Abstract
Background
Ibandronate is effective in reducing the risk of vertebral fractures, but experimental evidence offers conflicting results regarding nonvertebral fractures. Real-world evidence has been published evaluating the anti-nonvertebral fracture effect of ibandronate.
Aim
This meta-analysis of observational studies assessed the effectiveness of ibandronate in reducing the risk of nonvertebral fractures in women with osteoporosis.
Method
Pubmed/Embase databases were searched for observational studies. Risks of nonvertebral fractures and hip fractures were the outcomes. Meta-analyses were performed pooling rate ratios (RRs), using random-effects models. Data were reanalysed in sensitivity analyses considering Knapp–Hartung method and Bayesian random-effects.
Results
Six cohort studies were included. Overall, once-monthly 150 mg oral ibandronate reduced the risk of nonvertebral fractures (RR 0.84; 95% CI 0.76–0.94). Similar results were obtained when the comparison was restricted to once-monthly 150 mg risedronate, but no differences were found when the comparator was other oral bisphosphonates (weekly alendronate/risedronate). Ibandronate didn’t significantly change the risk of hip fractures (RR 1.25; 95% CI 0.89–1.76). The risk of hip fracture was comparable between once monthly, 150 mg oral ibandronate and other oral bisphosphonates. Intravenous ibandronate was not effective in reducing hip fractures comparing to intravenous zoledronate. The low number of studies diminished the robustness of sensitivity analyses.
Conclusion
Results suggest that once-monthly 150 mg oral ibandronate may be as effective as other oral bisphosphonates in reducing the risk of nonvertebral fractures. However, uncertainty associated to the small number of included studies, which are characterized by heterogeneous demographics and methodologies, precluded definitive conclusions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
Overall, once-monthly 150 mg oral ibandronate was associated with a reduction in the risk of overall nonvertebral fractures. Stratification of the results demonstrated that ibandronate may be as effective as other oral bisphosphonates.
-
While the risk of hip fracture was comparable between once monthly 150 mg oral ibandronate and other oral bisphosphonates, intravenous (IV) ibandronate was not as effective as IV zoledronate.
-
Although once-monthly 150 mg oral ibandronate seemed effective in preventing nonvertebral fractures, the small number of included studies, characterized by heterogeneous demographics and methodologies, diminished the robustness of sensitivity analyses, and precluded definitive conclusions.
-
These findings highlight the need for further real-world studies to clarify the effectiveness of ibandronate compared to other bisphosphonates in the prevention of osteoporotic nonvertebral fractures.
Introduction
Bisphosphonates are recommended as initial treatment for preventing osteoporotic fractures [1,2,3,4]. Ibandronate is indicated for the treatment of osteoporosis in women at increased risk of fracture, with the advantage of offering less frequent dosing intervals comparing to other oral bisphosphonates [5,6,7]. Two dosing regimens are approved: the once-monthly 150 mg tablet, and the 3 mg intravenous injection every 3 months [8].
Nonvertebral fractures are associated with high morbidity, health-related costs, and quality of life deterioration [9,10,11]. Current experimental evidence offers conflicting results on ibandronate preventing nonvertebral fractures. A post-hoc analysis of BONE trial concluded that ibandronate reduced the risk of nonvertebral fractures in a high-risk subgroup of patients (femoral neck BMD T score < − 3.0) [5]. One pooled analysis and one meta-analysis of randomized controlled trials (RCTs) demonstrated that high dose ibandronate (annual cumulative exposure [ACE] ≥ 10.8 mg) significantly reduced the risk of nonvertebral fractures [12, 13]. Though, meta-analyses of RCTs concluded that ibandronate was ineffective in preventing overall nonvertebral fractures, and hip and wrist fractures in particular [4, 14].
There are several observational studies evaluating the anti-nonvertebral fracture effect of ibandronate [15,16,17]. Observational data may contribute to clarify effectiveness of ibandronate in the prevention of nonvertebral fractures in populations under long-term treatment in clinical practice. However, it would be relevant to perform a meta-analysis of those studies, not only to assess the effectiveness of ibandronate in reducing the risk of nonvertebral fractures, but also to explore the existence of inconsistencies.
