which is reflected in the name given to this group (from the Latin word sapo = soap). Their amphiphilic character results from a combination in a molecule of the hydrophilic properties of the sugar part and the hydrophobic aglycone. As isoprenoids, they are synthesized from mevalonate via squalene, producing a wide diversity of sapogenins, either steroidal or triterpene, to which sugar chains (up to three) are linked with an ether or ester bond (Osbourn et al. 2011; Moses et al. 2014). Therefore the classification of saponins is based on the aglycone backbone and its respective structural types. Triterpene saponins are much more numerous and structurally diverse than steroidal compounds. They are usually divided into two classes, pentacyclic and tetracyclic, each of which comprises a large number of aglycone types. The most common pentacyclic sapogenins have oleanane, ursane, or lupane skeletons, while taraxastane or hopane are considered more rare. The vast majority of oleanane saponins are classified to Δ12 type, that comprises a large number of subgroups, such as eg. Δ12-oleanan-28,21β-olide, Δ12-oleanan-28,15β-olide, and others. Compounds with rearranged double bond (Δ13 or Δ14) are less common, as well as those without this structural feature such as 13,28-epoxyoleananes, which are distributed among a limited number of plant genera (Foubert et al. 2008; Dinda et al. 2010). Also, saponins with tetracyclic genins are generally less abundant in nature, the best known of these are derivatives of dammarane and cycloartane. Some more unique structural types of sapogenins contain nitrogen atom in an aminoacyl moiety attached to the triterpene skeleton (Arslan and Cenzano 2020).
Similarly to steroidal saponins, triterpene glycosides can have up to three sugar chains attached to the aglycone. Compounds with one sugar chain, known as monodesmosides, are most numerous, whereas those with three saccharide chains are fairly rare. The glycone part is usually composed of β-D-glucopyranose (Glc), β-D-galactopyranose (Gal), α-L-rhamnopyranose (Rha), β-L-arabinofuranose (Ara), β-D-xylopyranose (Xyl), β-D-fucopyranose (Fuc), β-D-mannopyranose (Man), while the presence of uronic acids, such as for example β-D-glucuronic acid, is considered a characteristic feature of saponins with triterpene genins as opposed to steroidal. Sugar chains may be linear or branched; they are quite often prenylated or acylated, and they are made of up to eleven monosaccharide units. Although in the case of triterpene monodesmosides the most common linkage site is through the ether bond at C-3 (less often at C-23 or by an ester bond at C-28), in bidesmosides the sugar chain attachment positions are usually C-3 + C-28 or C-3 + C-30.
Triterpene saponins are predominantly found in plants, however they have been reported also in marine organisms, such as, for example, sea cucumbers. As opposed to steroidal saponins, triterpene glycosides are typical of dicots and are distributed across a wide range of families, including Amaranthaceae, Araliaceae, Caryophyllaceae, Primulaceae, Sapindaceae, Sapotaceae and other. Their levels in plant material may be very high, exceeding 10–20%, as in horse chestnut seeds (Aesculus hippocastanum) or soapbark (Quillaja saponaria), although in most cases amount to 1–2% (eg, ginseng root). Plants rich in triterpene saponins have been used for medicinal purposes since antiquity. Saponin extracts and partially purified saponin fractions are also of industrial value, providing eco-friendly cosmetic formulations or being used in vaccine production as adjuvants (Dinda et al. 2010; Oleszek and Hamed 2010; Wang 2021).
The structural diversity and high polarity of triterpene saponins, as well as their occurrence in plant material, usually in complex mixtures, which only slightly differ in aglycone substituents or the composition of the sugar part, is an enormous challenge in terms of isolation of individual compounds. This is in turn essential for subsequent studies of pharmacological activity, molecular mechanisms of action, and structure activity relationships. Each year a large number of scientific reports appear in the literature that describe the structure elucidation of novel saponins, most often accompanied by studies of pharmacological activity (Garai 2014). It should be noted that within a very wide array of pharmacological effects known for triterpene saponins, including anti-inflammatory, gastroprotective, immunomodulatory, anti-leishmanial, and many others, the main focus has been on their cytotoxicity and potential role as antitumor agents.
Already in our previous review (Podolak et al. 2010) that covered the cytotoxicity data on triterpene saponins from 2005 to 2009, up to 193 active compounds have been reported. Since then, an increasing trend can be seen in evaluating their activity toward cancer cells, which is clearly reflected by a large number of new published results from in vitro and in vivo studies. This large reservoir of experimental data allows insight into the structure–activity relationships, mechanism of action, and versificated susceptibility of cancer cell lines to triterpene saponins. Although several reviews on triterpene saponins have appeared in the last decade, they generally listed cytotoxicity among other pharmacological activities (Osbourn et al. 2011; Thoppil and Bishayee 2011; Ali et al. 2012; Saraswati 2019) or were limited to a certain aspect related to cytotoxic activity (Wang et al. 2010; Tian et al. 2013b; Levitsky and Dembitsky 2015), focused exclusively on some structural type (Gauthier et al. 2011; Lacaille-Dubois et al. 2011; Patlolla and Rao 2015) or plant sources, such as licorice (Tang et al. 2015). Regarding botanical sources, more attention was given to potential antitumor effects of saponins typical of the Myrsinaceae family (Muhamad et al. 2021), Medicago species (Wang et al. 2021a), and most particularly ginseng and isolated ginsenosides (Nag et al. 2012; Choi et al. 2013; Vayghan et al. 2014; Wong et al. 2015; Nakhjavani et al. 2019; Wu et al. 2021b; Xu et al. 2021; Wang et al. 2021b). Also, reviews that focused on various natural compounds that targeted specific mechanisms relevant to cancer, including autophagy (Han and He 2021), tumor angiogenesis-associated cytokines (Li et al. 2021c) suppression of active Mdm2 (Kaur and Nayak 2021) listed examples of saponins among other active natural molecules.
In this second part of the current updated review of saponins as cytotoxic agents, we refer to the cytotoxic activity of triterpene saponins reported during the last decade (2010–2021). Similarly to the first part, which was devoted to steroidal saponins (Sobolewska et al. 2020), the emphasis is placed on structure–activity relationships and novel mechanisms of action. Electronic databases including SCOPUS, EMBASE, and MEDLINE/PubMed, were used to compile the literature. Keywords used were triterpene saponins, cancer, and cytotoxicity. Only English full texts were chosen for this review. Isolated plant saponins were taken into account, excluding total extracts and fractions, as well as compounds derived from marine sources, because respective reviews of these have recently been published (Park et al. 2014; Aminin et al. 2015; Honey-Escandón et al. 2015; Wargasetia and Widodo 2017; Eso et al. 2020). Among the primarily selected articles, which were numerous, only those studies in which the activities expressed as IC50 values were below 10 µM were finally included, making a total of 146 reports.
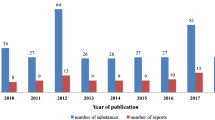
It should be noted that in the time frame of this review (2010–2021) up to 360 triterpene saponins that were tested for cytotoxic activity revealed such potent effects (IC50 < 10 µM). The results of these studies are summarized in Table S1 (see Supplementary Information). The graph below (Fig. 1) shows the number of these highly active compounds investigated per year and the number of published reports that appeared annually. As can be seen, there is steady interest from the scientific community in research focused on the cytotoxic activity of triterpene saponins.
Almost half of the investigated compounds (48%) were newly reported at the time of publication, the remaining 52% were already known structures (Fig. 2a). Most of these were saponins with an oleanane skeleton (85%), the activity of compounds belonging to other structural types was seldom reported (Fig. 2b). It is interesting to note that within the oleanane group, the largest number of cytotoxic saponins represented polyhydroxy compounds, 13,28-epoxy-derivatives, and oleanolic acid glycosides (Fig. 2a).
Among the reported compounds, several exerted significant activity compared to those of the reference drugs or were active toward rare and highly resistant tumor cell lines. One of such examples is azukisaponin IV (Table S1) isolated from Silene odontopetala, with IC50 = 0.09 μM against the A549 cell line, which was more potent compared to the reference doxorubicin (IC50 = 0.17 μM) (Ün et al. 2020). Also, ardicrenin, a triterpene saponin found in the roots of Ardisia crenata, exerted a significant effect, higher than paclitaxel used as a positive control, against several cancer cell lines (Song et al. 2021). These data surely warrant further more in-depth preclinical research that includes studies on the pharmacokinetic profile and possible ways to improve bioavailability. It should be mentioned here that several triterpene saponins that showed promising results in preliminary in vitro cytotoxicity studies have been the subject of extensive research since then; one of such examples is tubeimoside-I. Tubeimosides are saponins present in tubers of Bolbostemma paniculatum with anticancer activity via various mechanisms that were shown in numerous in vitro and in vivo studies (Islam et al. 2019). Some recent reviews cover data on other single saponins with well-studied anticancer potential, such as ginsenosides Rb2, Rg3, Rh2 (Wu et al. 2021b; Miao et al. 2022), chikusetsusaponins (Wang et al. 2021c), saporin-S6 (Chandra et al. 2021), saikosaponin D (Sun et al. 2020; Zhou et al. 2021) or raddeanin A (Naz et al. 2020).
The data summarized in Table S1 (Supplementary Information) reflect the varied effects of the investigated compounds on cell lines derived from different organs and tissues. Although the number of studies performed on human cells (96.1%) as opposed to animal cells (3.9%) has considerably increased compared to data covered by our previous review (Podolak et al. 2010), the percentage of experiments that simultaneously analyze the cytotoxic effect towards cancer and normal cells remains surprisingly low (Fig. 2c). Thus, the selectivity of action in the case of the majority of reported active compounds is still unknown. Nevertheless, a reference drug is included in most currently published articles (82%) which is a requirement that must be met when submitting manuscripts to highly impacted journals. It should be noted that cisplatin, doxorubicin and paclitaxel were chosen much more often than mitoxantrone or 5-FU.
Triterpene saponins with high activity (IC50 < 10 µM) that are included in the current review have been tested on a wide range of cell lines. The graph presented in Fig. 2d shows the percentage of experiments carried out in particular cancer and normal cell lines. Cytotoxicity studies that reported active triterpene saponins were predominantly focused on cell lines derived from colon, liver, and lung cancers that represented 17.13, 14.36 and 12.60%, respectively, of all cell lines tested. Careful analysis of the available data indicates that four human cancer cell lines were used most frequently, namely: A549—lung adenocarcinoma (10.0%), HL-60—leukemia (7.7%), HepG2—hepatocellular carcinoma (7.3%) and MCF-7—breast adenocarcinoma (5.8%).
The wide range of cell lines used in the cytotoxicity studies on triterpene saponins is listed in the legend to Table S1 (Supplementary Information). In the following parts of this paper, several aspects are discussed in more detail. These include structure–activity relationships (SAR), mechanisms of cytotoxic activity, and in vivo experiments. The cell lines that were used in these studies are listed below.
-
Human cancer cell lines: Breast: MCF-7, MDA-MB-231, ZR75-1, T47D; cervix: Bcap-37, HeLa, HeLa-S3, KB; cholangiocarcinoma: RBE, LIPF155C; colon: Caco-2, DLD-1, HT-29, HCT-8, HCT 116, SW480, SW620, HCT-116; esophagus: ESCC; glioma: T98G, U-87 MG, U118, U251 MG; leukemia: CCRF-CEM, HL-60, Jurkat, K562, U937, THP-1; liver: H22, Hep3B, HepG2, BEL-7402, SMMC-7721; lung: A549, H-460; melanoma: A375, A375-R A375.S2; neuroblastoma: SH-SY5Y, IMR-32; oral: YD-10B; osteosarcoma: MG63, U2OS, HOS; ovary: A2780, HEYA8, OVCAR-8, SK-OV-3; pancreas: MIA Paca-2, PANC-1, SW-1990; prostate: DU145, PC-3; retinoblastoma: RB Y79, RBL-13; sarcoma: RD; stomach: AGS, BCG, BGC-823, MKN-45, NCI-N87, SGC-7901; urinary bladder: EJ; thyroid: 8305C, SW1736.
-
Animal cancer cell lines: Glioma: C6 (rat), B16F10 (mouse); mammary gland: 4T1 (mouse); sarcoma: S180 (mouse)
-
Human normal cell lines: Fibroblasts: WL-1; kidney embryonic: HEK293; liver: HL-7702
Structure—activity correlation
Structure–activity correlations have always been an important aspect of studies that refer to the cytotoxicity of triterpene saponins. This issue was discussed in several previous review articles, which were devoted to specific classes of these compounds, such as acacic acid-type saponins (AATS) (Lacaille-Dubois et al. 2011), lupane-type triterpenoid saponins (Gauthier et al. 2011), glycyrrhetinic acid and its derivatives (Roohbakhsh et al. 2016), dammarane-type triterpenoids (Qi et al. 2010; Cao et al. 2012; Nag et al. 2012), ocotillol-type triterpenoids (Cao et al. 2020), or synthetic saponins (Juang and Liang 2020). A summary of the most important correlations derived from earlier studies was included in a book chapter (Loizzo et al. 2013).
The vast body of data on the cytotoxic activity of triterpene saponins that have been published in the time scope of this review (2010–2021) also includes some observations on the structure–activity relationship (SAR). Most of these were derived from in vitro studies (96.6%), only a small percentage of structure–activity observations was based on in vivo experiments (3.4%). A single report included a chemometric analysis of the obtained results (CA) (Sarraj-Laabidi and Semmar 2016).
Almost all SAR observations refer to human cancer cell lines, while studies of the potential impact on normal cells were not frequent. Furthermore, 67% of the publications, in addition to comparing the activity between individual structures, also refer to the standard chemotherapeutic drug. The vast majority of saponins, which were subjected to SAR research, were obtained by isolation from plant material (69.3%). Furthermore, in 5.7% of the analyzes, prosaponins obtained by alkaline hydrolysis of original saponins isolated from natural sources were also included.
Most literature reports on isolated saponins focused on oleanane derivatives (68%) and included mainly glycosides of oleanolic acid (OA), hederagenin (HE), and polyhydroxylated oleananes, as well as saponins with a 13,28-epoxy-oleanane skeleton. Data on dammarane (DT), cycloartane (CAT) or ursane type (UT) compounds were found more rarely, and accounted for 9%, 3% and 3% of the publications, respectively. A group of reports (10.6%) could be defined as "mixed" as they analyzed at the same time various types of saponins with the pentacyclic aglycone skeleton, i.e. derivatives of oleanane (OT), ursane (UT) or lupane-type (LT). Unfortunately, it was not always possible to compare the relationships between these main structural types due to too large differences in their structure, which in fact hindered drawing any SAR conclusions.
A substantial part of the reports devoted to SAR (24% of the papers) concern saponins obtained by chemical or enzymatic semisynthesis. Of these, 14.3% contain data on naturally occurring but artificially produced saponins. The remaining analyzes focused on nonnatural semisynthetic saponin derivatives with a modified backbone, which included mainly oleanolic acid derivatives (23% of the analyzes), hederagenin derivatives (12.8%), and a lower proportion of modified lupane, dammarane or ursane glycosides (these accounted respectively for 10%, 5%, 5% of all SAR reports). Although in some works the role of both the aglycone and sugar part was discussed, it is noteworthy that ca. 30% of all SAR studies focused exclusively on the modifications of 28-COOH in pentacyclic aglycone. Interestingly, 52% analyzed only the effect of the changes made in the sugar part. On the basis of the data collected from the literature for the purpose of this review, it can be seen that both the structure of the aglycone and the sugar portion are important for the activity of triterpene saponins. The main SAR observations that were discussed in the literature in years 2010–2019 are presented below.
The effect of key features of the aglycone part on the cytotoxic activity of saponins
The present research data confirm earlier assumptions that one of the important structural elements that influence the cytotoxic activity of triterpene saponins with the pentacyclic sapogenin skeleton is the free 28-COOH group. Numerous studies indicate a higher activity of monodesmosides of oleanolic acid, hederagenin and echinocystic acid, in which the sugar chain is attached at C-3 of the aglycone, compared to the corresponding bidesmosides with an ester-linked sugar chain in the 28-COOH group. The presence of a sugar moiety in the carbonyl group (28-COOH) significantly reduces or even eliminates cytotoxicity. A summary of studies for oleanane derivatives in which such a relationship was observed in relation to specific cell lines is presented in Table S2 (Supplementary Information).
Some reports indicate that the free COOH group is important for the activity of not only oleanane-derived saponins, but also ursane-type compounds, which was shown, eg, for quinic acid derivatives against Hela-S3 and KB (Kaennakam et al. 2018), as well as urs-12,18-diene-24,28-dioic acid derivatives from Ilex pubescens (Li et al. 2014a, b, c). A similar pattern was also observed for lupane-derived saponins (Xu et al. 2013a). 23-hydroxybetulinic acid monodesmosides showed higher activity against A549, SGC-7901 and HL-7702 cell lines than respective bidesmosides. Yang et al. also noted the importance of the free carboxyl group at C-17 (28-COOH) in lupane-type saponins (Yang et al. 2017).
However, there are SAR studies that indicate that the free 28-COOH group may not always be considered a beneficial structural feature of triterpene saponins as it is also responsible for their hemolytic activity. Therefore, attempts were made to modify the structure of cytotoxic natural triterpene saponins by converting the carbonyl group generally to an amide or ester, bearing various functional groups (Liu et al. 2010; Chen et al. 2015, 2018c; Fang et al. 2016, 2019). However, the final cytotoxic effect depended not only on the type of derivative obtained but predominantly on the sensitivity of a particular cell line.
Polyhydroxyoleanene sapogenins
Data available in the literature on polyhydroxyoleanene triterpenoid saponins also indicate that the free COOH group at C-17 may be an important structural element influencing the potency of these saponins. However, its effect is viewed as different from that observed for many saponins derived from OA or HE. When comparing the activity of bidesmosides and the corresponding monodesmosides of protobassic acid against the PC-3 cell line, Chen et al. (2018a) found that the free 28-COOH group reduces the cytotoxic activity. Similarly, Chun et al. (2013a) observed a complete loss of activity against A549, HepG2, Caco-2, HeLa, MCF-7, AGS, Jurkat cell lines of platycodigenin derivatives deglycosylated at C-28. In addition, bidesmosides (C-3, C-28) of polygalacic acid and bayogenin had higher topoisomerase II inhibitory activity than the corresponding monodesmosides (C-3) (Herrera-Martínez et al. 2012).
It should be noted that the common feature of all these derivatives is the presence of hydroxyl groups at the positions C-2 and C-23. It seems that the location and number of hydroxyl groups in the structure of the molecule may affect the observed activity of the compound. So far, it has been found, among others, that the presence of a hydroxyl at C-16 in the structure of saponins derived from 16α-hydroxyprotobassic acid significantly increases their cytotoxic activity against PC-3 compared to protobassic acid derivatives (Chen et al. 2018a). In turn, Chun et al. (2013a) noticed that a hydroxyl group at C-24 in platycodigenin derivatives appears to decrease the activity of these compounds, compared to dehydroxylated derivatives (polygalacic acid derivative).
The presence and localization of hydroxyl groups have been the subject of several studies on saponins, hydroxy derivatives of oleanolic acid (mono and dihydroxyoleanolic acid derivatives). Several reports indicate that the presence of additional hydroxyl groups at positions C-23, C-19, or C-27 of oleanolic acid reduces the cytotoxic activity of saponins with this type of aglycone. In conclusion, the derivatives of 23-hydroxyoleanolic acid (hederagenin), 19-hydroxyoleanolic acid (siaresinolic acid), and 27-hydroxyoleanolic acid were less effective than the corresponding glycosides of oleanolic acid, as shown in studies in the A549 cell line (Wang et al. 2013c; Xu et al. 2013a; Zhang et al. 2013b; Lv et al. 2021).
In general, OA derivatives were more cytotoxic compared to hederagenin glycosides against several other cell lines, namely HL-60 (Quang et al. 2012; Li et al. 2013b; Tian et al. 2013a; Wang et al. 2013b; Zhang et al. 2013b), SGC -7901 (Tian et al. 2013a; Xu et al. 2013a), PANC-1 (Lv et al. 2021), MDA-MB-231 (Lv et al. 2021), HL-7702 (Xu et al. 2013a), HeLa (Wang et al. 2013b). For siaresinolic acid derivatives, a decrease in activity was also observed in relation to OA saponins against MCF-7, HeLa and A375-S2 (Wang et al. 2012a), HepG2, HL-60, U87MG (Wang et al. 2013b). Similarly, compounds with 27-hydroxyoleanolic acid as sapogenin were found to be less cytotoxic than either OA or hederagenin glycosides against cell lines A549, PANC-1 and MDA-MB-231. Furthermore, the 23,27-dihydoxyoleanolic acid derivatives were less potent than the corresponding monohydroxy derivatives with an OH group located at C-23 or C-27 (Lv et al. 2021).
Some studies suggest that the presence of a hydroxyl group at the C-16 α position in oleanolic acid may also be important for cytotoxic activity. Miyase et al. (2010) concluded that a free hydroxyl at this position was crucial for the activity of echinocystic acid derivatives against HepG2, A549, HT29 and MCF-7, while its glycosidation had a detrimental effect. Additionally, Tchoukoua et al. (2017) indicate that the presence of C-16 is significant for the cytotoxicity toward the HL-60 cell line of N-acetylglucosamine-containing OA saponins.
Esterification of sapogenins
As noted by several authors, another important structural feature of triterpene sapogenins that influences the cytotoxic activity of saponins is their esterification. These observations are especially noticeable in the case of polyhydroxy oleanane derivatives, which are often esterified at C-21, C-22, C-16, or C-28, with angelic, tiglinic, acetic, or cinnamic acids. The presence of an angelic residue at position C-22 of camelliagenin A and barringtogenol C derivatives significantly increased the activity of such saponins against A549, HCT-8, BEL-7402 and A2780 cells, compared to saponins without the 22-hydroxy group (Tang et al. 2013). Grabowska et al. (2017) showed that a 16α-acetylcamelliagenin monodesmoside was more cytotoxic against A375, than its counterpart with a free hydroxyl group. The presence of two acetyl groups at C-1 and C-3 in imberbic acid seemed to determine the activity of 23-O-[α-L-rhamnopyranosyl]-1,3β-diacetyl-imberbic acid (Wu et al. 2010).
Several studies indicate that the potency of the cytotoxic effect is influenced by the type of esterifying acid (Magid et al. 2012; Yuan et al. 2012; Dai et al. 2017) and the esterification site. Yuan et al. (2013) noted that the activity of barringtogenol C derivatives was increased (toward A 549) when C-22 was substituted with an acetyl group and C-28 with a hydroxyl, however, the activity decreased after exchanging the positions of these two substituents. Similar observations were provided by Yu et al. (2012) for theasapogenol derivatives A and B against a broad panel of cell lines. Some authors indicate that acylation at both positions C-21 and C-22 is essential for the cytotoxic activity of theasapogenol A saponins (Magid et al. 2012; Yuan et al. 2012). In contrast, a study by Tang et al. (2013) showed that an additional angeloyl or acyl residue at C-21 of barringtogenol C decreased the cytotoxic activity of this saponin compared to the one that had only one angeloyl at position C-22.
