Abstract
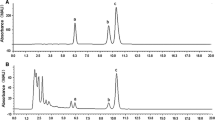
Glechoma longituba (Nakai) Kupr. is a perennial shade plant with pharmaceutical importance. The aim of this study was to investigate the effects of light intensity on the growth, photosynthesis, and accumulation of secondary metabolites in G. longituba grown under six different light environments. The high light intensity decreased the leaf size, specific leaf area, and aboveground dry mass, the number of grana per chloroplast, the number of lamella per granum, the thickness of the grana, the apparent quantum efficiency, the chlorophyll (Chl) content, the concentrations of ursolic and oleanolic acid. The high light increased the stomatal density, the stoma size, the number of chloroplast per a cell, the chloroplast size, the dark respiration rate, the light saturation point, the light compensation point, and the Chl a/b ratio. With the reduction in the light intensity, the light-saturated net photosynthetic rate, the aerial dry mass per plant, and the yields of ursolic and oleanolic acid decreased after an initial increase, peaking at 16 and 33% of sunlight levels. Overall, the 16 and 33% irradiance levels were the most efficient in improving the yields and qualities of the medicinal plant. The lower light demand and growth characteristics suggest that G. longituba is an extremely shade-tolerant plant and that appropriate light intensity management might be feasible to obtain higher yields of secondary metabolites in agricultural management.
Similar content being viewed by others
Abbreviations
- AQE:
-
apparent quantum efficiency
- Chl:
-
chlorophyll
- LCP:
-
light compensation point
- LSP:
-
light saturation point
- OA:
-
oleanolic acid
- P N :
-
net photosynthetic rate
- P Nmax :
-
light-saturated net photosynthetic rate
- R D :
-
dark respiration rate
- SLA:
-
specific leaf area
- UA:
-
ursolic acid
References
Almeida-Cortez J.S., Shipley B., Arnason J.T.: Do plant species with high relative growth rates have poorer chemical defenses? — Funct. Ecol. 16: 819–827, 1999.
Aoyagi H., Kobayashi Y., Yamada K. et al.: Efficient production of saikosaponins in Bupleurum falcatum root fragments combined with signal transducers. — Appl. Microbiol. Biotechnol. 57: 482–488, 2001.
Bilger W., Schreiber U., Bock M.: Determination of the quantum efficiency of photosystem II and of non-photochemical quanching of chlorophyll fluorescence in the field. — Oecologia 102: 425–432, 1995.
Bryant J.P., Chapin F.S., Klein D.R.: Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. — Oikos 40: 357–368, 1983.
Bussotti F.: Functional leaf traits, plant communities and acclimation processes in relation to oxidative stress in trees: a critical overview. — Global Change Biol. 14: 2727–2739, 2008.
Butterfass T.H.: Reproduction and continuity of chloroplasts in spermatophytes. — Bot. Rev. 61: 1–27, 1995.
Cai Z.Q., Qi X., Cao K.F.: [Response of stomatal characteristics and its plasticity to different light intensities in leaves of seven tropical woody seedlings.] — Chin. J. Appl. Ecol. 15: 201–204, 2004. [In Chinese]
Cai Z.Q., Wang W.H., Yang J., Cai C.T.: Growth, photosynthesis and root reserpine concentrations of two Rauvolfia species in response to a light gradient. — Ind. Crop Prod. 30: 220–226, 2009.
Ceulemans R., Praet L.V., Jiang X.N.: Effects of CO2 enrichment leaf position and clone on stomatal index and epidermal cell density in poplar (Populus). — New Phytol. 131: 99–107, 1995.
Chauser-Volfson E., Gutterman Y.: Content and distribution of anthrone C-glycosides in the South African arid plant species Aloe mutabilis growing in the direct sunlight and the shade in the Negev Desert of Israel. — J. Arid Environ. 40: 441–451, 1998.
Chen L.Q., Li C.S., Chaloner W.G. et al.: Assessing the potential for the stomatal characters of extant and fossil Ginkgo leaves to signal atmospheric CO2 change. — Am. J. Bot. 88: 1309–1315, 2001.