Aim
The aim of this systematic review and meta-analysis of observational studies was to assess the effectiveness of ibandronate in reducing the risk of nonvertebral fractures in women with osteoporosis.
Method
This systematic review and meta-analysis was conducted and reported according to the Centre for Reviews and Dissemination’s (CRD) guidance and the “Preferred Reported Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020” [18, 19].
Eligibility criteria
Studies were included if they met the following criteria:
-
Population: women with osteoporosis;
-
Intervention: ibandronate;
-
Comparators: any treatment (other antiosteoporosis treatments, placebo, insufficient dosing regimen of ibandronate, or non-use of ibandronate);
-
Outcomes: overall nonvertebral fractures; hip fractures;
-
Study design: observational, controlled studies (cohort, case–control);
-
Language: only English;
-
Timing: no restrictions.
Search strategy
Literature search followed the strategy from a systematic review evaluating ibandronate in preventing osteoporotic fractures [20]. The search strategy was applied to Pubmed and Embase, and duly updated through May 23rd, 2023 (Table S1). Bibliographic references lists of all relevant studies were hand searched to identify relevant studies.
Study selection
Two researchers independently screened by hand the titles and abstracts and selected full articles for inclusion. Disagreement was resolved by discussion and consensus with a third researcher.
Data collection
The following data were extracted from each study: reference, study design, population, intervention, comparator, outcomes, and results. Data were extracted from each included study by two researchers independently to a predeveloped form.
Risk of bias assessment
The Risk of Bias In non-randomized Studies of Interventions (ROBINS-I) tool, developed by Cochrane Collaboration, was used to assess the risk of bias in non-randomized studies [21]. Each assessment study can be graded into one of five categories: low risk of bias, moderate risk of bias, serious risk of bias, critical risk of bias, and no information.
Data analysis and data synthesis
A meta-analysis was performed by pooling rate ratios (RRs) with their 95% confidence intervals (CIs), using the DerSimonian and Laird random-effects model [22]. The most adjusted effect size estimate was used when more than one estimate was presented. Analyses were disaggregated according to different comparators. The I2 statistic test was used to assess for heterogeneity between studies, where an I2 > 50% was indicative of substantial heterogeneity [23]. Publication bias was assessed through funnel plots [24].
A sensitivity analysis was conducted to explore the robustness of the initial findings, using two methods to recalculate risks: a) the Knapp–Hartung method in combination with the Paule-Mandel estimator for the between-study variance [25]; b) a Bayesian random-effects meta-analysis [26]. A 95% prediction interval (PrI) was also estimated. The influence of the studies designs and methodological quality scores in the results was assessed.
Statistics were performed using R software (R version 4.1.2).
Results
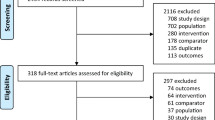
Figure 1 presents the flowchart of the systematic review of literature. A total of 1677 references were identified, of which 282 were considered duplicates. After reviewing titles and abstracts, further 1238 references were excluded. Lastly, 157 references were assessed for eligibility. From those, 6 observational studies were included [15,16,17, 27,28,29]. No studies were identified by hand search from reference lists.
Characteristics of the studies
Table 1 describes the characteristics of the studies, which are all retrospective cohorts. One study was conducted in Korea, one study in Germany, and four in the United States of America (USA). The sample size ranged between 2166 and 95,802 patients, while the median time of follow-up varied from 0.6 to 3 years.
Four studies assessed the effectiveness of once-monthly 150 mg oral ibandronate and two studies assessed the effectiveness of the intravenous (IV) ibandronate (Table 1). The control group was the use of other bisphosphonates in three studies and “no treatment” in three studies.”No treatment” was defined as medication possession ratio [MPR] < 50% by Siris et al., and as the initial 3-month period of therapy by Abelson et al. and by O’Kelly et al. [17, 27, 29]. Two studies used propensity scores to adjust for confounders [15, 16]. The number of covariates considered for the adjustment varied between the studies, with Yun et al. considering 13 covariates for adjustment and O’Kelly et al. only considering one (age) [16, 29]. The risks of fractures were estimated using Cox proportional hazards models in four studies [15,16,17, 28], and simple proportions in two studies [27, 29].