13,28-epoxy oleananes
Some important structure–activity observations have been reported for a relatively rare type of triterpene saponins with an oleanane skeleton and a 13,28-epoxy bridge. Several experiments have confirmed that the respective saponins without the bridge but with the same sugar chain were totally inactive (Li et al. 2012, 2015a; Mu et al. 2013). Furthermore, similarly to previously reported SAR observations for this group of oleanane derivatives (Podolak et al. 2010), a larger number of new data further confirm the apparent importance of a substituent at C-30 of the aglycone. Saponins with a methyl or aldehyde group at this position were more potent against HepG2 (Liang et al. 2011; Li et al. 2012; Mu et al. 2012; Zhang et al. 2013a), HT-29, BGC-823, A549, A357(Liang et al. 2011), MCF-7 (Zhang et al. 2013a; Mu et al. 2019), HeLa, EJ (Mu et al. 2012), BEL7402 (Li et al. 2012) cell lines compared to compounds with either a hydroxymethyl or the nor type (30-hydroxy moiety) structure. However, further studies that focused on this issue concluded that it is not possible to clearly indicate which substituent at C-30 guarantees the best activity, as this also depends on the susceptibility of a particular cell line and the influence of structural features of the sugar part.
It appears that in this group of saponins the presence of the C-28 lactone moiety or the C-16 ketone moiety also eliminates antiproliferative activity, as shown in the BEL-7402 and HepG2 cell lines (Li et al. 2012, 2015a). Additionally, a hydroxyl group at C-16 as well as its alpha configuration increased cytotoxicity towards A549, HepG2, Hep3B, BCAP-37, and MCF-7 (Li et al. 2015a). Mu et al. (2013) found that the presence of C = O at C-16 also seems to affect cytotoxic activity, as such compounds were more potent against HCT-8 and A549, however the remaining structural elements, such as the C-30 moiety and the 13,28-epoxy bridge itself, have a significant influence on the resultant activity.
Sapogenins with a double bond
Several studies indicate that the double C12/13 bond in the C-ring of pentacyclic sapogenins is of key importance for cytotoxic activity. Several experiments on Pulsatilla saponins A and D have shown that modifications leading to the elimination of the Δ12 double bond drastically reduced the cytotoxicity of such derivatives (Chen et al. 2015, 2018c; Tong et al. 2017). Pérez et al. (2017) noticed that also the location of double bonds within the aglycone may affect the potency of a saponin against some cell lines. Ursolic acid derivatives with an additional double bond at position C-18 (19) were more potent and selective against MCF-7 cells than saponins having an additional bond at position C-19 (20). The authors suggest that the presence of two conjugated double bonds in 2α,3β-dihydroxy-12,18(19)-ursadien-24,28-dioic acid may be an important factor that affects the potency and selectivity of the compound.
Rare types of sapogenins
Compared to pentacyclic saponins, relatively little data published in the scope of this review (2010–2021) refer to SAR issues in the cucurbitane-derived saponin group (CT). Few available studies have focused solely on the effect of sapogenin substituents. Gan et al. (2011) confirmed previously postulated conclusions that acetylation of the hydroxyl group at the C-25 position of sapogenin increases the cytotoxicity of cucurbitane derivatives. Chen et al. (2019a) indicate, however, that the presence of a carbonyl group (C = O) in C-11 or both C-11 and C-7 may be important for the activity against the MCF-7 and A-549 cell lines. It should be underlined that these studies were not focused on a comparison of a series of closely related compounds, thus making it difficult to draw any general conclusions.
Similarly, only a few reports dealt with SAR among cycloartane saponins (CATs). Interestingly, an attempt was made to analyze structure–activity relationships between cycloartane glycosides in the genus Astragalus using a chemometric approach (Sarraj-Laabidi et al. 2017). For this purpose the authors included literature data on up to 178 saponins. In conclusion, a general trend was observed between the cytotoxic potential and the aliphatic lateral chain in the cycloartane skeleton together with a single sugar chain linked at the C-3 position. These characteristics in monodesmosides made them more active than the respective 20,24-epoxycycloartane derivatives. In turn, Graziani et al. (2019), suggested that the cytotoxic activity towards Caco-2, HT-29 and HCT-116 cell lines of 20,24-epoxycycloartane saponins could be significantly improved provided some essential structural requirements are met. These include the presence of C3-xylopyranosyl, along with the C6-acetoxy group and the C25-free hydroxyl function.
In the years 2010–2021, several reviews on dammarane derivatives were published, in which the SAR relationships in this group of saponins were thoroughly discussed (Qi et al. 2010; Cao et al. 2012, 2020; Nag et al. 2012). Most of the original papers that included SAR observations focused mainly on the stereoisomerism of ginsenosides at the positions C-20 and C-24. These data indicate that ginsenoside epimers differ in activity, but this effect depends not only on the spatial configuration R or S, but also on the sensitivity of a specific tumor cell line (Jeong et al. 2019; Li et al. 2019a; Nakhjavani et al. 2019).
It should be noted that specially designed SAR studies comparing saponins that contain the same sugar chain but differ in the major type of aglycone (OT vs UT vs LT) are very rare. Xu et al. (2013a) showed that olean-12-ene monodesmosides, namely OA and HE derivatives, showed stronger activity against A549, SGC-7901, and HL-7702 cell lines than lupane (LT) derivatives bearing same sugar chains, such as betulinic acid and 23-hydroxybetulinic acid glycosides. According to Sylla et al. (2019), betulinic acid monorhamnosides revealed higher cytotoxicity against DLD-1 and WS-1 cells than ursolic acid monorhamnosides.
Gao et al. (2012) provided interesting data which analyzed both natural and nonnatural triterpenoid saponins that carried a unique synthetically obtained β-D-glucosyl/galactosyl-(1 → 3)-β-D-glucuronic acid methyl ester disaccharide moiety. It has been shown that oleanolic acid derivatives were much more cytotoxic to the MCF-7, HepG2 and K562 lines than ursolic acid derivatives containing an identical sugar chain. This shows that the position of the methyl group (C-29) is crucial for cytotoxic activity.
The effect of key features of the sugar part on the cytotoxic activity of saponins
It should be underlined that not only the type of sapogenin but also the presence, location, and structure of the sugar moiety are of great importance for the final cytotoxic potential of triterpene saponins. Undoubtedly, the attachment of even one monosaccharide to a triterpene backbone is an essential structural requirement. Many studies have confirmed that the loss of sugar residues linked to C-3 or C-28 dramatically reduced the cytotoxicity of saponins (Liang et al. 2010; Chun et al. 2013a; Ozer et al. 2018; Yu et al. 2019a, b).
SAR observations with respect to the sugar part have mainly focused on studying the influence of the number and type of sugar units. As far as the attachment site is concerned, one report dealt with the location of a glucose moiety. Dai et al. (2018) studied the cytotoxic activity of glycyrrhetinic acid (GA) monoglucosides (3-Glc, 30-Glc-GA) against HepG2 and MCF-7 cells. Interestingly, GA-30-O-β-D-glucoside showed stronger activity than GA-3-O-β-D-glucoside, which suggests that the binding position of the glucosyl moiety is important. Therefore it seems that within the GA derivatives the free OH group at C-3 enhances the cytotoxic effect more than the free 30-COOH group.
Number and type of sugar units
Numerous studies have confirmed that the sugar part composition is important in terms of the observed cytotoxic effect of triterpene saponins (Liang et al. 2010; Han et al. 2013; Wang et al. 2013b; Bai et al. 2017; Kaennakam et al. 2018; Lehbili et al. 2018). The role of the number of monosaccharide units was especially visible for ursane saponins (Shen et al. 2018) and 13,28-epoxy triterpenoid saponins, where tetra- to heptasaccharide chains were preferred structural features (Li et al. 2012; Mu et al. 2013, 2019).
Some important data on the impact of sugar type were provided by Schwarz et al. (2014). In their study, the cytotoxic activity of a significant number of synthetic saponins was compared, all of which were derivatives of methyl glycyrrhetinate, differing in the type of sugar attached at the C-3 position. Saponins with rhamnose, galactose, xylose, and arabinose were less active than compound with a mannosyl residue. Another study analyzed several natural and non-natural oleanane-type saponins bearing a unique synthetic β-D-glucosyl / galactosyl -(1 → 3)—β-D-glucuronic acid methyl ester disaccharide moiety. By changing the type of sugar, from glucose to galactose, an increase in cytotoxic activity was achieved against MCF-7, HepG2 and K562 cell lines (Gao et al. 2012).
Upon examination of bidesmosides isolated from Scabiosa stellata, Lehbili et al. (2018) observed that only saponins in which the xylose residue was linked at C-3 to oleanolic acid were active in contrast to saponins with an arabinose unit in the same position. Furthermore, Lanzotti et al. (Lanzotti et al. 2012) who analyzed the cytotoxic activity of paviosides A–H, a series of theasapogenol A (protoaescigenin) and B (barringtogenol C) glycosides isolated from Aesculus pavia, observed that the structure of the sugar part of the saponin had a greater influence on the activity than the type of aglycone (here the sapogenins differed by the presence or lack of a hydroxyl at C-23, or esterifying group at C-21). Compounds containing a three-sugar chain composed of two xyloses and glucuronic acid (3-O-[β-D-xylopyranosyl-(1 → 2)]-[-β-D-xylopyranosyl-(1 → 4)]-β-D-glucopyranosiduronic acid), located at C-3 of barrigtogenol C or protoaescigenin (24-hydroxylbarrigtogenol C) were more active than saponins that instead of xylose had glucose or galactose attached to the C-4 of GlcA.
The importance of the rhamnose moiety
A substantial number of reports have concluded that the presence of rhamnose moieties positively modulates the anticancer activity of triterpene saponins. Quinovic acid monodesmosides containing a rhamnose residue at C-3 of the aglycone showed higher activity than the corresponding glucosides (Kaennakam et al. 2018). The presence of a terminal L-rhamnose in the sugar chain was also important for the activity of the cytotoxic 16α-hydroxy-13,28-epoxy-oleanane saponins present in Ardisia gigantifolia (Mu et al. 2013).
A rhamnosyl residue may affect general trends observed with other sugar units. For example, the attachment of a sugar chain via the COOH group in oleanolic or echinocystic acid derivatives usually leads to a decrease of cytotoxicity compared to the corresponding monodesmosides with free COOH. However, some reports suggest that the composition of the sugar moiety attached to 28-COOR is crucial for the activity of such OT bidesmosides. Liu et al. (2013) showed that a decrease in cytotoxic activity against HL-60, A547 and A375 was not observed in the case of bidesmosides of oleanolic acid that possessed α-L-Rha at the terminal positions of both C-3 and C-28 sugar moieties. The activity of such bidesmosides was comparable to that of monodesmosides.
Regarding the number of rhamnosyl units in the sugar moieties of triterpene saponins, some conclusions can be drawn from experiments by Sylla et al. (2019) on ursolic acid monodesmosides and lupane (betulinic acid) derivatives. This study has shown that not only the presence of α-L-rhamnose is important, but also the number of these residues. The best activity against DLD-1 and WS-1 cells was observed for monodesmosides bearing either one rhamnose moiety or a disaccharide fragment: 3-O-α-L-rhamnopyranosyl-(1 → 4)-α-L-rhamnopyranosyl. On the other hand, glycosides made up of a larger number of rhamnose residues showed less activity.
Several authors suggest that even more valuable in terms of the cytotoxic potential of a compound is when the rhamnose unit is specifically linked to other monosaccharides. Various research groups have identified the following sequence, located at C-3 of a sapogenin, as the most promising: α-L-rhamnopyranosyl-(1 → 2)-[β-D-glucopyranosyl-(1 → 4)]-α-L-arabinopyranosyl. This sugar chain appears to be essential for the cytotoxic activity of OA, 27-OH -OA, HE, LT or PSD saponins (Xu et al. 2013a; Chen et al. 2015; Lv et al. 2021). Some studies concluded that only the α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranosyl disaccharide moiety located at C-3 may secure the cytotoxic activity of OT saponins (Ren et al. 2013; Grabowska et al. 2021), while other authors (Liu et al. 2013) were not entirely in favor of this observation. This research group has suggested that the sugar directly linked to sapogenin can be either β-D-Xyl, β-D-Gal, or β-D-Glc, but not necessarily L-Ara. However, of importance is the linkage site of the rhamnose unit which should be attached at C-2 of the sugar directly linked to sapogenin. It seems that for the cytotoxic activity of triterpene saponins, the hydroxyl group at the C-3 position of the first monosaccharide unit should remain free (Liu et al. 2013). This suggestion was further confirmed by research by Han et al. (2013).
Some studies point out that perhaps the spatial arrangement of various structural elements, including OH groups, may be essential for activity. For example, Ren et al. (2013) observed that for saponins with an α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranosyl disaccharide moiety located at C-3, the 4C1 chair conformation of the arabinose residue is of particular importance. A synthetic compound with abnormal conformation was devoid of activity against tumor cells (Ren et al. 2013). The cytotoxic activity of oleanolic acid saponins, bearing α-L-Ara in the sugar chain attached to the C-3 of the aglycone, was higher compared to compounds that contained β-L-Ara units (Liu et al. 2013). Also, Schwarz et al. (2014) reported that synthetic saponins, methyl glycyrrhetinate rhamnosides, containing β-L-Rha were slightly more active than those containing α-L-Rha. Further research is, however, needed to draw any solid conclusions.
Impact of esterification or other modifications within the sugar chain
Chun et al. (2013a) who studied Platycodon saponins (PSs), bidesmosides of platycodigenin and polygalacic acid, observed that O-acetylation at C-2 or C-3 of rhamnose significantly increased the cytotoxic activity of these compounds. As suggested by Dai et al. (2013) sugar acetylation increases the lipophilicity of the molecule, which may facilitate cellular uptake and may be responsible for higher cytotoxicity towards some cell lines. In turn, Wang et al. (2012a) observed that 6-O-methylation, and particularly 6-O-butylation of GlcA located at C-3 of OA, increases the cytotoxicity compared to non-esterified GlcA-saponins. The same tendency (against HL-60, MCF-7, HepG2 and A549) was observed in rare oleanane 30-nortriterpenoid saponins isolated from Salicornia bigelovii (Wang et al. 2012b).
However, studies on 13,28-epoxy triterpene saponins from Ardisia gigantifolia, a series of cyclamiretin A glycosides, gave contradictory results, as glucose acetylation at OH-6 had a detrimental effect on the cytotoxicity of these compounds towards Hela, EJ, HepG2 and BCG (Mu et al. 2012). Also, in the case of saponins from the roots of Bupleurum chinense DC, containing a disaccharide chain composed of glucose and fucose ((β-D-Glc-(1 → 3)-β-D-Fuc-) glucose acetylation at positions 2, 3 or 6 led to a decrease in cytotoxicity against A549, HepG2, Hep3B, BCAP-37 and MCF-7 cell lines (Li et al. 2015a). Similarly, a study on modified Pulsatilla D saponins showed that complete acetylation of sugars decreased their activity against A549 and MDA-MB-231 cells (Chen et al. 2015, 2018c).
The examples presented here show that the esterification of monosaccharides in the glycone part of triterpene saponins has an impact on their effect towards cancer cells, but whether it is an increase in activity or a decrease may depend on additional structural elements and the type of cell line. This statement is confirmed by research by Mu et al. (2013), who observed that the acetyl group at OH-6 of β-D-glucose in saponins isolated from Ardisia gigantifolia led to an increase in activity against A549 and HCT-8 lines, but a decrease in activity against BEL-7402 cells.
In addition to the esterification of monosaccharides, the presence of other nonsugar fragments of the molecule, such as, for example, monoterpene moieties, may also be important for the overall cytotoxic activity of triterpene saponins. Miyase et al. (2010) observed that with an increase in the number of monoterpene units linked to the terminal α-L-rhamnopyranosyl moiety of the 28-O-sugar chain in Gleditsia caspica echinocystic acid saponins, their potency against A549, HepG2 and HT29 cells was higher. Additionally, the linkage site of the monoterpene fragment in the sugar chain significantly affected cytotoxic activity, as was shown in a study on oleanane-type triterpenoid saponins from Albizia julibrissin (Han et al. 2017).
Most of the reports available in the literature refer to in vitro SAR analysis with only a few examples derived from in vivo studies. These are very valuable because they provide a closer look at the potential efficacy in the human body. Such studies typically include a comparison of a naturally occurring active saponin together with its semi-synthetically modified derivative. For example, Wei et al. (2012) published a comparison of two dammarane-type saponins, a natural Rh2 ginsenoside and its structurally modified dioctanoyl ester. Also, Fang et al. (2016, 2019) analyzed the activity of semi-synthetic derivatives of oleanane-type saponins: Pulsatilla saponin D (Fang et al. 2019) and hederacolchiside A1 (Fang et al. 2016), in H22 tumor-bearing mice. These are however only individual observations, which lack a broader SAR view.
There is no one-size-fits-all solution for different types of triterpene saponins, and definitely the sum of the individual structural features is essential for achieving high activity. Both the sapogenin and the sugar part affect the activity. Individual lines differ in their sensitivity to closely related compounds. Nevertheless, SAR studies are very important to determine the most active structures for specific types of cancer and to channel further research accordingly. The results derived from these experiments help to understand important processes in terms of the structure of the molecule and its interactions.
The most important SAR observations are summarized in a graph in Fig. 3.
Mechanisms of cytotoxic impact on cancer cells in vitro
The cytotoxic effect of triterpene saponins is based on several different mechanisms, which eventually lead to cell death. This involves apoptosis stimulation or non-apoptotic cell death pathways. The most important mechanisms of cytotoxicity reported for triterpene saponins are summarized in Table 1. The majority of compounds included in this review acted via the intrinsic apoptosis pathway, which is triggered by activation of different pro-apoptotic receptors on the cell surface (Fig. 4). What is interesting, none of the triterpene saponins was reported to selectively stimulate the extrinsic pathway of apoptosis, however several compounds regulated the expression of both intrinsic and extrinsic apoptosis-associated proteins (Liang et al. 2010; Chueh et al. 2012; Dong et al. 2015; Li et al. 2021a). With regard to the mechanisms included in our previous review (Podolak et al. 2010), some novel ways to induce cell death, triggered by triterpene saponins, were described, namely ferroptosis (Mbaveng et al. 2018; Wei et al. 2018, 2019), anoikis (Chun et al. 2013b), necroptosis (Tabata et al. 2012), and methuosis (Gong et al. 2018). However, a substantial number of compounds studied in the timeframe of this review acted via mechanisms similar to those described previously (Podolak et al. 2010). These include a decrease of cell membrane permeability (Lorent et al. 2013, 2016; Nishimura et al. 2021), inhibition of angiogenesis (Hong et al. 2013; Guan et al. 2015), sensitization of cancer cells to chemotherapeutics (Deng et al. 2017; Shen et al. 2017; Chen et al. 2019b; Guo et al. 2019), or inhibition of cancer cell migration (Jiang et al. 2017; Jia et al. 2018). An interesting example of the latter is a modification of the aquaporin water channel by some triterpene saponins (Pan et al. 2012; Nakhjavani et al. 2019). Furthermore, two authors have proved the influence of the tested compounds on topoisomerase activity (Wang et al. 2010; Herrera-Martínez et al. 2012), which is a mechanism previously unreported for saponins. Another rare mechanism of saponin-induced cytotoxicity involved their impact on cytoskeleton integration (Koczurkiewicz et al. 2013b; Yan et al. 2014). The most interesting examples of the cytotoxic mechanisms of triterpene saponins are described below.
Association of apoptosis and other cell death processes
Triterpene saponins that stimulate apoptosis and/or autophagy
Apoptosis is a programmed cell death, the initiation of which may be signaled either at the cell membrane (extrinsic pathway) or within the cell (intrinsic pathway). The extrinsic pathway is triggered by the activation of specific pro-apoptotic receptors on the cell surface, stimulated by pro-apoptotic ligands (Apo2L/TRAIL and CD95L/FasL), while the intrinsic pathway generally involves the activation of caspases by pro-apoptotic proteins, although it also depends on the balance between the pro- and anti-apoptotic proteins from the Bcl-2 superfamily (Podolak et al. 2010). During autophagy, stressed cells start to catabolize their own contents (proteins, organelles), which results in inhibition of tumor progression. The process is regulated by specific autophagy genes (Atg) and/or protein kinases mTOR and AMPK (Eisenberg-Lerner et al. 2009). As apoptosis and autophagy may be regulated by similar molecular mechanisms, a cross-talk between the two processes may manifest in three ways: i) partnership in inducing cell death, ii) antagonism, resulting from blocking the apoptotic pathway and promoting cell survival by autophagy, or iii) collateral stimulation, as autophagy may enable apoptosis by participating in some processes that occur during apoptotic cell death (Eisenberg-Lerner et al. 2009). All these types of interplay were described with respect to triterpene saponins cytotoxic activity, and the graphical summary of the mechanisms of the two processes, but also their relationships, is shown in Fig. 4. Apoptosis and autophagy both contributed to the death of human melanoma A375-R cells, treated with cumingianoside A (CUMA), isolated from leaves and twigs of Dysoxylum cumingianum (Cvetanova et al. 2019). Liang et al. (2016) described a similar effect for Pulsatilla chinensis raddeanoside R13 on human breast cancer ZR75-1 and MCF-7 cell lines, or Li et al. (2019b) for human RB Y79 and RBL-13 retinoblastoma cells treated with ginsenoside Rh2. Saxifragifolin D isolated from Androsace umbellata induced an interaction between apoptosis and autophagy in MCF-7 and MDA-MB-231 breast cancer cells, and stress of the endoplasmic reticulum appeared to be a mutual signaling pathway for both processes (Shi et al. 2013). Eclalbasaponin II from Eclipta prostrata induced apoptosis and autophagy in human SKOV3 and A2780 ovarian cancer cells by regulation of JNK, p38, and mTOR signaling. Furthermore, the use of the autophagy inhibitor BaF1 suppressed eclalbasaponin II-induced apoptosis (Cho et al. 2016). Similarly, commercially obtained momordin Ic simultaneously induced autophagy and apoptosis in human hepatocellular HepG2 cells by suppressing ROS-mediated PI3K/Akt and activating ROS-related JNK and p38 pathways (Mi et al. 2016). TBM stimulated apoptosis in colon cancer SW480 and SW620 cells but also induced autophagy by ROS-induced activation of AMPK and blocked autophagic flux by altering autophagolysosome degradation (Yan et al. 2019).