Coelho G.C., Rachwal M.F.G., Dedecek N.A. et al.: Effect of light intensity on methylxanthine contents of Ilex paraguariensis A. St. Hil. — Biochem. Syst. Ecol. 35: 75–80, 2007.
Coley P.D., Barone J.A.: Herbivory and plant defense in tropical forests. — Annu. Rev. Ecol. Syst. 27: 305–335, 1996.
Dai Y.J., Shen Z.G., Liu Y. et al.: Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. — Environ. Exp. Bot. 65: 177–182, 2009.
Eibl B., Fernandez R.A., Kozarik J.C. et al.: Agroforestry systems with Ilex paraguariensis (American holly or yerba mate) and native timber trees on small farms in Misiones. — Agroforest. Syst. 48: 1–8, 2000.
Fang L., Lin N.M., Wu Y.J.: [Simultaneous determination of four active components in Spica Prunellae by HPLC.] — China. J. Chin. Materia. Med. 35: 616–619, 2010. [In Chinese]
Galmés J., Medrano H., Flexas J.: Photosynthesis and photoinhibition in response to drought in a pubescent (var. minor) and a glabrous (var. palaui) variety of Digitalis minor. — Environ. Exp. Bot. 60: 105–111, 2007.
Gilmore A.D.: Excess light stress: probing excitation dissipation mechanisms through global analysis of time- and wavelengthresolved cholorophyll a fluorescence. — In: Papageorgiou G.C., Govindjee (ed.): Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration. Pp. 555–581. Springer, Dordrecht 2004.
Giner-Larza E.M., Máñez S., Recio M.C. et al.: Oleanonic acid, a 3-oxotriterpene from Pistacia, inhibits leukotriene synthesis and has anti-inflammatory activity. — Eur. J. Pharmacol. 428: 137–143, 2001.
Gregoriou K., Pontikis K., Vemmos S.: Effects of reduced irradiance on leaf morphology, photosynthetic capacity, and fruit yield in olive (Olea europaea L.). — Photosynthetica 45: 172–181, 2007.
Griffin J.J., Ranney T.G., Pharr D.M.: Photosynthesis, chlorophyll fluorescence, and carbohydrate content of Illicium taxa grown under varied irradiance. — J. Am. Soc. Hortic. Sci. 129: 46–53, 2004.
Guo Y.P., Guo D.P., Zhou H.F. et al.: Photoinhibition and xanthophyll cycle activity in bayberry (Myrica rubra) leaves induced by high irradiance. — Photosynthetica 44: 439–446, 2006.
Herms D.A., Mattson W.J.: The dilemma of plants: to grow or defend. — Q. Rev. Biol. 67: 283–335, 1992.
Höft M., Verpoorte R., Beck E.: Growth and alkaloid patterns of roots of Tabernaemontana pachysiphon and Rauvolfia mombasiana as influenced by environmental factors. — Bot. Acta 111: 222–230, 1998.
Hou J.L., Li W.D., Zheng Q.Y. et al.: Effect of low light intensity on growth and accumulation of secondary metabolites in roots of Glycyrrhiza uralensis Fisch. — Biochem. Syst. Ecol. 38: 160–168, 2010.
Ikeda Y., Murakami A., Nishizawa T., Ohigashi H.: Ursolic acid enhances cyclooxygenases and tumor necrosis factoralpha expression in mouse skin. — Biosci. Biotech. Bioch. 70: 1033–1037, 2006.
Kashiwada Y., Nagao T., Hashimoto A. et al.: Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. — J. Nat. Prod. 63: 1619–1622, 2000.
Kim G.T., Yano S., Kozuka T., Tsukaya H.: Photomorphogenesis if leaves: shade-avoidance and differentiation of sun and shade leaves. — Photoch. Photobio. Sci. 4: 770–774, 2005.
Kutík J.: The development of chloroplast structure during leaf ontogeny. — Photosynthetica 35: 481–505, 1998.
Lei T.T., Tabuchi R., Kitao M., Koike T.: The functional relationship between chlorophyll content, leaf reflectance, and light capturing efficiency of Japanese forest species under natural shade and open light regimes. — Physiol. Plantarum 96: 411–418, 1996.