Overall nonvertebral fractures were assessed in four studies and hip fractures in five (Table 1). Fracture outcomes were identified via International Classification of Diseases (ICD) codes in four studies [15, 17, 27, 28], and claims data and algorithms in two studies [16, 29].
Risk of bias
The results of the risk of bias assessment are illustrated in Fig. 2. All studies had serious risk of bias. Data on methods were not clear on how to avoid confounding in all studies. In addition, outcomes’ assessors were aware of the received intervention. However, it is not clear in which way this influenced the measurement of the outcome.
Risk of nonvertebral and hip fractures
The findings suggest that, overall, once-monthly 150 mg oral ibandronate reduces the risk of nonvertebral fractures (RR 0.84; 95% CI 0.76–0.94; I2 = 0%) (Fig. 3). The results were similar when the comparison was restricted to once-monthly 150 mg risedronate (RR 0.80; 95% CI 0.65–0.98; I2 = NA) but did not remain statistically significant when the comparator chosen was “no treatment” (RR 0.85; 95% CI 0.73–1.00; I2 = 0%) or other oral bisphosphonates (alendronate 35 mg/70 mg weekly, or risedronate 35 mg weekly) (RR 0.88; 95% CI 0.71–1.09; I2 = NA) (Fig. S3).
Ibandronate did not significantly change the risk of hip fractures (RR 1.25; 95% CI 0.89–1.76; I2 = 27%) (Fig. 4). The results remain similar when the comparison was restricted to other oral bisphosphonates (alendronate 35 mg/70 mg weekly, or risedronate 35 mg weekly) (RR 1.06; 95% CI 0.61–1.84; I2 = NA), 150 mg oral risedronate (RR 1.06; 95% CI 0.55–2.06; I2 = NA), or “no treatment” (RR 1.08; 95% CI 0.68–1.73; I2 = 0%). Comparing to IV zoledronic acid, IV ibandronate is not effective in reducing hip fractures (RR 2.37; 95% CI 1.15–4.50; I2 = NA) (Fig. S4).
Publication bias
No significant asymmetry was identified in the funnel plots of both meta-analyses (Figs. S1 and S2). The low number of studies limited these analyses.
Sensitivity analysis
According to the Knapp–Hartung method, the risk of nonvertebral fractures did not change; the 95% PrI was estimated as RR: 0.67–1.07 (Fig. S5). The risk of hip fracture did not significantly change comparing to the initial analysis (RR 1.25; 95% CI 0.76–2.06); the 95% PrI is wide (RR 95% PrI 0.51–3.09) (Fig. S6).
The results reported by the Bayesian meta-analyses did not identify statistically significant protective effects from ibandronate [nonvertebral fractures RR 0.85; 95% credible interval (CrI) 0.56–1.29); hip fractures RR 1.20; 95% CrI 0.66–2.20] (Figs. S7 and S8).
No further sensitivity analyses were conducted since all studies have similar design and methodological quality scores.
Discussion
This meta-analysis of observational studies evaluates the effectiveness of ibandronate in preventing osteoporotic nonvertebral fracture. There are reasons why this study stands out from a previous systematic review evaluating the antifracture benefit of ibandronate [20]. First, this meta-analysis is supported by an updated literature search which identified a new study [29]. Updating systematic reviews is a recommended procedure in clinical investigation, as newly identified studies can change the conclusions of previous publications [30]. Disregarding new evidence can threaten the validity of systematic reviews and misleading healthcare professionals, patients, and regulatory authorities at the time of decision-making [31, 32]. Second, the previous systematic review performed a qualitative evaluation of ibandronate antifracture effect. This meta-analysis provides a weighted average of the results of all individual studies and conducts a sensitivity analysis to assess the robustness of the results, helping readers understanding the conclusions. Statistical synthesis of the evidence adds value to this research topic since it provides objective risk estimates rather than qualitative descriptions [33].