Several authors described an antagonistic relationship between autophagy and apoptosis. Triterpenoid saponin isolated from Adenophora triphylla induced both apoptosis and autophagy in AGS cancer cells, but enhancement of the latter protected the cells from apoptotic cell death (Chun et al. 2014). A similar effect was also described for ginsenoside F2 in breast cancer stem cells (Mai et al. 2012) and human SGC-7901 gastric cancer cells treated with raddeanin A (Teng et al. 2016). Amplification of apoptosis was observed in human hepatocellular carcinoma BEL-7402 cells after co-treatment with platycodin D and autophagy inhibitors (Li et al. 2015b) in breast cancer MCF-7 and T47D cells treated with raddeanin A and autophagy inhibitors (Guan et al. 2018), while in Du145 prostate cancer cells inhibition of apoptosis significantly enhanced autophagy stimulated by afrocyclamin A, isolated from Androsace umbellata (Sachan et al. 2018). A saponin denoted SSPH I, which was isolated from Schizocapsa plantaginea rhizomes, inhibited autophagy by blocking autophagosome–lysosome fusion and at the same time stimulated apoptosis in HepG2 cells (Zhou et al. 2020). On the other hand, oleiferoside B from Camellia oleifera, activated autophagy and inhibited apoptosis in breast MCF-7 and liver SMMC 7221 cells (Feng et al. 2021a), and similar effect was also noted for ginsenoside CK in cervical HeLa cancer cells (Yin et al. 2021). Another interesting mechanism was described for eclalbasaponin I (EcI), a triterpene saponin isolated from Aralia elata, which was preincubated with SH-SY5Y neuroblastoma cells before the H2O2-induced oxidative stress. The compound induced prosurvival autophagy and suppressed oxidative stress-induced apoptosis through p38-mitogen activated protein kinase (p38) or extracellular regulated protein kinase ERK activation. In addition, this saponin also caused mitophagy in neuroblastoma cells, manifested by enhanced mitochondrial ROS generation, decreased endogenous antioxidant defence, and mitochondrial membrane potential (MMP), resulting in mitochondrial dysfunction (Wang et al. 2019a).
Much more limited data refer to the promotion of apoptosis by saponin-induced autophagy. One of such examples is Sasanquasaponin ΙΙΙ, isolated from Schima crenata Korth, which induced autophagy in human A375 melanoma cells by inhibiting the PI3K/Akt/mTOR pathway and the downstream pathway of the mTOR/p70S6K. The authors showed that both autophagy and apoptosis were blocked when an autophagy inhibitor was used in the study, which confirmed this relationship (Liang et al. 2019). In the case of Akebia saponin PA stimulation of autophagy in human gastric cancer AGS cells, it subsequently led to mitogen-activated protein kinases (MAPKs)-mediated apoptosis in cells (Xu et al. 2013b).
Triterpene saponins that stimulate apoptosis and ferroptosis
As mentioned above, a novel mechanism involved in the cytotoxicity of triterpene saponins reported in the last decade is ferroptosis. Ferroptosis is an iron-dependent and caspase-independent form of regulated cell death, usually induced by ROS accumulation and activation of the p53 pathway. The process differs from apoptosis, necrosis, or autophagy and is triggered by oncogenic RAS-selective lethal small molecule erastin (Dixon et al. 2012). Moreover, some ferroptosis promoters have been identified that induce accumulation of lipid peroxidation products and lethal amounts of ROS derived from glutathione peroxidase IV (GPX4) (Wei et al. 2019). Ferroptosis, which was reported as a cytotoxic mechanism of several triterpene saponins, was usually accompanied by apoptosis. One of the examples is albiziabioside A. The compound itself, conjugated with dichloroacetate, induced ferroptosis and apoptosis in human breast cancer MCF-7 cells, by suppressing the GPX4 pathway and stimulating lipid peroxidation (Wei et al. 2019). Another derivative of albiziabioside A showed a similar effect in HCT116 cells, however, by activating the p53 pathway (Wei et al. 2018). Another example is ardisiacrispin B, isolated from Ardisia kivuensis, which stimulated both apoptosis and ferroptosis in human leukemia CCRF–CEM cells. The latter effect was confirmed by the treatment of cells with the compound studied and the ferroptosis inhibitor ferrostatin-1 and the iron chelator deferoxamine, which significantly decreased the cytotoxic potential of ardisiacrisipin B (Mbaveng et al. 2018).
Triterpene saponins that stimulate necroptosis, anoikis, and methuosis
Necroptosis is a necrotic type of cell death, dependent on receptor-interacting protein kinase (RIPK). Signal transduction for necroptosis is known to be caspase-independent. Mitochondrial protein apoptosis-inducing factor (AIF) is also shown to be an important executor of necroptosis (Linkermann and Green 2014). Human neuroblastoma IMR-32 cells were incubated for 48 h with kuguaglycoside C, isolated from Momordica charantia. No activation of caspase-3 or caspase-9 was observed, but the compound decreased the expression of survivin and cleaved poly (ADP-ribose) polymerase (PARP) and increased the expression and cleavage of apoptosis-inducing factor (AIF). The authors suggest that kuguaglycoside C stimulated necroptosis in the treated cells (Tabata et al. 2012).
Anoikis is a special subtype of apoptosis, associated with loss of cell adhesion to the extracellular matrix and subsequent reorganization of the cell cytoskeleton. This results in changes in protein kinases expression, e.g., an inhibition of focal adhesion kinase (FAK) or activation of p38 mitogen-activated protein kinase (MAPK) and, as a consequence, altered expression of proteins directly involved in apoptosis (Frisch and Screaton 2001). Platycodin D-induced anoiksis in human gastric cancer cells AGS, manifested by externalization of phosphatidylserine, DNA fragmentation, increase in the sub-G1 phase, and caspase activation in a dose- and time-dependent manner. Additionally, the compound led to the phosphorylation of stress-activated protein kinases such as JNK and p38, followed by the activation of AP-1 (Chun et al. 2013b).
Methuosis is a process that leads to nonapoptotic cell death, which is manifested by displacement of the cytoplasm in the form of large fluid-filled vacuoles derived from macropinosomes (Maltese and Overmeyer 2014). Tubeimoside 1 strongly stimulated the accumulation of numerous phase-lucent cytoplasmic vacuoles (macropinosomes) in human colon carcinoma cell lines SW480, leading to cell death (Gong et al. 2018).
Triterpene saponins may inhibit topoisomerase activity
Topoisomerase inhibition is one of the important mechanisms of action of currently used oncological drugs. This mechanism has been reported for some triterpene saponins, however, it is definitely uncommon. In the study by Wang et al. (2010) among 50 triterpenoid glycosides isolated from Aesculus pavia, only 16 compounds inhibited the catalytic activity of topoisomerase I, by interacting directly with the free enzyme and preventing the formation of the DNA–TOP1 complex. None of the compounds tested was active against topoisomerase II. Interestingly, in a similar study by Herrera-Martínez et al. (2012) three out of ten saponins tested, isolated from Sicyos bulbosus and Microsechium helleri, exerted inhibitory activity against topoisomerase II activity, with IC50 0.3650, 0.3294 and 0.3691 µM, while the IC50 values for the reference drugs camptothecin and etoposide were 0.1435 and 0.0849 μM, respectively.
Triterpene saponins may sensitize cancer cells to cytostatic drugs or act synergistically
The combination of saponins with a cytostatic drug is another interesting aspect when considering their cytotoxic potential. Such an approach may lead to a reduction in the dose of the drug and also to a reduced risk of toxicity. What is interesting is that some of the reviewed studies also describe the synergy between two saponins combined together. However, only in a few cases did the authors provide information on the calculated combination index, which clearly defines the mutual relationship in the activity of both tested substances. The results of studies on the use of saponins with cytostatic drugs are presented in Table 2, while some of the most interesting examples, including interactions between two saponins administered together, are briefly described below.
An interesting synergy between two saponins isolated from Bacopa monnieri, namely bac I and bac II, was also described. Breast cancer MDA-MB-231 cells were treated with varying doses of bac I combined with a constant dose of bac II (2.5 µM), which was much below its IC50 value (18 µM). Co-treatment caused the decrease in cell viability and the IC50 for bac I decreased from 99 to 13 μM. The synergistic effect was also manifested by decreased cell proliferation, increased number of cells arrested in the G2/M phase, changes in cell morphology, and increased number of apoptotic cells (Palethorpe et al. 2019). He et al. (2021a) prepared a unique combination of ginsenoside Rg3 with Ganoderma lucidum polysaccharide and diterpenoid oridonin, in a special form of self-micro emulsifying drug delivery system, which was designed to increase the bioavailability and solubility of the three compounds. The mixture promoted apoptosis and inhibited p-EGFR and the activation of downstream AKT/GSK3 signaling pathways in hepatocellular carcinoma HepG2 and Huh7 cells, however the authors did not compare the effectiveness of the individual compounds.
Raddeanin A revealed a synergistic cytotoxic effect in human osteosarcoma MG63, U2OS, and HOS cells, in combination with doxorubicin. This was manifested by the increase in doxorubicin cellular uptake, ablating its efflux and downregulating multidrug resistance-associated protein 1 (MDRI1) in drug resistant cells, together with attenuation of signal transducer and activator of transcription-3 (STAT3) phosphorylation (Wang et al. 2019b). Huang et al. (2012) described an interesting experiment suggesting a decrease in multidrug resistance in 5-fluorouracil-sensitive and resistant human hepatic cancer cells Bel-7402 and Bel-7402/FU, respectively. Astragaloside II (0.08 mg/ml) significantly improved 5-fluorouracil cytotoxicity toward resistant Bel-7402/FU cells by increasing intracellular accumulation of rhodamine 123 by inhibiting the transport function of P-glycoprotein. Furthermore, this saponin downregulated the expression of the P-gp and mdr1 gene, and also suppressed phosphorylation of the extracellular signal regulated kinase 1/2, p38 and c-Jun N-terminal kinase.
Although TNF-α revealed a strong cytotoxic effect in primary tumors, its use for cancer treatment is limited due to undesirable activation of NF-κB signaling and its prometastatic nature. Therefore, interesting results were described for saikosaponin-d, co-administered with TNF-α. Saikosaponin-d significantly potentiated TNF-α-mediated cell death in HeLa and HepG2 cancer cells via suppression of TNF-α-induced NF-κB activation and its target gene expression involving cancer cell survival (Wong et al. 2013a, b).
Triterpene saponins may decrease migration ability of cancer cells
Migration of cancer cells plays an important role in their metastasis to other tissues, thus allowing the development and propagation of the disease. A β-hederin rhamnoside (DRβ-H) at noncytotoxic doses significantly inhibited the migration of breast cancer MDA-MB-231 cells, which was examined using different assays. The compound suppressed wound healing migration, migration through the chamber, and invasion through the Matrigel. Furthermore, this saponin up-regulated the expression of RNA-binding protein RNPC1 and the E-cadherin protein in MDA-MB-231 cells (Cheng et al. (2018a, b).
Combined doses of bacopasides bac I and bac II (5 µM each) significantly inhibited wound closure of breast cancer MDA-MB-231 cells (69% of inhibition at 20 h), while MCF7 cells were more resistant: 23% inhibition of migration occurred at 72 h with 10 µM bac I + 5 µM bac II (Palethorpe et al. (2019). Raddeanin A enhanced the potential of 5-fluorouracil in reducing cell colony formation in cholangiocarcinoma RBE and LIPF155C cell lines, when compared to the effect exerted by cytostatic alone (Guo et al. 2019).
Lclet4, when administered with mitoxantrone, synergistically decreased the invasive potential, the expression of metalloproteinase and the motility of prostate cancer DU145 cells (Koczurkiewicz et al. 2013b).
Aquaporin 1 (AQP1) is a member of the water channel protein family that, except for its functions in water transportation, can also facilitate cell migration. Overexpression of AQP1 is often associated with enhanced migration of cancer cells and their greater metastatic potential. Two different groups of authors reported that the ginsenoside Rg3 acts through this mechanism. Pan et al. (2012) described that the compound effectively decreased prostate cancer PC-3 M cell migration by downregulating AQP1 expression through the p38 MAPK pathway, while the results of Nakhjavani et al. (2019) indicated a similar effect on breast cancer MDA-MB-231 cells. Interestingly, the authors also showed that the difference in the activity of Rg3 epimers – 20(R)-Rg3 inhibited cell migration and invasion more effectively, compared to 20(S)-Rg3.
Triterpene saponins that reveal miscellaneous mechanisms
Exosomes are extracellular vesicles that contain a specific composition of proteins, lipids, RNA, and DNA. They can transfer signals to recipient cells, thus mediating a novel mechanism of cell-to-cell communication, including cancer cells. Therefore, inhibition of exosome excretion is one of the targets for the fight against cancer. Chen et al. (2018b) showed that a β-hederin rhamnoside, in addition to decreasing breast cancer MCF-7/S cells and stimulating their apoptosis, was also able to inhibit breast cancer-derived exosome secretion. The authors detected and knocked-down top five microRNAs in the exosomes, which decreased cell viability.
MicroRNAs (miRNAs) are small, noncoding RNA molecules that play a key role in cancer progression and prevention. MicroRNAs may bind to the 3' untranslated regions of their target genes, and inhibit protein translation and synthesis. Some of the miRNAs have been reported to determine malignancy of cancer cells while others may enhance cell drug sensitivity to anticancer agents. Ginsenoside Rh2 incubated with non-small cell lung cancer A549 induced a set of changes in the miRNA expression profile in the cells. The authors identified 44 and 24 miRNAs that show expression changes greater than two times in Rh2-treated A549 cells (An et al. 2013).
The peroxisome proliferator-activated receptors (PPARs) form a subfamily of the nuclear receptor superfamily PPARs regulate the expression of genes involved in the regulation of glucose, lipid, and cholesterol metabolism by binding to specific peroxisome proliferator response elements (PPREs) at the enhancer sites of the regulated genes.
Several triterpene saponins, derivatives of oleanolic acid and hederagenin, isolated from Kalopanax pictus, up-regulated PPAR transcriptional activity in a dose-dependent manner in liver cancer HepG2 cells, with values of EC50 in the range of 0.20–15.5 µM. Furthermore, two of the compounds examined, namely hederagenin bidesmosides, showed significant PPARα transactivation activity, with EC50 values of 10.3, and 17.3 µM, respectively. PPARα isoform regulates the expression of genes involved in lipid and glucose homeostasis, while PPAR controls genes involved in adipocyte differentiation and lipid metabolism. Interestingly, compounds the two above-mentioned saponins exhibited dose-dependent transactivation activity, with EC50 values of 17.1, and 16.3 µM. PPARβ(δ) is highly expressed in tissues that control lipid metabolism and its transcriptional activity was significantly up-regulated by the two saponins, with EC50 values of 15.7 and 17.7 µM, respectively. The authors conclude that saponins with this mechanism of action can be used in the treatment of diabetes, obesity, or metabolic syndrome (Quang et al. 2011a, b).
Pulsatilla saponin A, isolated from Pulsatilla sinensis, was shown to induce differentiation of acute myeloid leukemia (AML) cell lines (U937, K562, HL-60) and primary leukemia cells. This saponin stimulated the differentiation of U937, K562 and HL-60 cells, which was manifested by an increase in CD15 + cells in all three AML cell lines. Additionally, the cell morphology of these AML cells was changed: the cytoplasm/nuclei ratio increased, the basophilic cytoplasm decreased, and eccentric nuclei and granules were also observed. Similar effects of differentiation induction by Pulsatilla saponin A were observed in primary leukemia cells. The authors suggest that this saponin may stimulate the differentiation of leukemia cells by the MEK/ERK signaling pathway (Wang et al. 2016).
One of the commonly described mechanisms by which saponins can induce cancer cell death is membrane permeabilization. An interaction with membrane cholesterol of α-hederin was investigated in two malignant monocytic cell lines U937 and THP-1 cells, cholesterol-depleted or non-depleted. This saponin significantly reduced apoptotic cell death and membrane permeabilization in cholesterol-depleted cells. Permeabilization further led to extracellular calcium influx and nuclear fragmentation. In addition, a radical change in cell morphology was observed, including the disappearance of pseudopodes upon incubation with α-hederin. The authors suggest that the activity of α-hederin depends mainly on its interaction with membrane cholesterol and the consequent formation of pores (Lorent et al. 2016). A study by Nishimura et al. (2021) indicated that jegosaponins A and B, isolated from Styrax japonica, increased cell membrane permeability in prostate cancer PC-3 cells, which was proved with the use of a membrane nonpermeable fluorescent nucleus staining dye DRAQ7.
The nuclear factor-erythroid 2-related factor (Nrf2) participates in the response to cellular oxidative stress and is over-expressed in cancer cells, resulting in their increased survival and resistance to anticancer drugs. Hovenidulcioside C, as the only one out of six saponins isolated from Hovenia dulcis, dose-dependently inhibited the level of Nrf2 protein in lung cancer A549, and the authors conclude that the compound may be of interest in future studies on Nrf2 inhibitors (Cai et al. 2021).
Results of in vivo studies
In vivo models are an important step in the search for new drugs as they enable us to assess the activity and metabolism of a given compound in a living environment that differs significantly from the in vitro conditions. In studies on potential anticancer agents, several in vivo models are used, among which one of the most popular is the tumor xenograft model. In this assay, a human or murine cancer cell line is injected into rodents, usually mice or rats, and the effect of tested substances on tumor growth is compared with that of the untreated control group and/or with that of the reference chemotherapeutic agent. The tumor xenograft model allows for the evaluation of antitumor activity at the primary site and metastatic. The test is usually followed by immunohistochemical analysis of the tumor and/or serum of the sacrificed rodents, which can give insight into the mechanism of action of the investigated compounds.
Ehrlich’s ascites carcinoma (EAC) is another widely used mouse model to induce subcutaneous tumors. This model has several favorable characteristics, such as good transplantability, rapid proliferation without regression, 100% malignancy, shorter life span, and lack of tumor-specific transplantation antigens (Ozaslan et al. 2011). EAC cells are undifferentiated and tumor growth rate is rapid.
In other less commonly used models, cancerous changes are induced by the application of toxic substances, for example 7,12-dimethylbenz[a]anthracene (DMBA)-induced renal carcinogenesis in mice, DMBA-induced breast cancer in rats, diethylnitrosamine (DEN)-induced hepatocellular carcinoma in rats.
In various in vivo antitumor assays, the tested agents are administered chronically, but the exact dosage schedules can vary from daily application to 3 times a week (every second or third day). The test usually lasts 2 to 4 weeks, or sometimes even longer. Drug administration also differs between experiments, but the two most popular are intraperitoneal or oral routes (Table 3).
Triterpene saponins active in in vivo models of liver cancer
Liver cancer is one of the most common types of cancer in men and among the 10 most common types of cancer in women. Its mortality rate, one of the highest, reaching more than 90% in the general population is of great concern (WHO 2021). It should be noted that several triterpene saponins were investigated in vivo to evaluate their efficacy in models of liver cancer.
Promising results were obtained for a group of saponins found in the genus Pulsatilla. Pulsatilla saponin A was significantly effective in decreasing the volume of xenograft tumors induced by Bel-7402 cells from hepatocellular carcinoma in mice, with minimal toxicity. Its effect was comparable to that of cyclophosphamide (CTX) used at the same dose (Table 3) (Liu et al. 2014).
Another potentially promising saponin is platycodin D (PD) which was also investigated in the hepatocellular carcinoma xenograft model, although induced with the H22 cell line (Table 2) (Li et al. 2016a, b). The compound significantly and dose-dependently reduced tumor weight, without any effect on weight of the body or immune organs such as thymus and spleen. CTX, used as a reference, was more effective than PD but, on the other hand, it was more toxic, significantly reducing the weight of immune organs. It is worth noting that CTX was administered intraperitoneally whereas PD orally. The route of administration determines the pharmacokinetics of a given drug, and consequently it can affect its pharmacological effect. Further studies revealed that in the PD-treated groups serum cytokine levels, including IFN-γ, TNF-α, IL-6, and IL-2 were enhanced, while the production of VEGF in serum of H22-tumor mice was reduced. These changes were not observed in the CTX group.
Oleioferoside B, derived from Camellia oleifera, studied in the same H22-induced tumor mouse model, was able to reduce tumor growth at low doses (0.5 and 1 mg/kg/d), however, it was not as effective as CTX which inhibited tumor growth by almost 99%. On the other hand, the dose of the reference compound used in this study was much higher, what always provides the risk of side effects. However, the safety aspect was not checked in the study (Feng et al. 2021).
A weaker effect was reported in the same hepatocellular carcinoma model for chiisanoside, a lupane triterpenoid isolated from the leaves of Acanthopanax sessiliflorus. The saponin doses that significantly reduced tumor weight were much higher compared to CTX (Bian et al. 2017). However, chiisanoside appeared to improve liver function in contrast to the reference drug that induced significant hepatotoxicity. Furthermore, the authors suggested that the antitumor effect of chiisanoside was a result of its antiangiogenic and proapoptotic activity. Finally, this saponin was characterized by good pharmacokinetic parameters in rats: fast absorption, rapid elimination, and high distribution in the liver or intestines (Bian et al. 2017).
One of the saikosaponins, bioactive triterpene glycosides typical of Bupleurum falcatum, saikosaponin-d was shown, in turn, to prevent diethylnitrosamine (DEN)-induced liver carcinogenesis in rats by inhibiting COX-2 and C/EBPβ (Lu et al. 2012). Moreover, the compound reduced nodule formation, tumor metastasis and cell atypia increased by DEN compared to the control tumor group treated only with DEN. Similar observations were made by Jia et al. (2012), who investigated the effects of saikosaponin-d in the same model. Verma et al. (2018) reported the hepatoprotective properties of HEG (19-α-hydroxyurs-12(13)-ene-28-oic acid-3-O-β-D-glucopyranoside) in the same DEN-derived hepatocellular carcinoma model. HEG in a dose-dependent manner markedly decreased the number of tumor nodules found in rats treated with DEN, while showing a high level of safety.
Segetoside I was reported to be a potential anti-HepG2 agent. It is a bidesmodic saponin from Vaccaria segetalis (Neck) that effectively reduced tumor volume and prolonged the mean survival time, being even more effective than 5-fluorouracil, a reference drug used in the experiment (Firempong et al. 2016). It is noteworthy that the toxicity of the saponin was low.
The immune cytokine tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) pathway seems to be an attractive target in chemotherapy because of its ability to selectively trigger apoptosis in cancerous tissue cells, ensuring a better overall safety profile. Therefore, adjuvant agents capable of inducing sensitization to TRAIL seem to be an interesting solution to eliminate or overcome resistance to TRAIL chemotherapy (Lee et al. 2013). One of the interesting examples of triterpene saponins with the potential of TRAIL-sensitizing action is ginsenoside Rg3. Combination therapy: TRAIL + ginsenoside Rg3 in Huh 7-cells-induced hepatocellular carcinoma in the xenograft mouse model resulted in a decrease in tumor volume compared to a single TRAIL or Rg3 therapy (Lee et al. 2013). Unchanged body weight indicated that the therapy was safe. However, the difference between the TRAIL + ginsenoside Rg3 group and the TRAIL-alone group was not statistically significant.
