Abstract
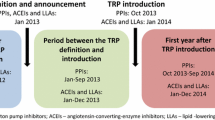
Background The therapeutic reference pricing (TRP) in Slovenia was implemented for proton pump inhibitors in 2013 and for angiotensin-converting enzyme inhibitors and lipid-lowering medicines in 2014. Objective The study aimed to assess patients’ knowledge and attitude towards the TRP system. Moreover, the patients’ willingness to pay was evaluated for patients who rejected the substitution of a current medicine within a therapeutic class by the reference medicine for which no co-payment is needed. Setting Invitation of patients to participate in a survey and filling in the first part of the questionnaire was run in the community pharmacies in Slovenia. The second part of the questionnaire was filled in at patients’ home. Method A representative sample of 676 patients that had been prescribed at least one medicine from the three therapeutic classes was surveyed. The survey was carried out from 15th May to 15th June 2014 in 40 community pharmacies with the help of the pharmacists, who filled in the first part of the questionnaire in the presence of the patients. The second part of the questionnaire was filled in by 475 patients at home and returned by prepaid mail. Main outcome measure Patients’ knowledge of and attitude to the TRP system implemented into Slovenian health care practice. Results Most of the statements describing patient’ rights and duties within the TRP system were known by approximately 50 % of the patients. Patients were inhomogeneous in their view about the necessity and benefits of the TRP system, most of them regarded it as an unnecessary burden. Among 50.4 % of the patients who were required to copay for their medicine, 46.7 % accepted and 3.7 % rejected co-payment. The average co-payment was € 6.92, while the expressed average willingness to co-pay was € 10.4 per 3 months of therapy. Conclusion Our results indicate that the implementation of the TRP system and potential upgrades represent a significant challenge for the patients.

Similar content being viewed by others
References
Puig-Junoy J. The impact of generic reference pricing interventions in the statin market. Health Policy. 2007;84(1):14–29.
Vrijens F, Van de Voorde C, Farfan-Portet MI, Vander Stichele R. Patient socioeconomic determinants for the choice of the cheapest molecule within a cluster: evidence from Belgian prescription data. Eur J Health Econ. 2012;13(3):315–25.
Health Insurance Institute of Slovenia. Therapeutic reference pricing system in Slovenia 2013 [cited 2016 Jun 5]. http://www.zzzs.si/zzzs/internet/zzzs.nsf/o/6A6BD0CD7CB327B3C1257BD900451900?OpenDocument.
Schneeweiss S. Reference drug programs: effectiveness and policy implications. Health Policy. 2007;81(1):17–28.
Schneeweiss S, Walker AM, Glynn RJ, Maclure M, Dormuth C, Soumerai SB. Outcomes of reference pricing for angiotensin-converting-enzyme inhibitors. N Engl J Med. 2002;346(11):822–9.
Health Insurance Institute of Slovenia [cited 2016 Jun 5]. http://www.zzzs.si/index.html.
Wallace LS. A view of health care around the world. Ann Fam Med. 2013;11(1):84.
Rules on inclusion of medicines on the list (Official Gazette of the Republic of Slovenia, No. 35/2013) 2013 [cited 2016 Jun 5]. https://www.uradnilist.si/1/content?id=112932.
Fiscal Balance Act. Legal information system of Slovenia; 2012 [cited 2016 Jun 5]. http://www.pisrs.si/Pis.web/pregledPredpisa?id=ZAKO6388#.
Dunne SS, Shannon B, Cullen W, Dunne CP. Beliefs, perceptions and behaviours of GPs towards generic medicines. Fam Pract. 2014;31(4):467–74.
Heikkila R, Mantyselka P, Hartikainen-Herranen K, Ahonen R. Customers’ and physicians’ opinions of and experiences with generic substitution during the first year in Finland. Health Policy. 2007;82(3):366–74.
Dylst P, Vulto A, Simoens S. Demand-side policies to encourage the use of generic medicines: an overview. Expert Rev Pharm Out. 2013;13(1):59–72.
Multimedia portal RTVSLO. Does health care become inaccessible or not? 2013 [cited 2016 Jun 5]. http://www.rtvslo.si/mmc-priporoca/zdravstvo-vse-bolj-nedostopno-pa-je-res/318648.
Quintal C, Mendes P. Underuse of generic medicines in Portugal: an empirical study on the perceptions and attitudes of patients and pharmacists. Health Policy. 2012;104(1):61–8.
Dunne S, Shannon B, Dunne C, Cullen W. Patient perceptions of generic medicines: a mixed-methods study. Patient. 2014;7(2):177–85.
Himmel W, Simmenroth-Nayda A, Niebling W, Ledig T, Jansen RD, Kochen MM, et al. What do primary care patients think about generic drugs? Int J Clin Pharmacol Ther. 2005;43(10):472–9.
Kersnik J, Peklar J. Attitudes of Slovene general practitioners towards generic drug prescribing and comparison with international studies. J Clin Pharm Ther. 2006;31(6):577–83.
Bayoumi AM. The measurement of contingent valuation for health economics. Pharmacoeconomics. 2004;22(11):691–700.