RCTs provide the most robust evidence regarding the efficacy and short-term safety of pharmacological interventions. Among their advantages, the strict inclusion/exclusion criteria, random allocation, continuous monitoring of patients, and precise definition of endpoints reduces the risk of bias and confounding [34]. However, real-world studies are essential to provide evidence of treatment effectiveness, by including patient populations that may be representative of clinical practice, larger sample sizes, and extended follow-up times when compared to RCTs. In this context, evidence of efficacy and safety of bisphosphonates from RCTs may not predict their actual effectiveness in clinical practice because of clinical, demographic, and suboptimal persistence/adherence differences between the populations. Therefore, there is a rational to conduct a meta-analysis of observational studies evaluating the effectiveness of ibandronate in preventing osteoporotic nonvertebral fractures. Moreover, meta-analyses of nonexperimental studies are frequently designed to explore eventual sources of heterogeneity among studies rather than to find evidence of causative associations [35].
These results suggest that ibandronate reduces the risk of nonvertebral fractures in general. Regarding this outcome, all the included studies evaluated ibandronate in reducing nonvertebral fractures as a once-monthly 150 mg oral regimen. However, the analysis of the results should take into consideration the different comparators used in the studies. The sensitivity analysis demonstrated that once-monthly 150 mg oral ibandronate reduced the risk of nonvertebral fractures compared with once-monthly 150 mg oral risedronate [15]. No differences were found when once-monthly 150 mg oral ibandronate was compared to weekly risedronate or weekly alendronate [28]. The comparison between ibandronate and “no treatment” returned a reduced risk, but without statistical significance [17, 27].
Although the results did not demonstrate an overall risk reduction of hip fracture, oral ibandronate seems to have a comparable risk of hip fracture to other oral bisphosphonates. No risk differences were found between once-monthly 150 mg oral ibandronate versus the other oral bisphosphonates (either once-monthly 150 mg oral risedronate, or weekly risedronate or weekly alendronate), nor versus “no treatment” [15, 27, 28]. However, the results suggest that IV zoledronate (once yearly) is more effective than IV ibandronate (once quarterly) to prevent hip fractures [16].
Both risedronate and alendronate are approved to prevent hip fractures in women with osteoporosis [36, 37]. Since the risk of hip fractures was comparable between once-monthly 150 mg oral ibandronate and the other oral bisphosphonates (once-monthly 150 mg oral risedronate, or weekly risedronate, and weekly alendronate), whether ibandronate could also be recommended to prevent such type of fracture may be matter of discussion. These results are in line with those from previous meta-analyses of RCTs, where no differences were found between these three bisphosphonates on the risk of hip fracture [38, 39]. Therefore, this meta-analysis of observational studies may contribute to clarifying the comparative effectiveness of ibandronate versus other bisphosphonates in the prevention of nonvertebral fractures in real-world clinical practice.
This study assessed hip fractures as the only outcome for site-specific fractures. Hip fractures are one of most severe type of osteoporotic fractures and are associated with higher mortality, reduced quality of life, and increased health resources consumption [40]. As hip fractures are an important endpoint considered in the design of RCTs of new antiosteoporosis drugs, studies assessing the effectiveness of the bisphosphonates in reducing this type of fractures in real-world clinical practice are valued by decision-makers [32, 41, 42]. Furthermore, only the study of O’Kelly et al. reported results for other type of fracture (wrist/forearm fracture) [29]. Given this, it was not possible to conduct a meta-analysis including only one study.
A strength of this meta-analysis is including only studies reporting results on definite nonvertebral fracture outcomes, and not studies reporting results for surrogate-type endpoints (e.g., changes in BMD). Although the incidence of osteoporotic fractures is usually associated with changes in BMD, this is still a predictive risk factor that do not replace the measurement of definite fracture outcomes [34, 35]. The marketing authorisations of bisphosphonates to prevent fractures in osteoporosis were granted based on statistically significant risk reductions of definite fracture outcomes (rather than solely on changes in BMD) [36]. Therefore, the summary of product characteristics of ibandronate highlights that the efficacy of the drug is yet to be proved in the prevention of nonvertebral fractures.