Finally, interesting results were reported for ardipusilloside I (ADS-I), a triterpene saponin from Ardisia pusilla. Lou et al. (2012) in an experiment in mice inoculated with human hepatocellular carcinoma, showed that the ADS-I-treated group developed fewer lung tumors, suggesting its antimetastatic potential. However, the effective dose was relatively high (Table 3).
Triterpene saponins active in in vivo models of breast cancer
Breast cancer is the most common cancer type worldwide with 30% mortality in women (WHO 2021). Several in vivo investigations of saponins have been dedicated to the search for anti-breast cancer agents. An interesting example is platycodin D (PD), which significantly suppressed tumor growth, compared to control, in MDA-MB-231 breast cancer tumor-bearing mice, with no significant toxicity observed (Chun and Kim 2013; Kong et al. 2016). In addition, downregulation of G0/G1 phase-related proteins, such as CDK2, CDK4, CDK6, cyclin E, was observed. Also, the levels of MDM2, MDMX, and mutant p53 were decreased, while the expression level of p21 and p27 was increased. In another study, a reduction in the expression of cell proliferation markers such as EGFR and Ki-67 was observed (Chun and Kim 2013).
The same breast cancer model was used to assess the potential of soyasaponin Ag derived from soybeans (Glycine max). Given daily with food, this saponin markedly reduced tumor size and weight with a decrease in MAPK1 and MAPK14 levels along with an increase in the level of the USP6 protein (Huang et al. 2021). A different breast cancer cell line, namely MDA-MB-157, was used to induce tumor for the purpose of investigating a triterpene saponin isolated from Ardisia gigantifolia, denoted AG36. A significant reduction in tumor growth was observed when this saponin was administered at doses as small as 0.75 and 1.5 mg/kg/every two days. Angiogenesis inhibition was suggested as a possible mechanism of the observed antitumor activity (Mu et al. 2020a).
Metastasis of breast cancer to bone results in the secretion of various osteolytic factors causing osteoclast-mediated bone resorption, often leading to severe bone destruction and increased mortality. Consequently, compounds with antimetastatic potential are much needed. PD was further examined in vivo in mice after intratibial injection with human metastatic breast cancer cell (Lee et al. 2015). PD suppressed MDA-MB-231-induced osteolysis of the tibiae, tumor growth in the tibial bone marrow, and bone loss. Fortunately, PD is not the only triterpene saponin with such a potential of action. Raddeanin A (RA), the oleanane-type saponin present in Anemone raddeana seems to be an interesting antiosteolytic agent in metastatic osteolysis associated with breast cancer. Its effect was investigated using tumor induced by MDA-MB-231 cells in the xenograft mouse model and mouse calvarial model which is a model commonly used to study osteolysis (Wang et al. 2018a, b). This saponin protected the bones from resorption resulting in a remarkably lower bone loss compared to the control group that received no treatment. Osteoclast formation seems to be inhibited by the SRC/AKT signaling pathway.
Another saponin for which anti-breast cancer and antimetastatic potential was reported was astragaloside IV (AS-IV), a compound derived from Astragalus membranaceus (Jiang et al. 2017). AS-IV reduced the growth of MDA-MB-231 breast cancer tumor by decreasing cell proliferation and migration and was shown to inhibit breast cancer metastasis to the lungs, in the tail vein metastasis model.
The tumor volume/size-decrease property was also reported for actein (Yue et al. 2016) and raddeanoside R13 in murine 4T1-induced breast cancer in a xenograft model (Liang et al. 2016). Interestingly, both agents exhibited an antimetastatic potential that inhibits the migration of cancer cells to the lungs and in the case of actein also to the liver. Finally, actein appeared to be safer than the reference drug, doxorubicine.
The antibreast cancer potential of saikosaponin-a (SSa) was evaluated in the 7,12-dimethyl-benz[a]anthracene (DMBA)-induced breast cancer model in rats (Zhao et al. 2019a, b). The tumor growth reduction effect was comparable to that of tamoxifen. The mechanism involved in the activity of SSa covered immunological changes, this saponin shifted the balance of lymphocyte helper cells Th1/Th2 towards Th1 by activating the IL-12/STAT4 pathway.
Triterpene saponins active in in vivo models of lung cancer
Lung cancer, and its different subtypes, is characterized by the second highest prevalence in the general population worldwide and high mortality, over 80% (WHO 2021). There are only few reports on the activity of saponins to suppress lung cancer, and with average effectiveness. One of the most effective was the ginsenoside Rk3, in a model induced by H460 cells from human non-small cell lung cancer (NSCLC) in mice (Duan et al. 2017). Histological analysis confirmed the antiproliferative effect of this compound, whereas immunohistochemistry analysis of CD34 proteins proved its antiangiogenic effect.
In addition, ardipusilloside I (ADS-I) inhibited tumor growth in xenograft tumor induced by human lung cancer A549 cells in mice, but the effect was much weaker than the reference drug, gefitinib (Wang et al. 2012c). Capilliposide, isolated from Lysimachia capillipes, is another saponin, recognized as safe, with a proven ability to reduce tumor volume, size, and weight of tumor induced by H460 cells of human non-small cell lung cancer in mice (Fei et al. 2014). However, again, the effect was much weaker compared to the reference drug, cisplatin.
An accepted method to support chemotherapy is to improve the immune system in the recognition and combat of cancerous cells. Indoleamine 2,3-dioxygenase (IDO), a tryptophan catabolizing enzyme that induces immune tolerance, participates in a tumor escape phenomenon from host immune systems in mice. Astragaloside IV (AS-IV) reduced tumor size in murine Lewis lung carcinoma in mice with IDO-overexpression (Zhang et al. 2014). Similar effects were shown for paclitaxel and the IDO inhibitor, 1-methyl-tryptophan. Furthermore, AS-IV and paclitaxel prolonged the survival rate in tumor-bearing mice. In a molecular study, AS-IV suppressed the overexpression of IDO in vitro and in vivo, which may explain its antitumor mechanism.
Triterpene saponins active in in vivo models of gastric, colon, and colorectal cancer
The antitumor activity of Pulsatilla saponin D (SB365) was also confirmed in the xenograft assay in mice with human colon cancer HT-29 cells (Son et al. 2013a, b). SB365 significantly reduced tumor weight and volume compared to the tumor control group. The effect was even stronger than that elicited by 5-FU at the same dose. Additionally, for SB365 and 5-FU no effect on body weight was observed, indicating low toxicity of the compounds. Further studies explained the possible mechanism of action of Pulsatilla saponin D. The fact that PCNA (proliferative marker) was poorly expressed in the SB365 group, in contrast to the control tumor group, suggested suppression of proliferation. Furthermore, the results of the TUNEL assay, as well as cleaved caspase-3, proved an induction of apoptosis by SB365. The levels of blood vessel markers, CD-34, and cells positive for VEGF were lower in the SB365 treated group than in the control, indicating antiangiogenic activity (Son et al. 2013a, b).
In other studies, SB365 has been reported to suppress tumor growth in gastric cancer MKN-45 by inhibiting cell proliferation and induction of apoptosis, which was revealed by histological investigation (Hong et al. 2013). As mentioned above, several ginseng saponins are widely investigated for potential antitumor activity (Nag et al. 2012; Choi et al. 2013; Vayghan et al. 2014; Wong et al. 2015; Nakhjavani et al. 2019). It should be noted that their metabolites have also gained attention; one of the best examples is 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol (20-GPPD, compound K). When tested in mice with CT-26-cells-induced tumor in colon cancer model, this compound showed a significant dose-dependent decrease in tumor volume and weight (Hwang et al. 2013). Noteworthy, the active doses of compound K in this experiment were relatively low compared to effective doses of other saponins with proven cytotoxic activity. A similar significant antitumor effect for 20-GPPD was obtained in the xenograft tumor assay with HCT-116 human colorectal cancer cells used (Zhang et al. 2013c).
Some more interesting reports have recently been published. One of the well-known triterpene saponins that was shown to be one of the relatively potent agents is α-hederin which dose-dependently reduced gastric tumors in mice (Wang et al. 2020a). Another widely studied compound, saikosaponin A, reduced the development of HCT15-colorectal-cancer-cells-induced tumors in mice (Zhang et al. 2021). The suggested mechanism was linked to inhibition of angiogenesis. A different mechanism of antitumor activity was reported for jujuboside B, isolated from the seeds of Zizyphus jujuba. The authors postulated that this compound induces apoptosis by interaction of the Bcl-2 and Bax proteins as well as reduction of cell proliferation (Li et al. 2021d).
It should be noted that a combination of 5-FU and anemoside B4 was found to be more efficient in suppressing tumor growth (derived from chemotherapy-resistant HCT116 colorectal cell line) than 5-FU given alone (He et al. 2021b). This study provides additional experimental data supporting the potential of triterpene saponins as adjuvants in chemotherapy.
Triterpene saponins active in in vivo models of prostate cancer
Although prostate cancer is the second most prevalent cancer type in men, in the past decade, there has been relatively little research that evaluated the efficacy of triterpene saponins against this cancer. Again, one of the most active compounds was platycodin D (PD). PD significantly suppressed human prostate cancer PC3 xenograft tumor growth in a dose-dependent manner (Zhou et al. 2014) Changes in protein expression indicated suppression of cell proliferation and induction of cell cycle arrest. Western blot analysis of excised tumors showed altered protein expression similar to that observed in vitro. PD increased the expression of the FOXO3a, p21 and p27 proteins and decreased the expression of the MDM2 protein in PC3 cells. Furthermore, the compound downregulated proteins involved in the cell cycle and apoptosis: CDK2, Cyclin D1, CDK1, Cyclin B1, Bcl2, and PARP, and up-regulated the expression of Bax. This suggested a possible mechanism of the antitumor activity of PD that included cell cycle arrest and apoptosis. No significant body weight loss was observed, indicating that there was no obvious toxicity of the compound.
Triterpene saponins active in in vivo models of pancreatic cancer
Relatively small numbers of triterpene saponins have been studied in in vivo models of pancreatic cancer. Three fairly effective compounds are those that were also studied against other tumors, namely ginsenoside Rg3, Pulsatilla saponins A and D. Ginsenoside Rg3 dose-dependently reduced tumor volume in a model of xenograft tumor induced by SW-1990 pancreatic cancer cells in mice (Guo et al. 2014) Furthermore, vascular mimicry, which is a network structure consisting of an extracellular matrix and cancer cells that facilitate neoangiogenesis and metastasis, associated with a poor prognosis of patients, was also significantly reduced in Rg3-treated groups. This made Rg3 a promising adjuvant in pancreatic cancer therapy that could potentially inhibit angiogenesis and tumor metastasis. Taking into account the results of the above-mentioned experiment, the role of Rg3 in chemotherapy as an adjuvant gains higher probability. Pulsatilla saponin A was also effective in the SW1990 pancreatic cancer model (Liu et al. 2014), while Pulsatilla saponin D (SB365), in turn, was shown to decrease tumor growth in the in vivo xenograft model of pancreatic cancer PANC-1 by blocking angiogenesis, reducing proliferation, and/or inducing apoptosis (Son et al. 2013a, b).
Triterpene saponins active in in vivo models of melanoma
Melanoma is the most frequent cause of death among people with skin cancer (WHO 2021). In addition, the number of patients diagnosed with the drug-resistant type of this cancer increases every year (Garbe et al. 2011). Corchorusin-D, a saikosaponin-like compound derived from Corchorus acutangulus, was studied in murine B16F10 melanoma cells-induced xenograft tumor in mice (Mallick et al. 2013). The compound significantly inhibited tumor growth, simultaneously increasing the survival rate in mice. Further histological analysis of the tumors showed a lower mitotic index and a higher level of annexin V-FITC and propidium iodide (PI)-positive tumor cells indicating an induction of apoptosis and a decrease in the microvascular density pointing to an antiangiogenic effect. Finally, corchorusin-D was recognized as safe.
As melanoma is found to be largely resistant to treatment, researchers hope to find more effective drugs by using models that involve cell lines that lack sensitivity to standard chemotherapy. An example of such a cell line is A375-R, a BRAFV600E mutant melanoma cell line resistant to PLX4032 (vemurafenib), an inhibitor of the mutated proto-oncogene BRAF, which in turn is the target of melanoma therapy (Cvetanova et al. 2019). Cumingianoside A (CUMA), isolated from Dysoxylum cumingianum, administered alone or in combination with PLX4032 revealed a strong statistically significant inhibition of tumor growth in the A375-R xenograft mouse model (Cvetanova et al. 2019). A negligible weight loss of the experimental animals indicated little toxicity of the treatment. Mice were sacrificed, and immunohistochemistry studies of tumors and organs showed that CUMA reduced proliferation. Also, an impairment of angiogenesis and apoptosis enhancement were suggested.
Triterpene saponins active in in vivo models of glioma
Glioma (glioblastoma multiforme) is the most common among intrinsic central nervous system tumors, with a high prevalence in adults and a high mortality rate (Liang et al. 2020). Only a few saponins have been studied as potential antiglioma agents in vitro and even less in vivo. Again, one of the saponins that have been mentioned already, that is, astragaloside IV (AS-IV), seems to be an interesting option for further research. Studies on AS-IV revealed attenuation of tumor growth when the compound was administered prior to inoculation of tumor cells in mice (Li et al. 2017). The proposed tumor inhibition mechanism was related to the MAPK/ERK signaling pathway, since immunochemistry analysis of tumor tissue revealed the reduction of PCNA expression (suppression of proliferation), phosphorylated MAPK, phosphorylated ERK and C-myc (Li et al. 2017). The MAPK/ERK signaling pathway in several studies was reported in several studies to be involved in the migration and invasion of glioma cells.
Another active compound is saponin 1, an oleanane-type structure from Anemone taipaiensis, which was examined in the tumor xenograft model in mice (induced with U251MG and U87MG cells). Saponin 1 inhibited the growth of both xenograft tumors, with U87MG cells being more sensitive to treatment (Li et al. 2013a). Also, another triterpene saponin, raddeanin A, which has a marked potential as an anticancer agent, exhibited antiglioma activity in U87-cells-induced tumor in mice, but at a very high dose of 100 mg/kg (Wu et al. 2021a).
Triterpene saponins active in in vivo models of miscellaneous cancers
Kalopanaxsaponin A, an oleanane triterpenoid from Kalopanax pictus, significantly reduced tumor volume in a dose-dependent manner compared to a group inoculated with human oral squamous cell carcinoma (OSCC) YD-10B cells but without saponin treatment (Hwang et al. 2012). Remarkable weight loss was observed in the tumor control group but not in mice treated with kalopanaxsaponin A, which proved its safety. Immunohistochemical analysis showed that tumors from cancer-cells- inoculated mice expressed protein markers: PCNA, MMP-9, TIMP-1, HuR, Rab1A which was not observed in the group that received kalopanaxsaponin A.
Saikosaponin-d, a compound studied in vivo against different types of cancers, was shown to perform a significant dose-dependent reduction in the volume and weight of anaplastic thyroid cancers induced by 8305C, SW1736 cells (Liu and Li 2014).
The effect on SKOV3 tumor growth in human ovarian cancer xenograft model in mice was examined using oldhamianoside, an oleanolic acid derivative from Gypsophila oldhamiana (Li et al. 2018a, b). As a result, this saponin inhibited tumor growth in a dose-dependent manner. Furthermore, the level of TNF-α, IL-6 and MCP-1 in murine plasma of the oldhamianoside-treated group was lower than in the control group, and the expression of VEGF and VEGFR2, as well as caspase-3 and Bax/Bcl-2 ratio, also decreased. The antitumor potential of oldhamianoside II was evaluated, in turn, in S180 cell-induced murine sarcoma in mice. The doses used reduced tumor growth by inducing apoptosis (Wang et al. 2013a).
The in vivo antitumor activity of cucurbitacine E and cucurbitacine I glucosides was evaluated using the EAC model (Ayyad et al. 2012). Both saponins induced a longer mean survival time and an increased life span compared to 5-fluorouracil but at 2.5 higher doses. Furthermore, no statistical analysis was performed so the significance of the effect is unclear. The analysis of hematological parameters showed an improvement in the saponin groups compared to the 5-FU group but again – only for the highest dose and the difference was not statistically significant. Furthermore, the level of alanine transferase, an indicator of liver dysfunction, was higher for saponins than for 5-FU.
Although the anti-leukemia activity of triterpene saponins is rarely investigated, interesting results were obtained for saikosaponin-d. In models that employ both murine cancer cells and human peripheral blood from patients with leukemia, this compound significantly suppressed white blood cell growth and splenomegaly (Sun et al. 2021).
In addition to the direct cytotoxic activity of anticancer agents, other mechanisms by which tumor growth may be suppressed are also investigated. One of the most frequent studies is the inhibition of angiogenesis. It is well known that overproliferated cells need oxygen, nutrients, and other factors that help them survive and proliferate further. These vital substances are supplied by blood vessels, and the more cancer cells, the more blood vessels are needed. Furthermore, neovascularization (promoting the formation of new blood vessels) is essential not only for the growth but also for the invasion and metastasis of solid tumors (Wang et al. 2012c). Cancer cells release certain pro-angiogenic agents such as vascular endothelial growth factor (VEGF) that binds to VEGF receptors (VEGFR) of types 1 and 2. A decrease in the number of VEGF-VEGFR complexes prevents incitement of cascade reactions leading to the proliferation of endothelial cells, their migration, and finally, blood vessel formation, which consequently limits tumor growth. That is why substances with antiangiogenic activity are being regarded as potential anticancer agents.
To assess the antiangiogenic properties of a compound, more specific models such as the chick chorioallantoic membrane (CAM) assay or the Matrigel plug assay are employed rather than the xenograft tumor model. In the CAM test, chicken eggs are utilized as an in vivo environment where the development of blood vessels can be easily observed. When it comes to the second method, the Matrigel containing HGF (angiogenic agent) is injected subcutaneously into the experimental animals, inducing neovascularization inside the Matrigel that becomes red due to blood cells that flow through newly formed vessels. Then each section of Matrigel plugs was taken for histological analysis and stained. An example of an ex vivo angiogenesis model is the HGF-induced microvessel sprouting from the rat aortic ring.
Two angiogenesis models were utilized to evaluate the effect of Pulsatilla saponin D (SB365) on new blood vessel formation. First, an ex vivo assay was performed. The saponin inhibited the HGF-induced sprouting of the aortic rings, completely suppressing it at a concentration of 10 μM. Then, Matrigel containing HGF placed in mice was shown to contain fewer newly formed blood vessels in the presence of SB365 than HGF-Matrigel without treatment (Hong et al. 2013).
In another study on ardipusilloside I (ADS-I) the microvessel density was significantly reduced by saponin itself as well as the reference drug—geftinib (Wang et al. 2012c). Furthermore, a beneficial effect of ardipusilloside I on microvessel formation in the CAM assay was confirmed. New, small and especially capillary blood vessels were promoted at a significantly lower rate compared to the group not treated with ADS-I. This was confirmed by CD31 immunochemistry analysis of microvessel density (MVD) in tumors obtained from experimental mice.
Another saponin with proven antiangiogenic activity is actein (Yue et al. 2016). In the mouse Matrigel plug model, an inhibition in vessel formation by actein given in situ (mixed with gel) or chronically was observed. The decrease in hemoglobin level in the actein-treated group confirmed its antiangiogenic effect. Ex vivo analysis of mice tumors revealed a reduction in the expression level of angiogenic proteins such as CD34 and Factor VIII as well as CXCR4 and down-regulation of metastatic-related VEGFR-1.
Investigation of ginsenoside Rk3, raddeanin A, saikosaponin A and oldhamianoside II in chick embryo CAM assay confirmed their antiangiogenic activity (Wang et al. 2013a; Duan et al. 2017; Wu et al. 2021a; Zhang et al. 2021). The latter also decreased microvessel density in the tumor in the xenograft model, while saikosaponin A additionally inhibited angiogenesis in a dose-dependent manner in the Matrigel plug assay (Zhang et al. 2021).
As was shown above, some saponins focus more research interest than others, raising hope to develop new chemotherapeutic agents. Still, the application of these compounds is difficult because of their poor bioavailability, which strongly limits the efficacy in vivo. To improve pharmacokinetic parameters, structural changes must be made or novel formulations need to be designed. Quite often structural modifications imply a better safety profile or selectivity of the investigated compounds, so semi-synthetic agents with plant-derived scaffolds are also prepared and tested.
Ginsenosides, which appear to be promising anticancer agents, are a good example to illustrate this need, as their absorption rate from the gastro-intestinal tract is very low. Some structural modifications have already been made to change physical–chemical properties, such as solubility or targetability (Dong et al. 2019). An example of these is the folic acid-modified ginsenoside Rg5-loaded bovine serum albumin nanoparticles (FA-Rg5-BSA NPs) that showed better antitumor activity in human breast cancer MCF-7 xenograft mouse model than Rg5 alone or without the folic acid moiety in the structure. Furthermore, real-time bioimaging showed that FA-Rg5-BSA NPs accumulated mainly in cancerous tissue with better targeting ability than other structures analyzed in the same experiment.
A comparison of the effectiveness of two Rh2 ginsenoside isomers: 20R and 20S in inhibiting xenograft tumor growth induced by H22 hepatocellular carcinoma cells in mice revealed a few differences with the same mechanism of inducing tumor cell apoptosis (Lv et al. 2016). However, in the study by Wei et al. (2012) ginsenoside Rh2, turned out to be toxic to normal liver cells. To minimize the toxicity, a chemical modification of the structure was carried out. The dioctanoyl ester of Rh2 (D-Rh2) showed cytotoxic activity similar to that of Rh2 in the xenograft model in mice with injected H22 liver cancer cells, but, in contrast, had a better safety profile. Furthermore, D-Rh2 stimulated lymphocytes to produce an antitumor response.
Another way to improve drug efficacy is to modify the formulation. In an experiment led by Xu et al. (2015) three different types of Rh2-loaded liposomes were designed: Rh2-loaded methoxy-poly(ethylene glycol)-poly(lactide) (mPEG-PLA) liposome (Rh2-PLP), Rh2-loaded normal liposome (Rh2-LP), Rh2-loaded cationic liposome (Rh2-CLP). Their effectiveness was compared with the activity of free Rh2 and tumor control. Rh2-PLP turned out to be the most effective in reducing tumor volume and weight. Furthermore, no weight loss was observed in all groups, indicating that the saponin was not overtly toxic.
To extend the half-life of ardipusilloside I (ADS-I) in serum, a new formulation was proposed. Saponin-loaded microspheres were compressed into wafers that were locally injected into G6-glioma tumors in rats. Interestingly, the effectiveness in inhibiting tumor growth and increasing a lifespan in the group that received wafers was significantly greater not only in comparison with the blank group, but also in the group that obtained the same dose of ADS-I but as a simple solution (Dang et al. 2015).
A novel formulation to target the anticancer agent more effectively in tumor tissue and simultaneously decrease its toxicity was designed for saikosaponin-d (SsD). It was found that the macrophage-biomimetic drug delivery system obtained by coating the macrophage membrane hybridized with T7 peptide on the surface of poly(latic-co-glycolic acid) nanoparticles (SCMNPs) loaded with SsD was significantly active in reducing the tumor induced by 4T1 murine breast cancer cells in mice at 1 and 5 mg/kg. With the higher dose, a 100% survival rate was achieved at the end of the experiment (50th day) and also a reversal in splenomegaly was observed compared to the negative control (Sun et al. 2020).