Li J., Guo W.J., Yang Q.Y.: Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. — World J. Gastroentero. 8: 493–495, 2002.
Li X.W., Dai Y., Chen S.L.: Growth and physiological characteristics of Fritillaria cirrhosa in response to high irradiance and shade in age-related growth phases. — Environ. Exp. Bot. 67: 77–83, 2009.
Li Y.L., Liu Y., Huang L., Liang S.L.: [An Illustrated Book of Chinese Common Flowers.] Pp. 139. — Henan Science and Technology Press, Zhengzhou 1999. [In Chinese]
Lichtenthaler H.K., Burkart S.: Photosynthesis and high light stress. — Bulg. J. Plant Physiol. 25: 3–16, 1999.
Lichtenthaler H.K.: Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. — Methods Enzymol. 148: 350–382, 1987.
Lichtenthaler H.K., Ac A., Marek M.V. et al.: Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. — Plant Physiol. Biochem. 45: 577–588, 2007.
Liu J.: Pharmacology of oleanolic acid and ursolic acid. — J. Ethnopharmacol. 49: 57–68, 1995.
Liu L., Zhu Z.B., Guo Q.S. et al.: Variation in contents of major bioactive compounds in Glechoma longituba related to harvesting time and geographic distribution. — J. Med. Plants Res. 6: 122–128, 2012.
Liu Y.J., Wang H.L., Qu S.H.: [Application of Garden Ornamental Flowers.] Pp. 688. — Liaoning Science and Technology Press, Shenyang 2008. [In Chinese]
Ma C., Nakamura N., Hattori M. et al.: Inhibitory effects on HIV-1 protease of constituents from the wood of Xanthoceras sorbifolia. — J. Nat. Prod. 63: 238–242, 2000.
Meier D., Lichtenthaler H.K.: Ultrastructural development of chloroplasts in radish seedlings grown at high- and low-light conditions and in the presence of the herbicide bentazon. — Protoplasma 107: 195–207, 1981.
Mooney H.A., Gulmon S.L., Johnson N.D.: Physiological constraints on plant chemical defenses. — In: Hedin A. (ed.): Plant Resistance to Insects. Pp. 21–36. American Chemical Society, Washington 1983.
Ni S.M., Qian D.W., Duan J.A. et al.: UPLC-QTOF/MS-based screening and identification of the constituents and their metabolites in rat plasma and urine after oral administration of Glechoma longituba extract. — J. Chromatogr. B 878: 2741–2750, 2010.
Niinemets Ü.: Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. — Ecology 82: 453–469, 2001.
Niinemets Ü., Kull O., Tenhunen J.D.: An analysis of light effects on foliar morphology, physiology and light interception in temperate deciduous woody species of contrasting shade tolerance. — Tree Physiol. 18: 681–696, 1998.
Ohigashi H., Takamura H., Koshimizu K. et al.: Search for possible antitumor promoters by inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation; ursolic acid and oleanolic acid from an antiinflammatory Chinese medicinal plant, Glechoma hederacea L. — Cancer Lett. 30: 143–151, 1986.
Ovesná Z., Kozics K., Slameňová D.: Protective effects of ursolic acid and oleanolic acid in leukemic cells. — Mutat. Res-Fund. Mol. M. 600: 131–137, 2006.
Park S.A., Kim M.G., Yoo M.H. et al.: Plant physiological responses in relation to temperature, light intensity, and CO2 concentration for the selection of efficient foliage plants on the improvement of indoor environment. — Korean J. Hortic. Sci. 28: 928–936, 2010.
Pyke K.A., Leech R.M.: The control of chloroplast number in wheat mesophyll cells. — Planta 170: 416–420, 1987.
Reinolds E.S.: The use of lead citrate of high pH as an electronopaque stain in electron microscopy. — J. Cell Biol. 17: 208–212, 1963.
Rosati A., Badeck F.W., Dejong T.M.: Estimating canopy light interception and absorption using leaf mass per unit leaf area in Solanum melongena. — Ann. Bot.-London. 88: 101–109, 2001.
Rousset O., Lepart J.: Positive and negative interactions at different life stages of a colonizing species (Quercus humilis). — J. Ecol. 88: 401–412, 2000.