Johannesson M. The contingent-valuation method. Med Decis Making. 1993;13(4):311–2.
Blomquist CH, Blumenshein K, Johannesson M. Eliciting willingness to pay without bias using follow-up certainty statements: comparisons between probably/definitely and a 10-point certainty scale. Environ Resour Econ. 2009;43(4):473–502.
van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample size. Tutor Quant Methods Psychol. 2007;3(2):43–50.
Rathe J, Larsen P, Andersen M, Paulsen M, Jarbol D, Thomsen J, et al. Associations between generic substitution and patients’ attitudes, beliefs and experiences. Eur J Clin Pharmacol. 2013;69(10):1827–36.
Heikkila R, Mantyselka P, Ahonen R. Do people regard cheaper medicines effective? Population survey on public opinion of generic substitution in Finland. Pharmacoepidemiol Drug Saf. 2011;20(2):185–91.
Slovene Chamber of Pharmacies. Pharmacies in Slovenia [cited 2016 May 20]. http://www.lzs.si/Uporabno/Statistika/Lekarne/tabid/110/Default.aspx.
Hassali MA, Alrasheedy AA, McLachlan A, Nguyen TA, Al-Tamimi SK, Ibrahim MI, et al. The experiences of implementing generic medicine policy in eight countries: a review and recommendations for a successful promotion of generic medicine use. Saudi Pharm J. 2014;22(6):491–503.
Kobayashi E, Karigome H, Sakurada T, Satoh N, Ueda S. Patients’ attitudes towards generic drug substitution in Japan. Health Policy. 2011;99(1):60–5.
Forum of International Research and Development Pharmaceutical Companies EIG. Therapeutic reference pricing is harmful to the patients 2013 [cited 2016 May 15]. http://www.firdpc.com/sl/Dogodki_in_novice/Terapevtske_skupine_zdravil/.
Slovene Chamber of Pharmacies. Therapeutic reference pricing will bring a lot of problems and no savings 2013 [cited 2016 May 15]. http://www.lekzbor.si/Aktualno/Sporocilazajavnost/tabid/133/smid/669/ArticleId/89/Default.aspx.
Rathe J, Sondergaard J, Jarbol DE, Hallas J, Andersen M. Patients’ concern about their medicine after a generic switch: a combined cross-sectional questionnaire and register study. Pharmacoepidemiol Drug Saf. 2014;23(9):965–73.
Toverud EL, Roise AK, Hogstad G, Wabo I. Norwegian patients on generic antihypertensive drugs: a qualitative study of their own experiences. Eur J Clin Pharmacol. 2011;67(1):33–8.
Babar ZU, Stewart J, Reddy S, Alzaher W, Vareed P, Yacoub N, et al. An evaluation of consumers’ knowledge, perceptions and attitudes regarding generic medicines in Auckland. Pharm World Sci. 2010;32(4):440–8.
Bulsara C, McKenzie A, Sanfilippo F, Holman CD, Emery JE. ‘Not the full Monty’: a qualitative study of seniors’ perceptions of generic medicines in Western Australia. Aust J Prim Health. 2010;16(3):240–5.
Dylst P, Vulto A, Simoens S. The impact of reference-pricing systems in Europe: a literature review and case studies. Expert Rev Pharm Out. 2011;11(6):729–37.
Galizzi MM, Ghislandi S, Miraldo M. Effects of reference pricing in pharmaceutical markets: a review. Pharmacoeconomics. 2011;29(1):17–33.
Dylst P, Vulto A, Simoens S. Reference pricing systems in Europe: characteristics and consequences. Generics Biosimilar Initiat J. 2012;1(3–4):127–31.
Analysis of the Faculty of pharmacy—assessed by data in National database on outpatient prescriptions for the year 2013–HIIS.
National Institute of Public Health. Health care statistics 2013 [cited 2016 may 20]. http://www.nijz.si/sites/www.nijz.si/files/uploaded/publikacije/letopisi/2013/7_ambulantno_predpisana_zdravila_18.pdf.
Acknowledgments
The authors would like to thank: The management and pharmacy staff of the following Public pharmaceutical institutes Zasavske Lekarne Trbolje, Lekarna Velenje, Pomurske lekarne, Gorenjske lekarne, Mestne lekarne, Lekarna Ljubljana, Dolenjske lekarne, Lekarna Ptuj, Lekarna Maribor, Goriške lekarne, Celjske lekarne and privately owned pharmacies Lekarna Apoteka pri Teatru and Lekarna Dravlje for the survey execution. Slovenian Pharmaceutical Society for the financial support. Wholesaler of medicines and medical devices–Kemofarmacija for the data on medicine prices and co-payments.
Funding
The study was performed as part of academic research at the University of Ljubljana, Faculty of Pharmacy. Funding support for the survey was provided by the Slovenian Pharmaceutical Society.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marđetko, N., Kos, M. Patients’ knowledge and attitude towards therapeutic reference pricing system in Slovenia. Int J Clin Pharm 38, 1301–1310 (2016). https://doi.org/10.1007/s11096-016-0370-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-016-0370-x