Some limitations must be considered. First, only 6 studies verified inclusion criteria. There are several observational studies evaluating the effectiveness of bisphosphonates published in the scientific literature, but without disaggregating the results at drug level [37]. A thorough literature search has been conducted but only a small of group studies evaluating the risk of nonvertebral fractures associated with ibandronate were found. Second, the studies used different control groups. Not only different bisphosphonates were used as active comparators but also three studies comparing ibandronate with “no treatment” adopted different approaches when defining the control groups. In the study of Siris et al. patients with a medication possession ratio (MPR) of less than 50% were considered the referent ‘‘untreated’’ population [17]. The studies of O’Kelly et al. and Abelson and et al. evaluated the risk of nonvertebral fractures by comparing the incidence of those fractures during an initial 3-month period of therapy with the incidence of fractures observed in the subsequent first year of therapy [27, 29]. Authors argued that the baseline fracture incidence during the initial 3 months of therapy may accurately reflect the underlying risk of the cohort, as bisphosphonates may take up to 3 months to reach maximum effectiveness [38]. The different design approaches between the studies using “no treatment” as a control group may be the reason why they did not reach concordant nonvertebral fracture risk estimates, resulting in a non-statistically significant risk reduction in the meta-analysis. Third, the results were only stratified according to different comparators, with all studies having the same design and risk of bias assessment. The influence of additional risk factors in the results needs to be accounted for when analysing these findings. However, most studies did not detail the results according to known risk factors for nonvertebral fractures, like age, prior use of bisphosphonates, and previous fractures. Fourth, there is no currently satisfactory methodology to perform meta-analysis including a small number of studies, particularly because between-studies heterogeneity cannot be reliably estimated. The initial analysis heterogeneity was most likely underestimated by the frequentist method. Therefore, additional analyses were conducted to better account the uncertainty. The Knapp–Hartung method (combined with the Paule-Mandel estimator) is recommended by Cochrane Collaboration for this specific scenario and the Bayesian random-effects meta-analysis is an alternative approach when having a low number of studies [25]. Fifth, the covariates used for the adjustment and approaches to control confounders varied significantly between the studies. Therefore, studies were assessed as having low methodological quality. Sixth, there are additional differences between the studies regarding their methods and demographics. Three studies included patients aged ≥ 45 years old, while three considered patients aged ≥ 65 years old. Additionally, sample sizes, median time of follow-up, and proportion of patients with previous fracture varied greatly between studies. While the heterogeneity found in these meta-analyses was not excessive (maximum I2 = 27%), the possibility of studies’ demographic and methodological discrepancies have influence in the risk estimates should not be ruled out. Seventh, we only searched Pubmed and Embase. However, these are the two most comprehensive biomedical literature databases and comprised more than 35 and 44 million citations, respectively [49, 50]. Eight, no protocol of this study was previously published, which could increase transparency and reduce potential for bias. Nonetheless, guidance to conduct and report this meta-analysis was followed.
Conclusion
These results suggest that once-monthly 150 mg oral ibandronate may be as effective as other oral bisphosphonates in reducing the risk of nonvertebral fractures. However, the small number of included studies are associated with uncertainty since they have heterogeneous demographics and methodologies. Thus, it was not possible to conduct a thorough assessment of the consistency of these findings and precaution is needed before taking definitive conclusions and offering recommendations. Further real-world studies are needed to clarify the effectiveness of ibandronate compared to other bisphosphonates in the prevention of osteoporotic nonvertebral fractures.
References
Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595–622.
Blake J, Cosman FA, Lewiecki EM, et al. Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause. 2021;28(9):973–97.
Drake MT, Clarke BL, Khosla S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83(9):1032–45.
Sanderson J, Martyn-St James M, Stevens J, et al. Clinical effectiveness of bisphosphonates for the prevention of fragility fractures: a systematic review and network meta-analysis. Bone. 2016;89:52–8.
Chesnut CH 3rd, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241–9.
Miller PD, McClung MR, Macovei L, et al. Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res. 2005;20(8):1315–22.
Delmas PD, Adami S, Strugala C, et al. Intravenous ibandronate injections in postmenopausal women with osteoporosis: one-year results from the dosing intravenous administration study. Arthritis Rheum. 2006;54(6):1838–46.
European Medicines Agency. Summary of Product Characteristics of Bonviva®. December 18, 2013. https://www.ema.europa.eu/en/documents/product-information/bonviva-epar-product-information_en.pdf. Accessed 4 Oct 2023.