Summary
Triterpene saponins constitute a vast reservoir of cytotoxic compounds which may be further developed as antitumor agents. Technological developments enable them to improve their pharmacokinetic parameters and overcome the problem of poor bioavailability. It is noteworthy that more and more in vivo studies are being conducted on saponins and their semisynthetic derivatives or novel formulations. Studies focused on the structure–activity relationship help to understand the importance of specific structural features within a wide array of saponin types. In the time scope of this review, a large number of such reports have appeared, which confirmed some already known correlations and established novel ones. However, this issue requires further research, as specially designed SAR studies that compare saponins that contain the same sugar chain but differ in the major type of the aglycone (OT vs. UT vs. LT) are still very rare.
A large number of in vitro studies published in the last decade show that the structural characteristics and susceptibility of a particular cell line and cancer type should be considered in equal measure. Although triterpene saponins have been tested in a large diversity of human cancer cell lines, their effect on normal cells of respective origin, which reflects selectivity of action, is generally omitted, as it was included in only 9% of the studies. Data regarding mechanisms of action have expanded in recent years, and novel mechanisms, such as ferroptosis, anoikis, necroptosis, and methuosis have been reported. Of interest is also the inhibition of topoisomerase by some saponins, the mechanism of action of currently used chemotherapeutic drugs, which has previously been undescribed for this group of compounds. Nevertheless, the general problem which can be observed after reviewing so many papers on the cytotoxic potential of triterpene saponins is quite a narrow approach to these studies. Many papers focus only on a selected, single aspect of saponin interference with a cancer cell, without a thorough, multidirectional view of their effect.
Change history
18 August 2022
The supplementary material is updated
Abbreviations
- AIF:
-
Apoptosis-inducing factor
- AKT:
-
Protein kinase B
- AMPK 5':
-
Adenosine monophosphate-activated protein kinase
- AP-1:
-
Activator protein 1
- Bax:
-
Bcl-2-associated X protein
- Bcl:
-
B-cell lymphoma protein
- BRAF:
-
Proto-oncogene encoding a protein B-Raf
- CA:
-
Correspondence analysis
- CAM:
-
Chick chorioallantoic membrane
- CDK:
-
Cyclin-dependent kinase
- C/EBPβ:
-
CCAAT/enhancer-binding protein β
- COX:
-
Cyclooxygenase
- CXCR:
-
Chemokine receptors
- EAC:
-
Ehrlich’s ascites carcinoma
- EGFR:
-
Epidermal growth factor receptor
- EMT:
-
Epithelial-to-mesenchymal transition
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular signal-regulated kinase
- 5-FU:
-
5-Fluorouracil
- FAK:
-
Focal adhesion kinase
- FAS:
-
Fas Ligand, a cell surface molecule belonging to the TNF family
- FOXO:
-
‘Forkhead box’ class O (forkhead transcription factor O)
- GPX4:
-
Glutathione peroxidase IV
- HCC:
-
Hepatocellular carcinoma
- HGF:
-
Hepatocyte growth factor
- HuR:
-
Human antigen R
- ICAM-1:
-
Intercellular adhesion molecule 1
- IDO:
-
Indoleamine 2,3-dioxygenase
- IFN:
-
Interferon
- IL:
-
Interleukin
- JNK:
-
C-Jun N-terminal kinases
- LC3:
-
Microtubule-associated protein 1A/1B-light chain 3
- MAPK:
-
Mitogen-activated protein kinase
- MDM:
-
Murine double-minute protein
- MDRI1:
-
Multidrug resistance-associated protein 1
- MMPs:
-
Matrix metalloproteinases
- MMP:
-
Mitochondrial membrane potential
- mTOR:
-
Mechanistic target of rapamycin
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NSCLC:
-
Non-small cell lung cancer
- p38:
-
P38-mitogen activated protein kinase
- PCNA:
-
Proliferating cell nuclear antigen
- PI3K:
-
Phosphoinositide 3-kinase
- PPAR:
-
Peroxisome proliferator-activated receptors
- PPRE:
-
Peroxisome proliferator response elements
- Rab:
-
Ras related proteins
- RIPK:
-
Receptor-interacting protein kinase
- ROS:
-
Reactive oxygen species
- RNPC1:
-
RNA-binding protein 1
- SRC:
-
Proto-oncogene: non-receptor tyrosine kinase
- STAT:
-
Signal transducer and activator of transcription
- Th:
-
Lymphocyte helper cells
- TIMP:
-
Tissue inhibitor of metalloproteinases
- TNF-α:
-
Tumor necrosis factor-α
- TRAIL:
-
Necrosis factor-related apoptosis-inducing ligand
- VCAM-1:
-
Vascular cell adhesion molecule 1
- VEGF:
-
Vascular endothelial growth factor
- VEGFR:
-
Vascular endothelial growth factor receptor
References
Adamska A, Stefanowicz-Hajduk J, Renata Ochocka J (2019) Alpha-hederin, the active saponin of Nigella sativa, as an anticancer agent inducing apoptosis in the SKOV-3 cell line. Molecules 24:2958. https://doi.org/10.3390/molecules24162958
Alam F, Najum us Saqib Q, Waheed A (2017) Cytotoxic activity of extracts and crude saponins from Zanthoxylum armatum DC. against human breast (MCF-7, MDA-MB-468) and colorectal (Caco-2) cancer cell lines. BMC Complement Altern Med 17:368https://doi.org/10.1186/s12906-017-1882-1
Ali R, Mirza Z, Ashraf GMD et al (2012) New anticancer agents: recent developments in tumor therapy. Anticancer Res 32:2999–3005
Aminin DL, Menchinskaya ES, Pisliagin EA et al (2015) Anticancer activity of sea cucumber triterpene glycosides. Mar Drugs 13:1202–1223. https://doi.org/10.3390/md13031202
An IS, An S, Kwon KJ et al (2013) Ginsenoside Rh2 mediates changes in the microRNA expression profile of human non-small cell lung cancer A549 cells. Oncol Rep 29:523–528. https://doi.org/10.3892/or.2012.2136
Arslan I, Cenzano AM (2020) N-triterpene saponins in cancer therapy: a review of mode of action. Rev Bras Farmacogn 30:1–6. https://doi.org/10.1007/s43450-020-00033-5
Ayyad SEN, Abdel-Lateff A, Alarif WM et al (2012) In vitro and in vivo study of cucurbitacins-type triterpene glucoside from Citrullus colocynthis growing in Saudi Arabia against hepatocellular carcinoma. Environ Toxicol Pharmacol 33:245–251. https://doi.org/10.1016/j.etap.2011.12.010
Bai C, Ye Y, Feng X et al (2017) Anti-proliferative effect of triterpenoidal glycosides from the roots of Anemone vitifolia through a pro-apoptotic way. Molecules 22:642. https://doi.org/10.3390/molecules22040642
Bian Q, Liu P, Gu J, Song B (2015) Tubeimoside-1 inhibits the growth and invasion of colorectal cancer cells through the Wnt/β-catenin signaling pathway. Int J Clin Exp Pathol 8:12517–12524
Bian X, Zhao Y, Guo X et al (2017) Chiisanoside, a triterpenoid saponin, exhibits anti-tumor activity by promoting apoptosis and inhibiting angiogenesis. RSC Adv 7:41640–41650. https://doi.org/10.1039/c7ra08041g
Cai W, Xu S, Ma T et al (2021) Five novel triterpenoid saponins from Hovenia dulcis and their Nrf2 inhibitory activities. Arab J Chem 14:103292. https://doi.org/10.1016/j.arabjc.2021.103292
Cao J, Zhao E, Zhu Q et al (2019) Tubeimoside-1 Inhibits glioblastoma growth, migration, and invasion via inducing ubiquitylation of MET. Cells 8:774. https://doi.org/10.3390/cells8080774
Cao M, Zhang J, Zhao Y et al (2012) Advances in ginsenoside Rh2 and its derivatives. Mod Tradit Chinese Med Mater Medica 14:2205–2211. https://doi.org/10.1016/s1876-3553(13)60018-6
Cao Y, Wang K, Xu S et al (2020) Recent advances in the semisynthesis, modifications and biological activities of ocotillol-type triterpenoids. Molecules 25:5562
Chai X, Zhang J, Li S et al (2021) Xanthoceraside induces cell apoptosis through downregulation of the PI3K/Akt/Bcl-2/Bax signaling pathway in cell lines of human bladder cancer. Indian J Pathol Microbiol 64:294–301
Cham BT, Linh NTT, Thao DT et al (2020) Cell growth inhibition of saponin XII from Dipsacus japonicus Miq. on acute myeloid leukemia cells. Molecules 25:3325. https://doi.org/10.3390/molecules25153325
Chandra S, Rawat DS, Bhatt A (2021) Phytochemistry and pharmacological activities of Saponaria officinalis L. a review. Not Sci Biol 13:10809. https://doi.org/10.15835/nsb13110809
Chen A, Zhang S, Zhang D, et al (2021a) Anti-proliferative and pro-apoptotic activities of hederacolchiside A1 on MCF-7 cells via ROS regulation and JAK2/STAT3 inactivation. Res Square https://doi.org/10.21203/rs.3.rs-408362/v1
Chen B, Jia XB (2013) Apoptosis-inducing effect of ginsenoside Rg6 on human lymphocytoma JK cells. Molecules 18:8109–8119. https://doi.org/10.3390/molecules18078109
Chen C, Lv Q, Li Y, Jin YH (2021b) The anti-tumor effect and underlying apoptotic mechanism of ginsenoside rk1 and rg5 in human liver cancer cells. Molecules 26:3926. https://doi.org/10.3390/molecules26133926
Chen DL, Xu XD, Li RT et al (2019a) Five new cucurbitane-type triterpenoid glycosides from the rhizomes of Hemsleya penxianensis with cytotoxic activities. Molecules 24:2937. https://doi.org/10.3390/molecules24162937
Chen HY, Liu JF, Tsai SF et al (2018a) Cytotoxic protobassic acid saponins from the kernels of Palaquium formosanum. J Food Drug Analys 26:557–564. https://doi.org/10.1016/j.jfda.2017.06.004
Chen L, Chen J, Xu H (2013) Sasanquasaponin from Camellia oleifera Abel. induces cell cycle arrest and apoptosis in human breast cancer MCF-7 cells. Fitoterapia 84:123–129. https://doi.org/10.1016/j.fitote.2012.11.009
Chen MF, Huang SJ, Huang CC et al (2016a) Saikosaponin d induces cell death through caspase-3-dependent, caspase-3-independent and mitochondrial pathways in mammalian hepatic stellate cells. BMC Cancer 16:532. https://doi.org/10.1186/s12885-016-2599-0
Chen WJ, Yu C, Yang Z et al (2012) Tubeimoside-1 induces G2/M phase arrest and apoptosis in SKOV-3 cells through increase of intracellular Ca 2+and caspase-dependent signaling pathways. Int J Oncol 40:535–543. https://doi.org/10.3892/ijo.2011.1218
Chen WX, Cheng L, Pan M et al (2018b) D Rhamnose β-hederin against human breast cancer by reducing tumor-derived exosomes. Oncol Lett 16:5172–5178. https://doi.org/10.3892/ol.2018.9254
Chen X, Wu QS, Meng FC et al (2016b) Chikusetsusaponin IVa methyl ester induces G1 cell cycle arrest, triggers apoptosis and inhibits migration and invasion in ovarian cancer cells. Phytomedicine 23:1555–1565. https://doi.org/10.1016/j.phymed.2016.09.002
Chen Z, Duan H, Tong X et al (2018c) Cytotoxicity, hemolytic toxicity, and mechanism of action of Pulsatilla saponin D and its synthetic derivatives. J Nat Prod 81:465–474. https://doi.org/10.1021/acs.jnatprod.7b00578
Chen Z, Duan H, Wang M et al (2015) Synthesis, cytotoxicity and haemolytic activity of Pulsatilla saponin A, D derivatives. Bioorg Med Chem Lett 25:2550–2554. https://doi.org/10.1016/j.bmcl.2015.04.049
Chen Z, Wei X, Shen L et al (2019b) 20(S)-ginsenoside-Rg3 reverses temozolomide resistance and restrains epithelial-mesenchymal transition progression in glioblastoma. Cancer Sci 110:389–400. https://doi.org/10.1111/cas.13881
Cheng L, Shi L, Wu J et al (2018a) A hederagenin saponin isolated from Clematis ganpiniana induces apoptosis in breast cancer cells via the mitochondrial pathway. Oncol Lett 15:1737–1743. https://doi.org/10.3892/ol.2017.7494
Cheng L, Xia TS, Shi L et al (2018b) D Rhamnose β-hederin inhibits migration and invasion of human breast cancer cell line MDA-MB-231. Biochem Biophys Res Commun 495:775780. https://doi.org/10.1016/j.bbrc.2017.11.081
Cheng L, Xia TS, Wang YF et al (2014a) The anticancer effect and mechanism of α-hederin on breast cancer cells. Int J Oncol 45:757–763. https://doi.org/10.3892/ijo.2014.2449
Cheng L, Xia TS, Wang YF et al (2014b) The apoptotic effect of D Rhamnose β-hederin, a novel oleanane-type triterpenoid saponin on breast cancer cells. PLoS ONE 9:e90848. https://doi.org/10.1371/journal.pone.0090848
Cho YJ, Woo JH, Lee JS et al (2016) Eclalbasaponin II induces autophagic and apoptotic cell death in human ovarian cancer cells. J Pharmacol Sci 132:6–14. https://doi.org/10.1016/j.jphs.2016.02.006
Choi JS, Chun KS, Kundu J, Kundu JK (2013) Biochemical basis of cancer chemoprevention and/or chemotherapy with ginsenosides (Review). Int J Mol Med 32:1227–1238. https://doi.org/10.3892/ijmm.2013.1519
Chueh FS, Hsiao YT, Chang SJ et al (2012) Glycyrrhizic acid induces apoptosis in WEHI-3 mouse leukemia cells through the caspase- and mitochondria-dependent pathways. Oncol Rep 28:2069–2076. https://doi.org/10.3892/or.2012.2029
Chun J, Ha IJ, Kim YS (2013a) Antiproliferative and apoptotic activities of triterpenoid saponins from the roots of Platycodon grandiflorum and their structure-activity relationships. Planta Med 79:639–645. https://doi.org/10.1055/s-0032-1328401
Chun J, Joo EJ, Kang M, Kim YS (2013b) Platycodin D induces anoikis and caspase-mediated apoptosis via p38 MAPK in AGS human gastric cancer cells. J Cell Biochem 114:456–470. https://doi.org/10.1002/jcb.24386
Chun J, Kang M, Kim YS (2014) A triterpenoid saponin from Adenophora triphylla var. japonica suppresses the growth of human gastric cancer cells via regulation of apoptosis and autophagy. Tumor Biol 35:12021–12030. https://doi.org/10.1007/s13277-014-2501-0
Chun J, Kim YS (2013) Platycodin D inhibits migration, invasion, and growth of MDA-MB-231 human breast cancer cells via suppression of EGFR-mediated Akt and MAPK pathways. Chem Biol Interact 205:212–221. https://doi.org/10.1016/j.cbi.2013.07.002
Chung KS, Cho SH, Shin JS et al (2013) Ginsenoside Rh2 induces cell cycle arrest and differentiation in human leukemia cells by upregulating TGF-β expression. Carcinogenesis 34:331–340. https://doi.org/10.1093/carcin/bgs341
Chung Y, Jeong S, Choi HS et al (2018) Upregulation of autophagy by ginsenoside Rg2 in MCF-7 cells. Animal Cells Syst 22:382–389. https://doi.org/10.1080/19768354.2018.1545696
Cvetanova B, Shen YC, Shyur LF (2019) Cumingianoside A, a phyto-triterpenoid saponin inhibits acquired BRAF inhibitor resistant melanoma growth via programmed cell death. Front Pharmacol 10:30. https://doi.org/10.3389/fphar.2019.00030
Dai L, Li J, Yang J et al (2018) Enzymatic synthesis of novel glycyrrhizic acid glucosides using a promiscuous Bacillus glycosyltransferase. Catalysts 8:615. https://doi.org/10.3390/catal8120615
Dai LM, Huang RZ, Zhang B et al (2017) Cytotoxic triterpenoid saponins from Lysimachia foenum-graecum. Phytochemistry 136:165–174. https://doi.org/10.1016/j.phytochem.2017.01.021
Dai Y, Harinantenaina L, Brodie PJ et al (2013) Two antiproliferative triterpene saponins from Nematostylis anthophylla from the Highlands of Central Madagascar. Chem Biodivers 10:233–240. https://doi.org/10.1002/cbdv.201200156
Dang H, Wang J, Cheng JX et al (2015) Efficacy of local delivery of ardipusilloside I using biodegradable implants against cerebral tumor growth. Am J Cancer Res 5:243–254
Deng S, Wong CKC, Lai HC, Wong AST (2017) Ginsenoside-Rb1 targets chemotherapy-resistant ovarian cancer stem cells via simultaneous inhibition of Wnt/β-catenin signaling and epithelial-to-mesenchymal transition. Oncotarget 8:25897–25914
Dinda B, Debnath S, Mohanta BC, Harigaya Y (2010) Naturally occurring triterpenoid saponins. Chem Biodivers 7:2327–2580. https://doi.org/10.1002/cbdv.200800070
Ding M, Wang X, Zhang Y et al (2019) New perspective on the metabolism of AD-1 in vivo: characterization of a series of dammarane-type derivatives with novel metabolic sites and anticancer mechanisms of active oleanane-type metabolites. Bioorg Chem 88:102961. https://doi.org/10.1016/j.bioorg.2019.102961
Dixon SJ, Lemberg KM, Lamprecht MR et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Dong XZ, Xie TT, Zhou XJ et al (2015) AG4, a compound isolated from Radix Ardisiae Gigantifoliae, induces apoptosis in human nasopharyngeal cancer CNE cells through intrinsic and extrinsic apoptosis pathways. Anticancer Drugs 26:331–342. https://doi.org/10.1097/CAD.0000000000000193
Dong Y, Fu R, Yang J et al (2019) Folic acid-modified ginsenoside Rg5-loaded bovine serum albumin nanoparticles for targeted cancer therapy in vitro and in vivo. Int J Nanomed 14:6971–6988. https://doi.org/10.2147/IJN.S210882
Duan Z, Deng J, Dong Y et al (2017) Anticancer effects of ginsenoside Rk3 on non-small cell lung cancer cells: in vitro and in vivo. Food and Funct 8:3723–3736. https://doi.org/10.1039/c7fo00385d
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 16:966–975. https://doi.org/10.1038/cdd.2009.33
Eso A, Uinarni H, Tommy T et al (2020) A reviews on use of sea cucumber as a treatment for oral cancer. Syst Rev Pharm 11:299–307. https://doi.org/10.31838/srp.2020.5.44
Fang Y, Hu D, Li H et al (2019) Synthesis, biological evaluation, and mode of action of Pulsatilla saponin D derivatives as promising anticancer agents. Front Pharmacol 10:1208. https://doi.org/10.3389/fphar.2019.01208
Fang Y, Yang Z, Ouyang H et al (2016) Synthesis and biological evaluation of Hederacolchiside A1derivatives as anticancer agents. Bioorg Med Chem Lett 26:4576–4579. https://doi.org/10.1016/j.bmcl.2016.08.077
Fei ZH, Wu K, Chen YL et al (2014) Capilliposide isolated from Lysimachia capillipes Hemsl induces ROS generation, cell cycle arrest, and apoptosis in human nonsmall cell lung cancer cell lines. Evidence-Based Complement Altern Med 2014:497456. https://doi.org/10.1155/2014/497456
Feng S, Zha Z, Wang Z et al (2021) Anticancer activity of oleiferoside B involving autophagy and apoptosis through increasing ROS release in MCF-7 cells and SMMC-7721 cells. Nat Prod Res 35:4865–4869. https://doi.org/10.1080/14786419.2020.1739039
Firempong CK, Zhang HY, Wang Y et al (2016) Segetoside I, a plant-derived bisdesmosidic gsaponin, induces apoptosis in human hepatoma cells in vitro and inhibits tumor growth in vivo. Pharmacol Res 110:101–110. https://doi.org/10.1016/j.phrs.2016.04.032
Foubert K, Theunis M, Apers S et al (2008) Chemistry, distribution and biological activities of 13,28-epoxy-oleanane saponins from the plant families Myrsinaceae and Primulaceae. Curr Org Chem 12:629–642. https://doi.org/10.2174/138527208784577376
Frisch SM, Screaton RA (2001) Anoikis mechanisms. Curr Opin Cell Biol 13:555–562. https://doi.org/10.1016/S0955-0674(00)00251-9
Fu R, Zhang L, Li Y et al (2020) Saikosaponin D inhibits autophagosome-lysosome fusion and induces autophagy-independent apoptosis in MDA-MB-231 breast cancer cells. Mol Med Rep 22:1026–1034. https://doi.org/10.3892/mmr.2020.11155
Gan M, Liu M, Liu B et al (2011) Cucurbitane glucosides from the root of Machilus yaoshansis. J Nat Prod 74:2431–2437. https://doi.org/10.1021/np200706n
Gao B, Shi HL, Li X et al (2014) P38 MAPK and ERK1/2 pathways are involved in the pro-apoptotic effect of notoginsenoside Ft1 on human neuroblastoma SH-SY5Y cells. Life Sci 108:63–70. https://doi.org/10.1016/j.lfs.2014.05.010
Gao J, Li X, Gu G et al (2012) Facile synthesis of triterpenoid saponins bearing β-Glu/Gal-(1→3)-β-GluA methyl ester and their cytotoxic activities. Bioorg Med Chem Lett 22:2396–2400. https://doi.org/10.1016/j.bmcl.2012.02.032
Garai S (2014) Triterpenoid saponins. Nat Prod Chem Res 2:148. https://doi.org/10.4172/2329-6836.1000148
Garbe C, Eigentler TK, Keilholz U et al (2011) Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 16:5–24. https://doi.org/10.1634/theoncologist.2010-0190
Gauthier C, Legault J, Piochon-Gauthier M, Pichette A (2011) Advances in the synthesis and pharmacological activity of lupane-type triterpenoid saponins. Phytochem Rev 10:521–544. https://doi.org/10.1007/s11101-010-9176-y
Gong X, Sun R, Gao Z et al (2018) Tubeimoside 1 acts as a chemotherapeutic synergist via stimulating macropinocytosis. Front Pharmacol 9:1044. https://doi.org/10.3389/fphar.2018.01044