Ryu S.Y., Oak M.H., Yoon S.K. et al.: Antiallergic and antiinflammatory triterpenes from the herb of Prunella vulgaris. — Planta Med. 66: 358–360, 2000.
Sims D.A., Pearcy R.W.: Photosynthetic characteristics of a tropical forest understory herb, Alocasia macrorrhiza, and a related crop species, Colocasia esculenta grown in contrasting light environments. — Oecologia 79: 53–59, 1989.
Sohn K.H., Lee H.Y., Chung H.Y. et al.: Anti-angiogenic activity of triterpene acids. — Cancer Lett. 94: 213–218, 1995.
Šesták Z.: Limitations for finding a linear relationship between chlorophyll content and photosynthetic activity. — Biol. Plantarum 8: 336–346, 1966.
Tao J.P., Zhong Z.C.: [Morphological responses to different nutrient supply in the stoloniferous herb Glechoma longituba.] — Acta Ecol. Sin. 20: 207–211, 2000. [In Chinese]
Temesgen H., Weiskittel A.R.: Leaf mass per area relationships across light gradients in hybrid spruce crowns. — Trees 20: 522–530, 2006.
Theis N., Lerdau M.: The evolution of function in plant secondary metabolites. — Int. J. Plant Sci. 164: 93–102, 2003.
Wang B.Y., Feng Y.L.: [Effects of growth light intensities on photosynthesis in seedlings of two tropical rain forest species.] — Acta Ecol. Sin. 25: 23–30, 2005. [In Chinese]
Wang H.H., Wang Z.Z., Guo W.B.: Comparative determination of ursolic acid and oleanolic acid of Macrocarpium officinalis (Sieb. et Zucc.) Nakai by RP-HPLC. — Ind. Crop Prod. 28: 328–332, 2008.
Wei S.L., Wang W.Q., Chen X.H. et al.: [Studies on the shadeendurance capacity of Glycyrrhiza uralensis.] — China J. Chin. Mater. Med. 30: 100–104, 2005. [In Chinese]
Wilson D., Cooper J.P.: Effect of light intensity during grown on leaf anatomy and subsequent light-saturated photosynthesis among contrasting Lolium genotypes. — New Phytol. 68: 1125–1135, 1969.
Wittmann C., Aschan G., Pfanz H.: Leaf and twig photosynthesis of young beech (Fagus sylvatica) and aspen (Populus tremula) trees grown under different light regime. — Basic Appl. Ecol. 2: 145–154, 2001.
Wu Z.Y., Chen X.Q., Li X.W. et al.: [Flora of China. 65]. Pp. 316–318, Science Press, Beijing 1977. [In Chinese]
Xiao P.G., Yu D.Q., Ding J. et al.: [Pharmacopoeia of People’s Republic of China.] Pp. 158–159. — Chemical Industry Press, Beijing 2010. [In Chinese]
Xu D.Q.: [Photosynthetic Efficiency.] Pp. 124–125. Shanghai Scientific and Technical Publishers, Shanghai 2002. [In Chinese]
Ye Z.P.: A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. — Photosynthetica 45: 637–640, 2007.
Ye Z.P.: [A new model of light-response of photosynthesis and its application.] — J. Biomath. 23: 710–716, 2008. [In Chinese]
Zhang S.R., Gao R.F.: [Light induces leaf orientation and chloroplast movements of hybrid poplar clones.] — Acta Ecol. Sin. 21: 68–74, 2001. [In Chinese]
Zhou S.B., Liu K., Zhang D. et al.: Photosynthetic performance of Lycoris radiata var. radiata to shade treatments. — Photosynthetica 48: 241–248, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: This study was supported by the projects of National Facilities and Information Infrastructure for Science and Technology (2005DKA21000) and National Natural Science Foundation of China (41471243).
Rights and permissions
About this article
Cite this article
Zhang, L.X., Guo, Q.S., Chang, Q.S. et al. Chloroplast ultrastructure, photosynthesis and accumulation of secondary metabolites in Glechoma longituba in response to irradiance. Photosynthetica 53, 144–153 (2015). https://doi.org/10.1007/s11099-015-0092-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-015-0092-7