Roux C, Wyman A, Hooven FH, et al. Burden of non-hip, non-vertebral fractures on quality of life in postmenopausal women: the Global Longitudinal study of Osteoporosis in Women (GLOW). Osteoporos Int. 2012;23(12):2863–71.
Adachi JD, Adami S, Gehlbach S, et al. Impact of prevalent fractures on quality of life: baseline results from the global longitudinal study of osteoporosis in women. Mayo Clin Proc. 2010;85(9):806–13.
Weycker D, Li X, Barron R, et al. Hospitalizations for osteoporosis-related fractures: economic costs and clinical outcomes. Bone Rep. 2016;5:186–91.
Cranney A, Wells GA, Yetisir E, et al. Ibandronate for the prevention of nonvertebral fractures: a pooled analysis of individual patient data. Osteoporos Int. 2009;20(2):291–7. https://doi.org/10.1007/s00198-008-0653-8.
Harris ST, Blumentals WA, Miller PD. Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studies. Curr Med Res Opin. 2008;24(1):237–45.
Jin YZ, Lee JH, Xu B, et al. Effect of medications on prevention of secondary osteoporotic vertebral compression fracture, non-vertebral fracture, and discontinuation due to adverse events: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2019;20(1):399.
Lee DR, Lee J. Comparison of the efficacy between once-monthly oral ibandronate and risedronate among Korean women with osteoporosis: a nationwide population-based study. Osteoporos Int. 2019;30(3):659–66.
Yun H, Delzell E, Saag KG, et al. Fractures and mortality in relation to different osteoporosis treatments. Clin Exp Rheumatol. 2015;33(3):302–9.
Siris ES, Pasquale MK, Wang Y, et al. Estimating bisphosphonate use and fracture reduction among US women aged 45 years and older, 2001–2008. J Bone Miner Res. 2011;26(1):3–11.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
University of York, Centre for Reviews and Dissemination. Systematic reviews: CRD’s guidance for undertaking reviews in health care. January 2009. https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed 4 Oct 2023.
Mendes D, Penedones A, Alves C, et al. Ibandronate in the prevention of vertebral and nonvertebral osteoporotic fractures: a systematic review of experimental and observational studies. J Clin Rheumatol. 2023;29(2):78–83.
Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355: i4919.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Publication bias. In: Borenstein M, Hedges LV, JPT H, Rothstein HR, editors. Introduction to meta-analysis. Wiley, Chichester, pp 277–291. 2009.
Bender R, Friede T, Koch A, et al. Methods for evidence synthesis in the case of very few studies. Res Synth Methods. 2018;9(3):382–92.
Röver C, Friede T. Using the bayesmeta R package for Bayesian random-effects meta-regression. Comput Methods Programs Biomed. 2023;229: 107303.
Abelson A, Ringe JD, Gold DT, et al. Longitudinal change in clinical fracture incidence after initiation of bisphosphonates. Osteoporos Int. 2010;21(6):1021–9.
Harris ST, Reginster JY, Harley C, et al. Risk of fracture in women treated with monthly oral ibandronate or weekly bisphosphonates: the eValuation of IBandronate Efficacy (VIBE) database fracture study. Bone. 2009;44(5):758–65.
O'Kelly J, Bartsch R, Kossack N, et al. Real-world effectiveness of osteoporosis treatments in Germany [published correction appears in Arch Osteoporos. 2022;17(1):129]. Arch Osteoporos 2022;17(1):119.
Cochrane Editorial and Publishing Policy Resource. Policy: Cochrane Review updates. June 30, 2021. https://documentation.cochrane.org/display/EPPR/Policy%3A+Cochrane+Review+updates. Accessed 4 Oct 2023.
Garner P, Hopewell S, Chandler J, et al. When and how to update systematic reviews: consensus and checklist. BMJ. 2016;354: i3507.
Laird C, Williams KA, Benson H. Perceptions and practices of aged care pharmacists regarding osteoporosis management: a qualitative study. Int J Clin Pharm. 2023;45(4):913–21.
Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4. August 2023. www.training.cochrane.org/handbook. Accessed October 4, 2023.
Houle S. An introduction to the fundamentals of randomized controlled trials in pharmacy research. Can J Hosp Pharm. 2015;68(1):28–32.
Alves C, Mendes D, Marques FB. Fluoroquinolones and the risk of tendon injury: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2019;75(10):1431–43.
European Medicines Agency. Summary of Product Characteristics of Fosavance®. April 24, 2015. https://www.ema.europa.eu/en/documents/product-information/fosavance-epar-product-information_en.pdf. Accessed 4 Oct 2023.
Medicines & Healthcare products Regulatory Agency. Summary of Product Characteristics of Actonel®. January 14, 2016. https://products.mhra.gov.uk/product/?product=ACTONEL%20ONCE%20A%20WEEK%2035%20MG%20FILM-COATED%20TABLETS. Accessed 4 Oct 2023.
Barrionuevo P, Kapoor E, Asi N, et al. Efficacy of Pharmacological Therapies for the Prevention of Fractures in Postmenopausal Women: A Network Meta-Analysis. J Clin Endocrinol Metab. 2019;104(5):1623–30.
Jansen JP, Bergman GJ, Huels J, et al. The efficacy of bisphosphonates in the prevention of vertebral, hip, and nonvertebral-nonhip fractures in osteoporosis: a network meta-analysis. Semin Arthritis Rheum. 2011;40(4):275–84.e1–2.
Kanters TA, van de Ree CLP, de Jongh MAC, et al. Burden of illness of hip fractures in elderly Dutch patients. Arch Osteoporos. 2020;15(1):11.
European Medicines Agency. Committee for Medicinal Products for Human Use. Guideline on the Evaluation of Medicinal Products in the Treatment of Primary Osteoporosis. November 16, 2006. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-medicinal-products-treatment-primary-osteoporosis_en.pdf. Accessed 4 Oct 2023.
Kempen TGH, Koumi R, Sporrong SK. Pharmacists in general practice: what do they do? A qualitative case study. Int J Clin Pharm. 2023. doi: https://doi.org/10.1007/s11096-023-01619-4.
Black DM, Bauer DC, Vittinghoff E, et al. Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(8):672–82.
Eastell R, Vittinghoff E, Lui LY, et al. Validation of the Surrogate Threshold Effect for Change in Bone Mineral Density as a Surrogate Endpoint for Fracture Outcomes: The FNIH-ASBMR SABRE Project. J Bone Miner Res. 2022;37(1):29–35.
European Medicines Agency. Evaluation of new medicinal products in the treatment of primary osteoporosis. CPMP/EWP/552/95 Rev. 2. May 31, 2007. https://www.ema.europa.eu/en/evaluation-new-medicinal-products-treatment-primary-osteoporosis. Accessed 4 Oct 2023.
Tsuda T, Hashimoto Y, Okamoto Y, et al. Meta-analysis for the efficacy of bisphosphonates on hip fracture prevention. J Bone Miner Metab. 2020;38(5):678–86.
Barton DW, Smith CT, Piple AS, et al. Timing of Bisphosphonate Initiation After Fracture: What Does the Data Really Say? Geriatr Orthop Surg Rehabil. 2020;11:2151459320980369.
Banefelt J, Åkesson KE, Spångéus A, et al. Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos Int. 2019;30(3):601–9.
Elsevier. Embase content coverage. August 2023. https://www.elsevier.com/solutions/embase-biomedical-research/coverage-and-content. Accessed 4 Oct 2023.
National Institute of Health (NIH). National Library of Medicine. PubMed Overview. August 15, 2023. https://pubmed.ncbi.nlm.nih.gov/about/. Accessed 4 Oct 2023.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was supported by the Tecnimede, SA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
António Donato and Tânia Oliveira are employees of Tecnimede, SA. There is no other conflict of interests do declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alves, C., Mendes, D., Penedones, A. et al. The effectiveness of ibandronate in reducing the risk of nonvertebral fractures in women with osteoporosis: systematic review and meta-analysis of observational studies. Int J Clin Pharm 46, 357–367 (2024). https://doi.org/10.1007/s11096-023-01666-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-023-01666-x