Grabowska K, Pecio Ł, Galanty A et al (2021) Serjanic acid glycosides from Chenopodium hybridum L. with good cytotoxicity and selectivity profile against several panels of human cancer cell lines. Molecules 26:4915. https://doi.org/10.3390/molecules26164915
Grabowska K, Podolak I, Galanty A et al (2017) Two new triterpenoid saponins from the leaves of Impatiens parviflora DC. and their cytotoxic activity. Ind Crops Prod 96:71–79. https://doi.org/10.1016/j.indcrop.2016.11.022
Graziani V, Esposito A, Scognamiglio M et al (2019) Spectroscopic characterization and cytotoxicity assessment towards human colon cancer cell lines of acylated cycloartane glycosides from Astragalus boeticus L. Molecules 24:1725. https://doi.org/10.3390/molecules24091725
Gu G, Qi H, Jiang T et al (2017) Investigation of the cytotoxicity, apoptosis and pharmacokinetics of raddeanin A. Oncol Lett 13:1365–1369. https://doi.org/10.3892/ol.2017.5588
di Guan Y, Jiang SL, Yu P et al (2018) Suppression of eEF-2K-mediated autophagy enhances the cytotoxicity of raddeanin A against human breast cancer cells in vitro. Acta Pharmacol Sin 39:642–648. https://doi.org/10.1038/aps.2017.139
Guan F, Wang H, Shan Y et al (2013) Bigelovii A induces apoptosis of HL-60 human acute promyelocytic leukaemia cells. Mol Med Rep 7:1585–1590. https://doi.org/10.3892/mmr.2013.1353
Guan YY, Liu HJ, Luan X et al (2015) Raddeanin A, a triterpenoid saponin isolated from Anemone raddeana, suppresses the angiogenesis and growth of human colorectal tumor by inhibiting VEGFR2 signaling. Phytomedicine 22:103–110. https://doi.org/10.1016/j.phymed.2014.11.008
Guo JQ, Zheng QH, Chen H et al (2014) Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int J Oncol 45:1065–1072. https://doi.org/10.3892/ijo.2014.2500
Guo SS, Wang Y, Fan QX (2019) Raddeanin A promotes apoptosis and ameliorates 5-fluorouracil resistance in cholangiocarcinoma cells. World J Gastroenterol 25:3380–3391. https://doi.org/10.3748/wjg.v25.i26.3380
Han B, He C (2021) Targeting autophagy using saponins as a therapeutic and preventive strategy against human diseases. Pharmacol Res 166:105428. https://doi.org/10.1016/j.phrs.2021.105428
Han H, Yang Y, Wu Z et al (2021) Capilliposide B blocks VEGF-induced angiogenesis in vitro in primary human retinal microvascular endothelial cells. Biomed Pharmacother 133:110999. https://doi.org/10.1016/j.biopha.2020.110999
Han LT, Fang Y, Cao Y et al (2016) Triterpenoid saponin flaccidoside II from Anemone flaccida triggers apoptosis of NF1-associated malignant peripheral nerve sheath tumors via the MAPK-HO-1 pathway. Onco Targets Ther 9:1969–1979. https://doi.org/10.2147/OTT.S95597
Han LT, Fang Y, Li MM et al (2013) The antitumor effects of triterpenoid saponins from the Anemone flaccida and the underlying mechanism. Evidence-Based Complement Altern Med 2013:517931. https://doi.org/10.1155/2013/517931
Han Q, Qian Y, Wang X et al (2017) Cytotoxic oleanane triterpenoid saponins from Albizia julibrissin. Fitoterapia 121:183–193
He S, Tian S, He X et al (2021a) Multiple targeted self-emulsifying compound RGO reveals obvious anti-tumor potential in hepatocellular carcinoma. Mol Ther Oncolytics 22:604–616. https://doi.org/10.1016/j.omto.2021.08.008
He X, Tang J, Yan H-Z et al (2021b) Anemoside B4 sensitizes human colorectal cancer to fluorouracil-based chemotherapy through src-mediated cell apoptosis. AGIN 13:25365–25376
Herrera-Martínez M, Ramírez-Mares MV, Burgueño-Tapia E et al (2012) Screening of antitopoisomerase, antioxidant, and antimicrobial activities of selected triterpenes and saponins. Revi Latinoamer Quim 40:165–177
Honey-Escandón M, Arreguín-Espinosa R, Solís-Marín FA, Samyn Y (2015) Biological and taxonomic perspective of triterpenoid glycosides of sea cucumbers of the family Holothuriidae (Echinodermata, Holothuroidea). Comp Biochem Physiol Part B Biochem Mol Biol 180:16–39. https://doi.org/10.1016/j.cbpb.2014.09.007
Hong SW, Jung KH, Lee HS et al (2013) SB365, Pulsatilla saponin D, targets c-Met and exerts antiangiogenic and antitumor activities. Carcinogenesis 34:2156–2169. https://doi.org/10.1093/carcin/bgt159
Huang C, Xu D, Xia Q et al (2012) Reversal of P-glycoprotein-mediated multidrug resistance of human hepatic cancer cells by Astragaloside II. J Pharm Pharmacol 64:1741–1750. https://doi.org/10.1111/j.2042-7158.2012.01549.x
Huang S, Huang P, Wu H et al (2021) Soyasaponin Ag inhibits triple-negative breast cancer progression via targeting the DUSP6/MAPK signaling. Folia Histochem Cytobiol 59:291–301. https://doi.org/10.5603/FHC.a2021.0029
Hwang JA, Hwang MK, Jang Y et al (2013) 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginseng, inhibits colon cancer growth by targeting TRPC channel-mediated calcium influx. J Nutr Biochem 24:1096–1104. https://doi.org/10.1016/j.jnutbio.2012.08.008
Hwang YS, Chung WY, Kim J et al (2011) Buddlejasaponin IV induces cell cycle arrest at G2/M phase and apoptosis in immortalized human oral keratinocytes. Phytother Res 25:1503–1510. https://doi.org/10.1002/ptr.3406
Hwang YS, Park KK, Chung WY (2012) Kalopanaxsaponin A inhibits the invasion of human oral squamous cell carcinoma by reducing metalloproteinase-9 mRNA stability and protein trafficking. Biol Pharm Bull 35:289–300. https://doi.org/10.1248/bpb.35.289
Islam MS, Wang C, Zheng J et al (2019) The potential role of tubeimosides in cancer prevention and treatment. Eur J Med Chem 162:109–121. https://doi.org/10.1016/j.ejmech.2018.11.001
Jang HJ, Han IH, Kim YJ et al (2014) Anticarcinogenic effects of products of heat-processed ginsenoside Re, a major constituent of ginseng berry, on human gastric cancer cells. J Agric Food Chem 62:2830–2836. https://doi.org/10.1021/jf5000776
Jeong Y, Ku S, You HJ, Ji GE (2019) A stereo-selective growth inhibition profile of ginsenoside Rh2 on human colon cancer cells. CyTA - J Food 17:488–493. https://doi.org/10.1080/19476337.2019.1607562
Jia LY, Xia HL, da Chen Z et al (2018) Anti-proliferation effect of theasaponin E1 on the ALDH-positive ovarian cancer stem-like cells. Molecules 23:1469. https://doi.org/10.3390/molecules23061469
Jia X, Dang S, Cheng Y et al (2012) Effects of saikosaponin-D on syndecan-2, matrix metalloproteinases and tissue inhibitor of metalloproteinases-2 in rats with hepatocellular carcinoma. J Tradit Chinese Med 32:415–422. https://doi.org/10.1016/s0254-6272(13)60048-5
Jiang K, Lu Q, Li Q et al (2017) Astragaloside IV inhibits breast cancer cell invasion by suppressing Vav3 mediated Rac1/MAPK signaling. Int Immunopharmacol 42:195–202. https://doi.org/10.1016/j.intimp.2016.10.001
Jo H, Jang D, Park SK et al (2021) Ginsenoside 20(S)-protopanaxadiol induces cell death in human endometrial cancer cells via apoptosis. J Ginseng Res 45:126–133. https://doi.org/10.1016/j.jgr.2020.02.002
Juang YP, Liang PH (2020) Biological and pharmacological effects of synthetic saponins. Molecules 25:4974. https://doi.org/10.3390/molecules25214974
Kabe Y, Koike I, Yamamoto T et al (2021) Glycyrrhizin derivatives suppress cancer chemoresistance by inhibiting progesterone receptor membrane component 1. Cancers 13:3265. https://doi.org/10.3390/cancers13133265
Kaennakam S, Rassamee K, Siripong P, Tip-pyang S (2018) Catomentosaponin, a new triterpene saponin from the roots of Catunaregam tomentosa. Nat Prod Res 32:1499–1505. https://doi.org/10.1080/14786419.2017.1385009
Kaur S, Nayak SK (2021) Natural products suppressors of active Mdm2 as Anti-cancer agents. Zeichen J 7:270–297
Kim JH, Choi JS (2016) Effect of ginsenoside Rh-2 via activation of caspase-3 and Bcl-2-insensitive pathway in ovarian cancer cells. Physiol Res 65:1031–1037. https://doi.org/10.33549/physiolres.933367
Kim KH, Kim JY, Kwak JH et al (2016) Different apoptotic effects of saxifragifolin C in human breast cancer cells. Arch Pharm Res 39:577–589. https://doi.org/10.1007/s12272-016-0729-5
Kim KH, Kim JY, Kwak JH, Pyo S (2015) Different anticancer effects of saxifragifolin A on estrogen receptor-positive and estrogen receptor-negative breast cancer cells. Phytomedicine 22:820–828. https://doi.org/10.1016/j.phymed.2015.05.057
Kim KR, Park KK, Chung WY, Hwang YS (2013) The inhibitory effect of buddlejasaponin IV on the growth of YD-10B human oral squamous cell carcinoma cells. J Cancer Prev 18:330–336. https://doi.org/10.15430/jcp.2013.18.4.330
Koczurkiewicz P, Podolak I, Skrzeczyńska-Moncznik J et al (2013a) Triterpene saponosides from Lysimachia ciliata differentially attenuate invasive potential of prostate cancer cells. Chem Biol Interact 206:6–17. https://doi.org/10.1016/j.cbi.2013.08.003
Koczurkiewicz P, Podolak I, Wójcik KA et al (2013) Lclet 4 enhances pro-apoptotic and anti-invasive effects of mitoxantrone on human prostate cancer cells - in vitro study. Acta Biochim Pol 60:331–338. https://doi.org/10.18388/abp.2013_1989
Kong Y, Lu ZL, Wang JJ et al (2016) Platycodin D, a metabolite of Platycodin grandiflorum, inhibits highly metastatic MDA-MB-231 breast cancer growth in vitro and in vivo by targeting the MDM2 oncogene. Oncol Rep 36:1447–1456. https://doi.org/10.3892/or.2016.4935
Kwon J, Ko K, Zhang L et al (2019) An autophagy inducing triterpene saponin derived from Aster koraiensis. Molecules 24:4489. https://doi.org/10.3390/molecules24244489
Lacaille-Dubois MA, Pegnyemb DE, Noté OP, Mitaine-Offer AC (2011) A review of acacic acid-type saponins from Leguminosae-Mimosoideae as potent cytotoxic and apoptosis inducing agents. Phytochem Rev 10:565–584. https://doi.org/10.1007/s11101-011-9218-0
Lai M, Ge Y, Chen M et al (2020) Saikosaponin d inhibits proliferation and promotes apoptosis through activation of MKK4–JNK signaling pathway in pancreatic cancer cells. Onco Targets Ther 13:9465–9479. https://doi.org/10.2147/OTT.S263322
Lanzotti V, Termolino P, Dolci M, Curir P (2012) Paviosides A-H, eight new oleane type saponins from Aesculus pavia with cytotoxic activity. Bioorgan Med Chem 20:3280–3286. https://doi.org/10.1016/j.bmc.2012.03.048
Lee JY, Jung KH, Morgan MJ et al (2013) Sensitization of TRAIL-induced cell death by 20(S)- ginsenoside Rg 3 via CHOP-mediated DR5 upregulation in human hepatocellular carcinoma cells. Mol Cancer Ther 12:274–285. https://doi.org/10.1158/1535-7163.MCT-12-0054
Lee SK, Park KK, Kim HJ et al (2015) Platycodin D blocks breast cancer-induced bone destruction by inhibiting osteoclastogenesis and the growth of breast cancer cells. Cell Physiol Biochem 36:1809–1820. https://doi.org/10.1159/000430152
Lehbili M, Alabdul Magid A, Kabouche A et al (2018) Triterpenoid saponins from Scabiosa stellata collected in North-Eastern Algeria. Phytochemistry 150:40–49. https://doi.org/10.1016/j.phytochem.2018.03.005
Levitsky DO, Dembitsky VM (2015) Anti-breast cancer agents derived from plants. Nat Prod Bioprospect 5:1–16. https://doi.org/10.1007/s13659-014-0048-9
Li B, Tong T, Ren N et al (2021a) Theasaponin E1 inhibits platinum-resistant ovarian cancer cells through activating apoptosis and suppressing angiogenesis. Molecules 26:1681. https://doi.org/10.3390/molecules26061681
Li B, Wang F, Liu N et al (2017) Astragaloside IV inhibits progression of glioma via blocking MAPK/ERK signaling pathway. Biochem Biophys Res Commun 491:98–103. https://doi.org/10.1016/j.bbrc.2017.07.052
Li DQ, Wu J, Liu LY et al (2015a) Cytotoxic triterpenoid glycosides (saikosaponins) from the roots of Bupleurum chinense. Bioorg Med Chem Lett 25:3887–3892. https://doi.org/10.1016/j.bmcl.2015.07.053
Li J, Tang H, Zhang Y et al (2013a) Saponin 1 induces apoptosis and suppresses NF-κB-mediated survival signaling in glioblastoma multiforme (GBM). PLoS ONE 8:e81258. https://doi.org/10.1371/journal.pone.0081258
Li J, Wu DD, Zhang JX et al (2018a) Mitochondrial pathway mediated by reactive oxygen species involvement in α-hederin-induced apoptosis in hepatocellular carcinoma cells. World J Gastroenterol 24:1901–1910. https://doi.org/10.3748/wjg.v24.i17.1901
Li KK, Xu F, Li SS et al (2019a) Cytotoxic epimeric ginsenosides from the flower buds of Panax ginseng. Steroids 143:1–5. https://doi.org/10.1016/j.steroids.2018.12.002
Li L, Feng LS, He YX (2014a) Cytotoxic triterpene saponins from Ilex pubescens. J Asian Nat Prod Chem Res 16:830–835. https://doi.org/10.1080/10286020.2014.920012
Li M, Zhang D, Cheng J et al (2019b) Ginsenoside Rh2 inhibits proliferation but promotes apoptosis and autophagy by down-regulating microRNA-638 in human retinoblastoma cells. Exp Mol Pathol 108:17–23. https://doi.org/10.1016/j.yexmp.2019.03.004
Li N, Sun YN, Zhang L et al (2016a) NF-κB inhibitory activities of triterpenoid glycosides from the stems of Acanthopanax senticosus (Rupr. & Maxim.) Harms. Phytochem Lett 15:210–214. https://doi.org/10.1016/j.phytol.2016.01.008
Li Q, Li W, Hui LP et al (2012) 13,28-Epoxy triterpenoid saponins from Ardisia japonica selectively inhibit proliferation of liver cancer cells without affecting normal liver cells. Bioorg Med Chem Lett 22:6120–6125. https://doi.org/10.1016/j.bmcl.2012.08.027
Li R, Song X, Guo Y et al (2021b) Natural products: a promising therapeutics for targeting tumor angiogenesis. Front Oncol 11:772915. https://doi.org/10.3389/fonc.2021.772915
Li R, Zhang L, Zhang L et al (2014b) Capilliposide C derived from Lysimachia capillipes Hemsl inhibits growth of human prostate cancer PC3 cells by targeting caspase and MAPK pathways. Int Urol Nephrol 46:1335–1344. https://doi.org/10.1007/s11255-013-0641-6
Li T, Xu WS, Wu GS et al (2014c) Platycodin D induces apoptosis, and inhibits adhesion, migration and invasion in HepG2 hepatocellular carcinoma cells. Asian Pacific J Cancer Prev 15:1745–1749. https://doi.org/10.7314/APJCP.2014.15.4.1745
Li T, Xu XH, Tang ZH et al (2015b) Platycodin D induces apoptosis and triggers ERK- and JNK-mediated autophagy in human hepatocellular carcinoma BEL-7402 cells. Acta Pharm Sinic 36:1503–1513. https://doi.org/10.1038/aps.2015.99
Li W, Ding Y, Sun YN et al (2013b) Oleanane-type triterpenoid saponins from the roots of Pulsatilla koreana and their apoptosis-inducing effects on HL-60 human promyelocytic leukemia cells. Arch Pharm Res 36:768–774. https://doi.org/10.1007/s12272-013-0042-5
Li W, Tian YH, Liu Y et al (2016b) Platycodin D exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. J Toxicol Sci 41:417–428. https://doi.org/10.2131/jts.41.417
Li X, Chen M, Yao Z et al (2021c) Jujuboside B induces mitochondrial-dependent apoptosis in colorectal cancer through ROS-mediated PI3K/Akt pathway in vitro and in vivo. J Funct Foods 87:104796. https://doi.org/10.1016/j.jff.2021.104796
Li Y, Li Z, Jia Y et al (2020) In vitro anti-hepatoma activities of notoginsenoside R1 through downregulation of tumor promoter miR-21. Dig Dis Sci 65:1364–1375. https://doi.org/10.1007/s10620-019-05856-4
Li Z, Zuo Y, Hou L et al (2018b) Oldhamianoside inhibits the growth of ovarian cancer both in vitro and in vivo via adjusting inflammation and angiogenesis signals. Onco Targets Ther 11:6031–6037. https://doi.org/10.2147/OTT.S174528
Liang C, Ding Y, Nguyen HT et al (2010) Oleanane-type triterpenoids from Panax stipuleanatus and their anticancer activities. Bioorg Med Chem Lett 20:7110–7115. https://doi.org/10.1016/j.bmcl.2010.09.074
Liang D, Hao ZY, Zhang GJ et al (2011) Cytotoxic triterpenoid saponins from Lysimachia clethroides. J Nat Prod 74:2128–2136. https://doi.org/10.1021/np2004038
Liang J, Lv X, Lu C et al (2020) Prognostic factors of patients with gliomas- an analysis on 335 patients with glioblastoma and other forms of gliomas. BMC Cancer 20:35. https://doi.org/10.1186/s12885-019-6511-6
Liang QP, Xu TQ, Liu BL et al (2019) Sasanquasaponin ΙΙΙ from Schima crenata Korth induces autophagy through Akt/mTOR/p70S6K pathway and promotes apoptosis in human melanoma A375 cells. Phytomedicine 58:152769. https://doi.org/10.1016/j.phymed.2018.11.029
Liang Y, Xu X, Yu H et al (2016) Raddeanoside R13 inhibits breast cancer cell proliferation, invasion, and metastasis. Tumor Biol 37:9837–9847. https://doi.org/10.1007/s13277-015-4748-5
Lin X, Li W, Ye C et al (2014) Research on the interaction between tubeimoside 1 and HepG2 cells using the microscopic imaging and fluorescent spectra method. Comput Math Methods Med 2014:470452. https://doi.org/10.1155/2014/470452
Linkermann A, Green DR (2014) Necroptosis. New Engl J Med 23:455–465. https://doi.org/10.1056/NEJMra1310050
Liu C, Dong L, Sun Z et al (2018) Esculentoside A suppresses breast cancer stem cell growth through stemness attenuation and apoptosis induction by blocking IL-6/STAT3 signaling pathway. Phytother Res 32:2299–2311. https://doi.org/10.1002/ptr.6172
Liu J, Wei X, Wu Y et al (2016) Giganteaside D induces ROS-mediated apoptosis in human hepatocellular carcinoma cells through the MAPK pathway. Cell Oncol 39:333–342. https://doi.org/10.1007/s13402-016-0273-9
Liu M, Zhang G, Naqvi S et al (2020a) Cytotoxicity of Saikosaponin A targets HEKa cell through apoptosis induction by ROS accumulation and inflammation suppression via NF-κB pathway. Intern Immunopharmacol 86:106751. https://doi.org/10.1016/j.intimp.2020.106751
Liu Q, Chen W, Jiao Y et al (2014) Pulsatilla saponin A, an active molecule from Pulsatilla chinensis, induces cancer cell death and inhibits tumor growth in mouse xenograft models. J Surg Res 188:387–395. https://doi.org/10.1016/j.jss.2014.01.026
Liu Q, Liu H, Zhang L et al (2013) Synthesis and antitumor activities of naturally occurring oleanolic acid triterpenoid saponins and their derivatives. Eur J Med Chem 64:1–15. https://doi.org/10.1016/j.ejmech.2013.04.016
Liu RY, Li JP (2014) Saikosaponin-d inhibits proliferation of human undifferentiated thyroid carcinoma cells through induction of apoptosis and cell cycle arrest. Eur Rev Med Pharmacol Sci 18:2435–2443
Liu X, Xing Y, Li M et al (2021) Licochalcone A inhibits proliferation and promotes apoptosis of colon cancer cell by targeting programmed cell death-ligand 1 via the NF-κB and Ras/Raf/MEK pathways. J Ethnopharmacol 273:113989. https://doi.org/10.1016/J.JEP.2021.113989
Liu Y, Lu WX, Yan MC et al (2010) Synthesis and tumor cytotoxicity of novel amide derivatives of β-hederin. Molecules 15:7871–7883. https://doi.org/10.3390/molecules15117871
Liu Y, Zhang L, Wei S et al (2020b) Pulchinenosides from Pulsatilla chinensis increase P-glycoprotein activity and induce P-glycoprotein expression. Evidence-Based Compl Altern Med 2020:4861719. https://doi.org/10.1155/2020/4861719
Loizzo MR, Menichini F, Tundis R (2013) Recent insights into the emerging role of triterpenoids in cancer therapy: Part I. In: Studies in Natural Products Chemistry, 1st ed., pp 1–31
Lorent J, Le Duff CS, Quetin-Leclercq J, Mingeot-Leclercq MP (2013) Induction of highly curved structures in relation to membrane permeabilization and budding by the triterpenoid saponins, α- and δ-hederin. J Biol Chem 288:14000–14017. https://doi.org/10.1074/jbc.M112.407635
Lorent JH, Léonard C, Abouzi M et al (2016) α-Hederin induces apoptosis, membrane permeabilization and morphologic changes in two cancer cell lines through a cholesterol-dependent mechanism. Planta Med 82:1532–1539. https://doi.org/10.1055/s-0042-114780
Lou L, Ye W, Chen Y et al (2012) Ardipusilloside inhibits survival, invasion and metastasis of human hepatocellular carcinoma cells. Phytomedicine 19:603–608. https://doi.org/10.1016/j.phymed.2012.01.003
Lu XL, He SX, Ren MD et al (2012) Chemopreventive effect of saikosaponin-d on diethylinitrosamine-induced hepatocarcinogenesis: involvement of CCAAT/enhancer binding protein β and cyclooxygenase-2. Mol Med Rep 5:637–644. https://doi.org/10.3892/mmr.2011.702
Lu Z, Song W, Zhang Y et al (2021) Combined anti-cancer effects of Platycodin D and sorafenib on androgen-independent and PTEN-deficient prostate cancer. Front Oncol 11:648985. https://doi.org/10.3389/fonc.2021.648985
Lu Z, Wang H, Zhu M et al (2018) Ophiopogonin D’, a natural product from Radix Ophiopogonis, induces in vitro and in vivo RIPK1-dependent and caspase-independent apoptotic death in androgen-independent human prostate cancer cells. Front Pharmacol 9:432. https://doi.org/10.3389/fphar.2018.00432
Lv C, Zhao Y, Zhao B et al (2021) New 23, 27-dihydroxy-oleanane-type triterpenoid saponins from Anemone raddeana Regel. Nat Prod Res 35:384–391. https://doi.org/10.1080/14786419.2019.1634708
Lv Q, Rong N, Liu L et al (2016) Antitumoral activity of (20R)- and (20S)-ginsenoside Rh2 on transplanted hepatocellular carcinoma in mice. Planta Med 82:705–711
Magid AA, Lalun N, Long C et al (2012) Triterpene saponins from Antonia ovata leaves. Phytochemistry 77:268–274. https://doi.org/10.1016/j.phytochem.2012.01.025
Mai TT, Moon JY, Song YW et al (2012) Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett 321:144–153. https://doi.org/10.1016/j.canlet.2012.01.045
Mallick S, Pal BC, Kumar D et al (2013) Effect of corchorusin-D, a saikosaponin like compound, on B16F10 melanoma cells (in vitro and in vivo). J Asian Nat Prod Chem Res 15:1197–1203
Mallick S, Pal BC, Vedasiromoni JR et al (2012) Corchorusin-D directed apoptosis of K562 cells occurs through activation of mitochondrial and death receptor pathways and suppression of AKT/PKB pathway. Cell Physiol Biochem 30:915–926. https://doi.org/10.1159/000341469
Maltese WA, Overmeyer JH (2014) Methuosis: nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments. Am J Pathol 184:1630–1642. https://doi.org/10.1016/j.ajpath.2014.02.028
Mbaveng AT, Ndontsa BL, Kuete V et al (2018) A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine 43:78–85. https://doi.org/10.1016/j.phymed.2018.03.035
Mi Y, Xiao C, Du Q et al (2016) Momordin Ic couples apoptosis with autophagy in human hepatoblastoma cancer cells by reactive oxygen species (ROS)-mediated PI3K/Akt and MAPK signaling pathways. Free Radic Biol Med 90:230–242. https://doi.org/10.1016/j.freeradbiomed.2015.11.022
Miao L, Yang Y, Li Z et al (2022) Ginsenoside Rb2: a review of pharmacokinetics and pharmacological effects. J Ginseng Res 46:206–213. https://doi.org/10.1016/j.jgr.2021.11.007
Mihoub M, Pichette A, Sylla B et al (2018) Bidesmosidic betulin saponin bearing L-rhamnopyranoside moieties induces apoptosis and inhibition of lung cancer cells growth in vitro and in vivo. PLoS ONE 13:e0193386. https://doi.org/10.1371/journal.pone.0193386
Miyase T, Melek FR, Ghaly NS et al (2010) Echinocystic acid 3,16-O-bisglycosides from Albizia procera. Phytochemistry 71:1375–1380. https://doi.org/10.1016/j.phytochem.2010.05.004
Mo S, Xiong H, Shu G et al (2013) Phaseoloideside E, a novel natural triterpenoid saponin identified from Entada phaseoloides, induces apoptosis in Ec-109 esophageal cancer cells through reactive oxygen species generation. J Pharmacol Sciences 122:163–175. https://doi.org/10.1254/jphs.12193FP
Moses T, Papadopoulou KK, Osbourn A (2014) Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit Rev Biochem Mol Biol 49:439–462. https://doi.org/10.3109/10409238.2014.953628
Mu LH, Huang CL, Bin ZW et al (2013) Methanolysis of triterpenoid saponin from Ardisia gigantifolia stapf. and structure-activity relationship study against cancer cells. Bioorg Med Chem Lett 23:6073–6078. https://doi.org/10.1016/j.bmcl.2013.09.029
Mu LH, Wang LH, Wang YN et al (2020a) Antiangiogenic effects of AG36, a triterpenoid saponin from Ardisia gigantifolia stapf. J Nat Med 74:732–740. https://doi.org/10.1007/s11418-020-01427-4
Mu LH, Wang LH, Yu TF et al (2020b) Triterpenoid saponin AG8 from Ardisia gigantifolia stapf induces triple negative breast cancer cells apoptosis through oxidative stress pathway. Oxidative Med Cell Longevit 2020:7963212. https://doi.org/10.1155/2020/7963212
Mu LH, Wei NY, Liu P (2012) Cytotoxic triterpenoid saponins from Ardisia gigantifolia. Planta Med 78:617–621. https://doi.org/10.1055/s-0031-1298254
Mu LH, Yan H, Wang YN et al (2019) Triterpenoid saponins from Ardisia gigantifolia and mechanism on inhibiting proliferation of MDA-MB-231 cells. Biol Pharm Bull 42:194–200. https://doi.org/10.1248/bpb.b18-00569
Muhamad M, Choo CY, Leow CY (2021) Cytotoxic phytochemicals from Myrsinaceae family and their modes of action: a review. J Biol Active Prod Nat 11:298–324
Muthoni DK, Samarakoon SR, Piyathilaka PC et al (2021) Identification of 3-O-α-l-arabinosyl oleanolic acid, a triterpenoid saponin, as a new breast cancer stem cell growth inhibitor. Nat Prod Res. https://doi.org/10.1080/14786419.2021.1933971
Nag SA, Qin JJ, Wang W et al (2012) Ginsenosides as anticancer agents: in vitro and in vivo activities, structure-activity relationships, and molecular mechanisms of action. Front Pharmacol 3:25. https://doi.org/10.3389/fphar.2012.00025
Nakhjavani M, Palethorpe HM, Tomita Y et al (2019) Stereoselective anti-cancer activities of ginsenoside Rg3 on triple negative breast cancer cell models. Pharmaceuticals 12:117. https://doi.org/10.3390/ph12030117
Naz I, Ramchandani S, Khan MR et al (2020) Anticancer potential of Raddeanin A, a natural triterpenoid isolated from Anemone raddeana Regel. Molecules 25:1035. https://doi.org/10.3390/molecules25051035
Nhiem NX, Thu VK, van Kiem P et al (2012) Cytotoxic oleane-type triterpene saponins from Glochidion eriocarpum. Archiv Pharm Res 35:19–26. https://doi.org/10.1007/s12272-012-0102-2
Ninomiya K, Motai C, Nishida E et al (2016) Acylated oleanane-type triterpene saponins from the flowers of Bellis perennis show anti-proliferative activities against human digestive tract carcinoma cell lines. J Nat Med 70:435–451. https://doi.org/10.1007/s11418-016-0998-9
Nishimura M, Fuchino H, Takayanagi K et al (2021) Toxicity of jegosaponins A and B from Styrax japonica Siebold et al Zuccarini in prostate cancer cells and zebrafish embryos resulting from increased membrane permeability. Int J Mol Sci 22:6354. https://doi.org/10.3390/ijms22126354
Noté OP, Kamto ELD, Toukea DD et al (2018) Pro-apoptotic activity of new triterpenoid saponins from the roots of Albizia adianthifolia (Schumach.) W.Wight. Fitoterapia 129:34–41. https://doi.org/10.1016/j.fitote.2018.06.008
Noté OP, Messi LM, Mbing JN et al (2017) Pro-apoptotic activity of acylated triterpenoid saponins from the stem bark of Albizia chevalieri harms. Phytochem Lett 22:95–101. https://doi.org/10.1016/j.phytol.2017.09.008
Obasi TC, Braicu C, Iacob BC et al (2018) Securidaca–saponins are natural inhibitors of AKT, MCL-1, and BCL2L1 in cervical cancer cells. Cancer Manag Res 10:5709–5724. https://doi.org/10.2147/CMAR.S163328
Oleszek W, Hamed A (2010) Saponin-based surfactants. In: Kjellin M, Johansson I (eds) Surfactants from renewable resources, pp 239–249
Ortega-Muñoz M, Rodríguez-Serrano F, de los Reyes-Berbel E et al (2018) Biological evaluation and docking studies of synthetic oleanane-type triterpenoids. ACS Omega 3:11455–11468. https://doi.org/10.1021/acsomega.8b01034
Osbourn A, Goss RJM, Field RA (2011) The saponins-polar isoprenoids with important and diverse biological activities. Nat Prod Rep 28:1261–1268. https://doi.org/10.1039/c1np00015b
Ozaslan M, Karagoz ID, Kilic IH, Guldur ME (2011) Ehrlich ascites carcinoma. African. J Biotechnol 10:2375–2378. https://doi.org/10.4314/ajb.v10i13
Ozer O, Sarikahya NB, Nalbantsoy A, Kirmizigul S (2018) Increased cytotoxic potential of infrequent triterpenoid saponins of Cephalaria taurica obtained through alkaline hydrolysis. Phytochemistry 152:29–35. https://doi.org/10.1016/j.phytochem.2018.04.015
Palethorpe HM, Smith E, Tomita Y et al (2019) Bacopasides I and II Act in synergy to inhibit the growth, migration and invasion of breast cancer. Molecules 24:3539. https://doi.org/10.3390/molecules24193539
Pan XY, Guo H, Han J et al (2012) Ginsenoside Rg3 attenuates cell migration via inhibition of aquaporin 1 expression in PC-3M prostate cancer cells. Eur J Pharmacol 683:27–34. https://doi.org/10.1016/j.ejphar.2012.02.040
Park JI, Bae HR, Kim CG et al (2014) Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers. Front Chem 2:77. https://doi.org/10.3389/fchem.2014.00077
Park SE, Lee SY, Shin DY et al (2013) Pro-apoptotic effects of platycodin D isolated from Platycodon grandiflorum in human. J Life Sci 23:389–398. https://doi.org/10.5352/JLS.2013.23.3.389
Patlolla JMR, Rao CV (2015) Anti-inflammatory and anti-cancer properties of β-escin, a triterpene saponin. Curr Pharmacol Reports 1:170–178. https://doi.org/10.1007/s40495-015-0019-9
Pérez AJ, Pecio Ł, Kowalczyk M et al (2017) Cytotoxic triterpenoids isolated from sweet chestnut heartwood (Castanea sativa) and their health benefits implication. Food Chem Toxicol 109:863–870. https://doi.org/10.1016/j.fct.2017.03.049
Phi LTH, Wijaya YT, Sari IN et al (2018) The anti-metastatic effect of ginsenoside Rb2 in colorectal cancer in an EGFR/SOX2-dependent manner. Cancer Med 7:5621–5631. https://doi.org/10.1002/cam4.1800
Podolak I, Galanty A, Sobolewska D (2010) Saponins as cytotoxic agents: a review. Phytochem Rev 9:425–474
Qi LW, Wang CZ, Yuan CS (2010) American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol 80:947–954. https://doi.org/10.1016/j.bcp.2010.06.023
Qin H, Du X, Zhang Y, Wang R (2014) Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, inducesG2/M arrest and apoptosis in human hepatoma HepG2 cells by modulating the PI3K/Akt pathway. Tumor Biol 35:1267–1274. https://doi.org/10.1007/s13277-013-1169-1
Qu L, Zhang H, Yang Y et al (2016) Corosolic acid analogue, a natural triterpenoid saponin, induces apoptosis on human hepatocarcinoma cells through mitochondrial pathway in vitro. Pharm Biol 54:1445–1457. https://doi.org/10.3109/13880209.2015.1104699
Quang TH, Ngan NTT, Minh C et al (2011a) Anti-inflammatory triterpenoid saponins from the stem bark of Kalopanax pictus. J Nat Prod 74:1908–1915. https://doi.org/10.1021/np200382s
Quang TH, Ngan NTT, Van MC et al (2012) Cytotoxic triterpene saponins from the stem bark of Kalopanax pictus. Phytochem Lett 5:177–182. https://doi.org/10.1016/j.phytol.2011.12.005
Quang TH, Ngan NTT, Van MC et al (2011b) Effect of triterpenes and triterpene saponins from the stem bark of Kalopanax pictus on the transactivational activities of three PPAR subtypes. Carbohydr Res 346:2567–2575. https://doi.org/10.1016/j.carres.2011.08.029
Rasul A, Shen X, Wang B et al (2014) Tubeimoside-1 upregulates p21 expression and induces apoptosis and G2/M phase cell cycle arrest in human bladder cancer T24 cells. Bangladesh J Pharmacol 9:595–603. https://doi.org/10.3329/bjp.v9i4.19989
Rasul A, Song R, Wei W et al (2012) Tubeimoside-1 inhibits growth via the induction of cell cycle arrest and apoptosis in human melanoma A375 cells. Bangladesh J Pharmacol 7:150–156. https://doi.org/10.3329/bjp.v7i3.11507
Ren L, Liu YX, Lv D et al (2013) Facile synthesis of the naturally cytotoxic triterpenoid saponin Patrinia-glycoside B-II and its conformer. Molecules 18:15193–15206. https://doi.org/10.3390/molecules181215193
Ren M, McGowan E, Li Y et al (2019) Saikosaponin-d suppresses COX2 through p-STAT3/C/EBPβ signaling pathway in liver cancer: a novel mechanism of action. Front Pharmacol 10:623. https://doi.org/10.3389/fphar.2019.00623
Roohbakhsh A, Iranshahy M, Iranshahi M (2016) Glycyrrhetinic acid and its derivatives: anti-cancer and cancer chemopreventive properties, mechanisms of action and structure- cytotoxic activity relationship. Curr Med Chem 23:498–517. https://doi.org/10.2174/0929867323666160112122256
Ruan C, You L, Qiu Y et al (2020) Tubeimoside I induces autophagy in HepG2 cells by activating the AMP-activated protein kinase signaling pathway. Oncol Lett 20:623–630. https://doi.org/10.3892/ol.2020.11604
Sachan R, Kundu A, Jeon Y et al (2018) Afrocyclamin A, a triterpene saponin, induces apoptosis and autophagic cell death via the PI3K/Akt/mTOR pathway in human prostate cancer cells. Phytomedicine 51:139–150. https://doi.org/10.1016/j.phymed.2018.10.012
Samarakoon SR, Ediriweera MK, Nwokwu CDU et al (2017) A study on cytotoxic and apoptotic potential of a triterpenoid saponin (3-O-α-L-arabinosyl oleanolic acid) isolated from Schumacheria castaneifolia Vahl in human non-small-cell lung cancer (NCI-H292) cells. BioMed Res Int. https://doi.org/10.1155/2017/9854083
Saraswati G (2019) Triterpenoid saponins discovery research 2013–2016. Int J Ayurvedic Herb Med 9:3492–3513. https://doi.org/10.31142/ijahm/v9i3.02
Sarraj-Laabidi A, Messai H, Hammami-Semmar A, Semmar N (2017) Chemometric analysis of inter- and intra-molecular diversification factors by a machine learning simplex approach. A review and research on Astragalus saponins. Curr Top Med Chem 17:2820–2848. https://doi.org/10.2174/1568026617666170719165552
Sarraj-Laabidi A, Semmar N (2016) Chemometrical analysis of structure-structure and structure-activity trends of cycloartane-based saponins in Astragalus genus. MOL2NET 2:3870. https://doi.org/10.3390/mol2net-02-03870
Schwarz S, Siewert B, Xavier NM et al (2014) A “natural” approach: synthesis and cytoxicity of monodesmosidic glycyrrhetinic acid glycosides. Eur J Med Chem 72:78–83. https://doi.org/10.1016/j.ejmech.2013.11.024
Seo YS, Kang OH, Kong R et al (2018) Polygalacin D induces apoptosis and cell cycle arrest via the PI3K/Akt pathway in non-small cell lung cancer. Oncol Rep 39:1702–1710. https://doi.org/10.3892/or.2018.6230
Shaheen U, Ragab EA, Abdalla AN, Bader A (2018) Triterpenoidal saponins from the fruits of Gleditsia caspica with proapoptotic properties. Phytochemistry 145:168–178. https://doi.org/10.1016/j.phytochem.2017.11.007
Shen S, Li W, Ouyang M, Wang J (2018) Structure-activity relationship of triterpenes and derived glycosides against cancer cells and mechanism of apoptosis induction. Nat Prod Rep 32:654–661. https://doi.org/10.1080/14786419.2017.1335725
Shen Z, Xu L, Li J, Zhang N (2017) Capilliposide C sensitizes esophageal squamous carcinoma cells to oxaliplatin by inducing apoptosis through the PI3K/Akt/mTOR pathway. Med Sci Monitor 23:2096–2103
Shi H, Bi H, Sun X et al (2018a) Antitumor effects of tubeimoside-1 in NCI-H1299 cells are mediated by microRNA-126-5p-induced inactivation of VEGF-A/VEGFR-2/ERK signaling pathway. Mol Med Rep 17:4327–4336. https://doi.org/10.3892/mmr.2018.8459
Shi JM, Bai LL, Zhang DM et al (2013) Saxifragifolin D induces the interplay between apoptosis and autophagy in breast cancer cells through ROS-dependent endoplasmic reticulum stress. Biochem Pharmacol 85:913–926. https://doi.org/10.1016/j.bcp.2013.01.009
Shi W, Xu D, Gu J et al (2018b) Saikosaponin-d inhibits proliferation by up-regulating autophagy via the CaMKKβ–AMPK–mTOR pathway in ADPKD cells. Mol Cell Biochem 449:219–226. https://doi.org/10.1007/s11010-018-3358-0
Shin JE, Kim HJ, Kim KR et al (2015) Type I saikosaponins A and D inhibit osteoclastogenesis in bone marrow-derived macrophages and osteolytic activity of metastatic breast cancer cells. Evid Based Complement Altern Med 2015:582437. https://doi.org/10.1155/2015/582437
Simo LM, Noté OP, Mbing JN et al (2017) New cytotoxic triterpenoid saponins from the roots of Albizia gummifera. Chem Biodivers 14:e1700260. https://doi.org/10.1002/cbdv.201700260
Sobolewska D, Galanty A, Grabowska K et al (2020) Saponins as cytotoxic agents: an update (2010–2018). Part I—steroidal saponins. Phytochem Rev 19:139–189. https://doi.org/10.1007/s11101-020-09661-0
Son MK, Jung KH, Hong SW et al (2013a) SB365, Pulsatilla saponin D suppresses the proliferation of human colon cancer cells and induces apoptosis by modulating the AKT/mTOR signalling pathway. Food Chem 136:26–33. https://doi.org/10.1016/j.foodchem.2012.07.096
Son MK, Jung KH, Lee HS et al (2013b) SB365, Pulsatilla saponin D suppresses proliferation and induces apoptosis of pancreatic cancer cells. Oncol Rep 30:801–808. https://doi.org/10.3892/or.2013.2517
Song NN, Yang LM, Zhang MJ et al (2021) Triterpenoid saponins and phenylpropanoid glycoside from the roots of Ardisia crenata and their cytotoxic activities. Chinese J Nat Med 19:63–69. https://doi.org/10.1016/S1875-5364(21)60007-9
Song X, Wang W, Zhang X et al (2015) Deglucose chikusetsusaponin IVa isolated from Rhizoma Panacis Majoris induces apoptosis in human HepG2 hepatoma cells. Mol Med Rep 12:5494–5500. https://doi.org/10.3892/mmr.2015.4035
Sun D, Shen W, Zhang F et al (2018) α-Hederin inhibits interleukin 6-induced epithelial-to-mesenchymal transition associated with disruption of JAK2/STAT3 signaling in colon cancer cells. Biomed Pharmacother 101:107–114. https://doi.org/10.1016/j.biopha.2018.02.062
Sun HY, Liu BB, Hu JY et al (2016) Novel cycloartane triterpenoid from Cimicifuga foetida (Sheng ma) induces mitochondrial apoptosis via inhibiting Raf/MEK/ERK pathway and Akt phosphorylation in human breast carcinoma MCF-7 cells. Chinese Med 11:1. https://doi.org/10.1186/s13020-015-0073-6
Sun J, Feng Y, Wang Y et al (2019) α-Hederin induces autophagic cell death in colorectal cancer cells through reactive oxygen species dependent AMPK/mTOR signaling pathway activation. Int J Oncol 54:1601–1612. https://doi.org/10.3892/ijo.2019.4757
Sun K, Du Y, Hou Y et al (2021) Saikosaponin D exhibits anti-leukemic activity by targeting FTO/m6A signaling. Theranostics 11:5831–5846. https://doi.org/10.7150/thno.55574
Sun K, Yu W, Ji B et al (2020) Saikosaponin D loaded macrophage membrane-biomimetic nanoparticles target angiogenic signaling for breast cancer therapy. Appl Mater Today 18:100505. https://doi.org/10.1016/j.apmt.2019.100505
Sylla B, Lavoie S, Legault J et al (2019) Synthesis, cytotoxicity and anti-inflammatory activity of rhamnose-containing ursolic and betulinic acid saponins. RSC Adv 9:39743–39757. https://doi.org/10.1039/c9ra09389c
Tabata K, Hamano A, Akihisa T, Suzuki T (2012) Kuguaglycoside C, a constituent of Momordica charantia, induces caspase-independent cell death of neuroblastoma cells. Cancer Sci 103:2153–2158. https://doi.org/10.1111/cas.12021
Tang J, Yu L, Fu H et al (2013) Cytotoxic triterpenoid saponins from the stems of Gordonia longicarpa. Planta Med 79:353–360. https://doi.org/10.1055/s-0032-1328245
Tang ZH, Li T, Tong YG et al (2015) A systematic review of the anticancer properties of compounds isolated from licorice (Gancao). Planta Med 81:1670–1687. https://doi.org/10.1055/s-0035-1558227
Tchoukoua A, Tabopda TK, Uesugi S et al (2017) Triterpene saponins from the roots of Acacia albida Del. (Mimosaceae). Phytochemistry 136:31–38. https://doi.org/10.1016/j.phytochem.2016.12.019
Teng YH, Li JP, Liu SL et al (2016) Autophagy protects from raddeanin A-induced apoptosis in SGC-7901 human gastric cancer cells. Evid-Based Complement Altern Med 2016:9406758. https://doi.org/10.1155/2016/9406758
Thoppil RJ, Bishayee A (2011) Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J Hepatol 3:228–249. https://doi.org/10.4254/wjh.v3.i9.228
Tian L, Shen D, Li X et al (2016) Ginsenoside Rg3 inhibits epithelial-mesenchymal transition (EMT) and invasion of lung cancer by down-regulating FUT4. Oncotarget 7:1619–1632. https://doi.org/10.18632/oncotarget.6451
Tian X, Feng J, Tang H et al (2013a) New cytotoxic triterpenoid saponins from the whole plant of Clematis lasiandra Maxim. Fitoterapia 90:233–239. https://doi.org/10.1016/j.fitote.2013.07.017
Tian X, Tang H, Lin H et al (2013b) Saponins: the potential chemotherapeutic agents in pursuing new anti-glioblastoma drugs. Mini-Reviews Med Chem 13:1709–1724. https://doi.org/10.2174/13895575113136660083
Tong B, Lu D, Wei Z et al (2013) Gleditsioside B, a triterpene saponin isolated from the anomalous fruits of Gleditsia sinensis Lam., abrogates bFGF-induced endothelial cell migration through preventing the activation of MMP-2 and FAK via inhibiting ERK and PI3K/AKT signaling pathways. Vascul Pharmacol 58:118–126. https://doi.org/10.1016/j.vph.2012.09.006
Tong X, Han L, Duan H et al (2017) The derivatives of Pulsatilla saponin A, a bioactive compound from Pulsatilla chinensis: their synthesis, cytotoxicity, haemolytic toxicity and mechanism of action. Eur J Med Chem 129:325–336. https://doi.org/10.1016/j.ejmech.2017.02.025
Ün RN, Masullo M, Karayildirim T et al (2020) Triterpene glycosides from Silene odontopetala. Phytochemistry 176:112404. https://doi.org/10.1016/j.phytochem.2020.112404
Vayghan HJ, Ghadimi SS, Nourazarian AR (2014) Preventive and therapeutic roles of ginseng - focus on colon cancer. Asian Pacific J Cancer Prev 15:585–588. https://doi.org/10.7314/APJCP.2014.15.2.585
Verma A, Singh D, Anwar F et al (2018) Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kB pathway. Inflammopharmacology 26:133–146. https://doi.org/10.1007/s10787-017-0350-3
de Wang X, Su GY, Zhao C et al (2018a) Anticancer activity and potential mechanisms of 1C, a ginseng saponin derivative, on prostate cancer cells. J Ginseng Res 42:133–143. https://doi.org/10.1016/j.jgr.2016.12.014
Wang FL, Sun JY, Wang Y et al (2013a) Oldhamianoside II, a new triterpenoid saponin, prevents tumor growth via inducing cell apoptosis and inhibiting angiogenesis. Oncol Res 20:369–376. https://doi.org/10.3727/096504013X13657689382978
Wang G, Wang J, Liu W et al (2021a) Biological activities and chemistry of triterpene saponins from Medicago species: an update review. Evid Based Complement Altern Med 2021:ID6617916
Wang H, Zheng Y, Sun Q et al (2021b) Ginsenosides emerging as both bifunctional drugs and nanocarriers for enhanced antitumor therapies. J Nanobiotechnol 19:322
Wang J, Deng H, Zhang J et al (2020a) α-Hederin induces the apoptosis of gastric cancer cells accompanied by glutathione decrement and reactive oxygen species generation via activating mitochondrial dependent pathway. Phytother Res 34:601–611. https://doi.org/10.1002/ptr.6548
Wang J, Lu J, Lv C et al (2012a) Three new triterpenoid saponins from root of Gardenia jasminoides Ellis. Fitoterapia 83:1396–1401. https://doi.org/10.1016/j.fitote.2012.07.004
Wang J, Yuan L, Xiao H et al (2013b) Momordin Ic induces HepG2 cell apoptosis through MAPK and PI3K/Akt-mediated mitochondrial pathways. Apoptosis 18:751–765. https://doi.org/10.1007/s10495-013-0820-z
Wang K, Tu Y, Wan J-B et al (2020b) Synergistic anti-breast cancer effect of pulsatilla saponin D and camptothecin through interrupting autophagic-lysosomal function and promoting p62-mediated ubiquitinated protein aggregation. Carcinogenesis 41:804–816. https://doi.org/10.1093/carcin/bgz140
Wang P (2021) Natural and synthetic saponins as vaccine adjuvants. Vaccines 9:222. https://doi.org/10.3390/vaccines9030222
Wang P, Ownby S, Zhang Z et al (2010) Cytotoxicity and inhibition of DNA topoisomerase I of polyhydroxylated triterpenoids and triterpenoid glycosides. Bioorganic Med Chem Lett 20:2790–2796. https://doi.org/10.1016/j.bmcl.2010.03.063
Wang Q, Mo J, Zhao C et al (2018b) Raddeanin A suppresses breast cancer-associated osteolysis through inhibiting osteoclasts and breast cancer cells. Cell Death Dis 9:376. https://doi.org/10.1038/s41419-018-0417-0
Wang QZ, Liu XF, Shan Y et al (2012b) Two new nortriterpenoid saponins from Salicornia bigelovii Torr. and their cytotoxic activity. Fitoterapia 83:742–749. https://doi.org/10.1016/j.fitote.2012.02.013
Wang R, Gu Y, Zhang WD et al (2012c) Inhibition of tumor-induced angiogenesis and its mechanism by ardipusilloside I purified from Ardisia pusilla. J Asian Nat Prod Chem Res 14:55–63. https://doi.org/10.1080/10286020.2011.631182
Wang T, Gong F, Zhang R et al (2016) Pulsatilla saponin A induces differentiation in acute myeloid leukemia in vitro. Hematology 21:182–186. https://doi.org/10.1080/10245332.2015.1101967
Wang W, Yao GD, Shang XY et al (2019a) Eclalbasaponin I causes mitophagy to repress oxidative stress-induced apoptosis via activation of p38 and ERK in SH-SY5Y cells. Free Radic Res 53:655–668. https://doi.org/10.1080/10715762.2019.1620937
Wang XJ, Xie Q, Liu Y et al (2021c) Panax japonicus and chikusetsusaponins: a review of diverse biological activities and pharmacology mechanism. Chinese Herbal Med 13:64–77
Wang XY, Gao H, Zhang W et al (2013c) Bioactive oleanane-type saponins from the rhizomes of Anemone taipaiensis. Bioorg Med Chem Lett 23:5714–5720. https://doi.org/10.1016/j.bmcl.2013.08.006
Wang Z, Shen J, Sun W et al (2019b) Antitumor activity of Raddeanin A is mediated by Jun amino-terminal kinase activation and signal transducer and activator of transcription 3 inhibition in human osteosarcoma. Cancer Sci 110:1746–1759. https://doi.org/10.1111/cas.14008
Wang Z, Wang C, Zuo D et al (2019c) Attenuation of STAT3 phosphorylation promotes apoptosis and chemosensitivity in human osteosarcoma induced by raddeanin A. Int J Biol Sci 15:668–679. https://doi.org/10.7150/ijbs.30168
WargasetiaWidodo TL (2017) Mechanisms of cancer cell killing by sea cucumber-derived compounds. Invest New Drugs 35:820–826. https://doi.org/10.1007/s10637-017-0505-5
Wei G, Sun J, Hou Z et al (2018) Novel antitumor compound optimized from natural saponin Albiziabioside A induced caspase-dependent apoptosis and ferroptosis as a p53 activator through the mitochondrial pathway. Eur J Med Chem 157:759–772. https://doi.org/10.1016/j.ejmech.2018.08.036
Wei G, Sun J, Luan W et al (2019) Natural product albiziabioside A conjugated with pyruvate dehydrogenase kinase inhibitor dichloroacetate to induce apoptosis-ferroptosis-M2-TAMs polarization for combined cancer therapy. J Med Chem 62:8760–8772. https://doi.org/10.1021/acs.jmedchem.9b00644
Wei GQ, Zheng YN, Li W et al (2012) Structural modification of ginsenoside Rh2 by fatty acid esterification and its detoxification property in antitumor. Bioorg Med Chem Lett 22:1082–1085. https://doi.org/10.1016/j.bmcl.2011.11.104
WHO (2021) Global Cancer Observatory. In: International Agency for Research on Cancer. Global Cancer Observatory. https://gco.iarc.fr/. Accessed 25 Jul 2021
Wong AST, Che CM, Leung KW (2015) Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep 32:256–272. https://doi.org/10.1039/c4np00080c
Wong VKW, Li T, Law BYK et al (2013a) Saikosaponin-d, a novel SERCA inhibitor, induces autophagic cell death in apoptosis-defective cells. Cell Death Dis 4:e720. https://doi.org/10.1038/cddis.2013.217
Wong VKW, Zhang MM, Zhou H et al (2013b) Saikosaponin-d enhances the anticancer potency of TNF- α via overcoming its undesirable response of activating NF-kappa B signalling in cancer cells. Evid Based Complement Altern Med 2013:745295. https://doi.org/10.1155/2013/745295
Wu B, Zhu J, Dai X et al (2021a) Raddeanin A inhibited epithelial-mesenchymal transition (EMT) and angiogenesis in glioblastoma by downregulating β-catenin expression. Int J Med Sci 18:1609–1617. https://doi.org/10.7150/ijms.52206
Wu J, Niu S, Li G et al (2021b) Antitumor effect of ginsenosides: a systematic review. Yangtze Med 5:207–225. https://doi.org/10.4236/ym.2021.53020
Wu JP, Kang NX, Zhang MY et al (2018a) Oleiferoside W from the roots of Camellia oleifera C. Abel, inducing cell cycle arrest and apoptosis in A549 cells. J Asian Nat Prod Chem Res 20:793–806. https://doi.org/10.1080/10286020.2017.1347640
Wu T, Cui H, Xu Y et al (2018b) The effect of tubeimoside-1 on the proliferation, metastasis and apoptosis of oral squamous cell carcinoma in vitro. Onco Targets Ther 11:3989–4000. https://doi.org/10.2147/OTT.S164503
Wu X-P, Han C-R, Chen G-Y et al (2010) Cytotoxic pentacyclic triterpenoids from Combretum oliviforme. Nat Prod Commun 5:1027–1030
Xianjun F, Xirui X, Jie T et al (2021) Momordin Ic induces G0/1 phase arrest and apoptosis in colon cancer cells by suppressing SENP1/c-MYC signaling pathway. J Pharmacol Sci 146:249–258. https://doi.org/10.1016/j.jphs.2021.04.007
Xu C, Sun G, Yuan G et al (2014) Effects of platycodin D on proliferation, apoptosis and PI3K/Akt signal pathway of human glioma U251 cells. Molecules 19:21411–21423. https://doi.org/10.3390/molecules191221411
Xu FY, Shang WQ, Yu JJ et al (2016) The antitumor activity study of ginsenosides and metabolites in lung cancer cell. Am J Transl Res 8:1708–1718
Xu J, Pan Y, Liu Y et al (2021) A review of anti-tumour effects of ginsenoside in gastrointestinal cancer. J Pharm Pharmacol 73:1292–1301
Xu K, Shu Z, Xu QM et al (2013a) Cytotoxic activity of Pulsatilla chinensis saponins and their structure-activity relationship. J Asian Nat Prod Chem Res 15:680–686. https://doi.org/10.1080/10286020.2013.790901
Xu L, Xiao S, Yuan W et al (2018) Synthesis and anticancer activity evaluation of hydrolyzed derivatives of Panax notoginseng saponins. Molecules 23:3021. https://doi.org/10.3390/molecules23113021
Xu L, Yu H, Yin S et al (2015) Liposome-based delivery systems for ginsenoside Rh2: in vitro and in vivo comparisons. J Nanoparticle Res 17:415. https://doi.org/10.1007/s11051-015-3214-z
Xu MY, Lee DH, Joo EJ et al (2013b) Akebia saponin PA induces autophagic and apoptotic cell death in AGS human gastric cancer cells. Food Chem Toxicol 59:703–708. https://doi.org/10.1016/j.fct.2013.06.059
Xu XF, Zhang TL, Jin S et al (2013c) Ardipusilloside I induces apoptosis by regulating Bcl-2 family proteins in human mucoepidermoid carcinoma Mc3 cells. BMC Complement Altern Med 13:322. https://doi.org/10.1186/1472-6882-13-322
Xu Y, Wang G, Chen Q et al (2013d) Intrinsic apoptotic pathway and G2/M cell cycle arrest involved in tubeimoside I-induced EC109 cell death. Chinese J Cancer Res 25:312–321. https://doi.org/10.3978/j.issn.1000-9604.2013.06.03
Xue G, Zou X, Zhou JY et al (2013) Raddeanin A induces human gastric cancer cells apoptosis and inhibits their invasion in vitro. Biochem Biophys Res Commun 439:196–202. https://doi.org/10.1016/j.bbrc.2013.08.060
Xue S, Zhou Y, Zhang J et al (2019) Anemoside B4 exerts anti-cancer effect by inducing apoptosis and autophagy through inhibiton of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am J Transl Res 11:2580–2589
Yan H, Wang X, Niu J et al (2014) Anti-cancer effect and the underlying mechanisms of gypenosides on human colorectal cancer SW-480 cells. PLoS ONE 9:e95609. https://doi.org/10.1371/journal.pone.0095609
Yan J, Dou X, Zhou J et al (2019) Tubeimoside-I sensitizes colorectal cancer cells to chemotherapy by inducing ROS-mediated impaired autophagolysosomes accumulation. J Exp Clin Cancer Res 38:353. https://doi.org/10.1186/s13046-019-1355-0
Yang H, Kim H, Kim Y, Sung S (2017) Cytotoxic activities of naturally occurring oleanane-, ursane-, and lupane-type triterpenes on HepG2 and AGS cells. Pharmacogn Mag 13:118–122. https://doi.org/10.4103/0973-1296.196308
Yang H, Yoo G, Kim HS et al (2012) Implication of the stereoisomers of ginsenoside derivatives in the antiproliferative effect of HSC-T6 cells. J Agric Food Chem 60:11759–11764. https://doi.org/10.1021/jf303714c
Yang J, Qian S, Cai X et al (2016a) Chikusetsusaponin IVa butyl ester (CS-IVa-Be), a novel IL6R antagonist, inhibits IL6/STAT3 signaling athway and induces cancer cell apoptosis. Mol Cancer Ther 15:1190–1200. https://doi.org/10.1158/1535-7163.MCT-15-0551
Yang JB, Khan M, He YY et al (2016b) Tubeimoside-1 induces oxidative stress-mediated apoptosis and G0 /G1 phase arrest in human prostate carcinoma cells in vitro. Acta Pharm Sinic 37:950–962. https://doi.org/10.1038/aps.2016.34
Yang X, Li X, Luo M et al (2021) Tubeimoside I promotes angiogenesis via activation of eNOS-VEGF signaling pathway. J Ethnopharmacol 267:113642. https://doi.org/10.1016/J.JEP.2020.113642
Yao M, Yang J, Cao L et al (2014) Saikosaponin-D inhibits proliferation of DU145 human prostate cancer cells by inducing apoptosis and arresting the cell cycle at G0/G1 phase. Mol Med Rep 10:365–372. https://doi.org/10.3892/mmr.2014.2153
Ye B, Zhou Y, Liu Y et al (2021) Pulsatilla saponin A induces apoptosis and differentiation of myeloma cells. Anti-Cancer Agents Med Chem 21:919–926. https://doi.org/10.2174/1871520620666200721125036
Yin Q, Chen H, Ma RH et al (2021) Ginsenoside CK induces apoptosis of human cervical cancer HeLa cells by regulating autophagy and endoplasmic reticulum stress. Food Funct 12:5301–5316. https://doi.org/10.1039/d1fo00348h
Yu J, Li G, Mu Y et al (2019a) Anti-breast cancer triterpenoid saponins from the thorns of Gleditsia sinensis. Nat Prod Res 33:2308–2313. https://doi.org/10.1080/14786419.2018.1443092
Yu JH, Yu ZP, Wang YY et al (2019b) Triterpenoids and triterpenoid saponins from Dipsacus asper and their cytotoxic and antibacterial activities. Phytochemistry 162:241–249. https://doi.org/10.1016/j.phytochem.2019.03.028
Yu L, Wang X, Wei X et al (2012) Triterpenoid saponins from Xanthoceras sorbifolia Bunge and their inhibitory activity on human cancer cell lines. Bioorg Med Chem Lett 22:5232–5238. https://doi.org/10.1016/j.bmcl.2012.06.061
Yuan W, Wang P, Deng G, Li S (2012) Cytotoxic triterpenoid saponins from Aesculus glabra Willd. Phytochemistry 75:67–77. https://doi.org/10.1016/j.phytochem.2011.11.012
Yuan W, Wang P, Su Z et al (2013) Cytotoxic triterpenoid saponins from husks of Aesculus californica (Spach) Nutt. Phytochemistry 90:95–105. https://doi.org/10.1016/j.phytochem.2013.02.003
Yue GGL, Xie S, Lee JKM et al (2016) New potential beneficial effects of actein, a triterpene glycoside isolated from Cimicifuga species, in breast cancer treatment. Sci Rep 6:35263. https://doi.org/10.1038/srep35263
Zhang A, Zheng Y, Que Z et al (2014) Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol 140:1883–1890. https://doi.org/10.1007/s00432-014-1744-x
Zhang CX, Zhang DM, Chen MF et al (2013a) Antiproliferative triterpenoid saponins from the stem of Psychotria sp. Planta Med 79:978–986. https://doi.org/10.1055/s-0032-1328650
Zhang J, Akihisa T, Kurita M et al (2018) Melanogenesis-inhibitory and cytotoxic activities of triterpene glycoside constituents from the bark of Albizia procera. J Nat Prod 81:2612–2620. https://doi.org/10.1021/acs.jnatprod.8b00167
Zhang P, Lai X, Zhu MH et al (2021) Saikosaponin A, a triterpene saponin, suppresses angiogenesis and tumor growth by blocking VEGFR2-mediated signaling pathway. Front Pharmacol 12:713200. https://doi.org/10.3389/fphar.2021.713200
Zhang X, Shi G, Wu X, Zhao Y (2020a) Gypensapogenin H from hydrolyzate of total Gynostemma pentaphyllum saponins induces apoptosis in human breast carcinoma cells. Nat Prod Res 34:1642–1646. https://doi.org/10.1080/14786419.2018.1525370
Zhang X, Zhai T, Hei Z et al (2020b) Effects of Platycodin D on apoptosis, migration, invasion and cell cycle arrest of gallbladder cancer cells. Oncol Lett 20:311. https://doi.org/10.3892/OL.2020.12174
Zhang X, Zhao M, Chen L et al (2011a) A triterpenoid from Thalictrum fortunei induces apoptosis in BEL-7402 cells through the P53-induced apoptosis pathway. Molecules 16:9505–9519. https://doi.org/10.3390/molecules16119505
Zhang XS, Zhao C, Tang WZ et al (2015) Gypensapogenin H, a novel dammarane-type triterpene induces cell cycle arrest and apoptosis on prostate cancer cells. Steroids 104:276–283. https://doi.org/10.1016/j.steroids.2015.10.014
Zhang Y, Peng Y, Li L et al (2013b) Studies on cytotoxic triterpene saponins from the leaves of Aralia elata. Food Chem 138:208–213. https://doi.org/10.1016/j.foodchem.2012.10.041
Zhang Y, Xu X, He P (2011b) Tubeimoside-1 inhibits proliferation and induces apoptosis by increasing the Bax to Bcl-2 ratio and decreasing COX-2 expression in lung cancer A549 cells. Mol Med Rep 4:25–29. https://doi.org/10.3892/mmr.2010.379
Zhang Z, Du GJ, Wang CZ et al (2013c) Compound K, a ginsenoside metabolite, inhibits colon cancer growth via multiple pathways including p53–p21 interactions. Int J Mol Sci 14:2980–2995. https://doi.org/10.3390/ijms14022980
Zhang Z, Feng Y, Li ZY, Cao XZ (2019) Antiproliferative and apoptotic activity of glycyrrhizinic acid in MCF-7 human breast cancer cells and evaluation of its effect on cell cycle, cell migration and m-TOR/PI3K/Akt signalling pathway. Arch Med Sci 15:174–182. https://doi.org/10.5114/aoms.2018.79429
Zhang Z, Zhao M, Zheng W, Liu Y (2017) Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, suppresses the growth and invasion of human oral squamous cell carcinoma cells via the NF-κB pathway. J Biochem Mol Toxicol 31:e21934. https://doi.org/10.1002/jbt.21934
Zhao LIN, Li J, Sun ZB et al (2019a) Saikosaponin d inhibits proliferation of human osteosarcoma cells via the p53 signaling pathway. Exp Ther Med 17:488–494. https://doi.org/10.3892/etm.2018.6969
Zhao R, Chen M, Jiang Z et al (2015) Platycodin-D induced autophagy in non-small cell lung cancer cells via PI3K/Akt/mTOR and MAPK signaling pathways. J Cancer 6:623–631. https://doi.org/10.7150/jca.11291
Zhao X, Liu J, Ge S et al (2019b) Saikosaponin A inhibits breast cancer by regulating Th1/Th2 balance. Front Pharmacol 10:624. https://doi.org/10.3389/fphar.2019.00624
Zheng J, Chen J, Zou X et al (2019) Saikosaponin d causes apoptotic death of cultured neocortical neurons by increasing membrane permeability and elevating intracellular Ca 2+ concentration. Neurotoxicology 70:112–121. https://doi.org/10.1016/j.neuro.2018.11.006
Zhong Y, Li XY, Zhou F et al (2021) Ziyuglycoside II inhibits the growth of digestive system cancer cells through multiple mechanisms. Chinese J Nat Med 19:351–363. https://doi.org/10.1016/S1875-5364(21)60033-X
Zhou JL, Huang XY, Qiu HC et al (2020) SSPH I, a novel anti-cancer saponin, inhibits autophagy and induces apoptosis via ROS accumulation and ERK1/2 signaling pathway in hepatocellular carcinoma cells. Onco Targets Ther 13:5979–5991. https://doi.org/10.2147/OTT.S253234
Zhou P, Shi W, He XY et al (2021) Saikosaponin D: review on the antitumour effects, toxicity and pharmacokinetics. Pharm Biol 59:1478–1487. https://doi.org/10.1080/13880209.2021.1992448
Zhou R, Lu Z, Liu K et al (2014) Platycodin D induces tumor growth arrest by activating FOXO3a expression in prostate cancer in vitro and in vivo. Curr Cancer Drug Targets 14:860–871. https://doi.org/10.2174/1568009614666141128104642
Zhu X, Wang K, Zhang K et al (2013) Ziyuglycoside II inhibits the growth of human breast carcinoma MDA-MB-435 cells via cell cycle arrest and induction of apoptosis through the mitochondria dependent pathway. Int J Mol Sci 14:18041–18055. https://doi.org/10.3390/ijms140918041
Zong J, Wang D, Jiao W et al (2016) Oleiferasaponin C6 from the seeds of Camellia oleifera Abel.: a novel compound inhibits proliferation through inducing cell-cycle arrest and apoptosis on human cancer cell lines in vitro. RSC Adv 6:91386–91393. https://doi.org/10.1039/c6ra14467e
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Podolak, I., Grabowska, K., Sobolewska, D. et al. Saponins as cytotoxic agents: an update (2010–2021). Part II—Triterpene saponins. Phytochem Rev 22, 113–167 (2023). https://doi.org/10.1007/s11101-022-09830-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-022-09830-3