Abstract
Objectives
To evaluate the economic outcomes that arose from the introduction of therapeutic reference pricing (TRP) into Slovenian practice in 2013, based on the first three therapeutic classes, namely proton-pump inhibitors (PPIs), angiotensin-converting-enzyme inhibitors (ACEIs), and lipid-lowering agents (LLAs).
Methods
National health claims data on prescription medicines from January 2011 to December 2015 were analyzed. Monthly medicine expenditure, medicine consumption, changes in medicine use, and market competition (Herfindahl–Hirschman index) were determined to assess the TRP impact on market dynamics. Interrupted time series analysis was used to assess the TRP cost-saving potential.
Results
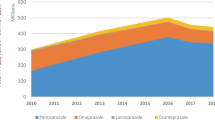
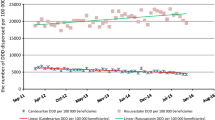
Medicine expenditure in all three therapeutic classes was decreasing prior to TRP; however, with the TRP introduction, the cost for ACEIs and LLAs fell 25 and 45%, respectively. The costs for PPIs decreased by 10%, but the cost reductions before TRP were greater. After TRP introduction, the downward trend for monthly medicine expenditure was less steep; coefficient changes from −20,798 to −363 for PPIs (p < 0.001), from −18,175 to −4862 for ACEIs (p = 0.001) and from −10,669 to −2761 for LLAs (p = 0.105) were observed. Consumption of any therapeutic class or their market competition were not changed significantly. An increased use of the reference pantoprazole (PPIs) was observed and the market position of ezetimibe was deteriorated significantly after TRP introduction. However, the demand for the references simvastatin (LLAs) and ramipril (ACEIs) did not increase.
Conclusions
The Slovenian TRP system was established as an effective cost-containment measure. However, pitfalls arising from a country-specific TRP should be considered when introducing this policy.





Similar content being viewed by others
References
von der Schulenburg, F., Vandoros, S., Kanavos, P.: The effects of drug market regulation on pharmaceutical prices in Europe: overview and evidence from the market of ACE inhibitors. Health Econ. Rev. 1(1), 18 (2011). doi:10.1186/2191-1991-1-18
Galizzi, M.M., Ghislandi, S., Miraldo, M.: Effects of reference pricing in pharmaceutical markets: a review. Pharmacoeconomics 29(1), 17–33 (2011). doi:10.2165/11537860-000000000-00000
Ess, S.M., Schneeweiss, S., Szucs, T.D.: European healthcare policies for controlling drug expenditure. Pharmacoeconomics 21(2), 89–103 (2003)
Usher, C., Barry, M.: A reference pricing system for Ireland. Expert Rev. Pharm. Out. Res. 12(6), 675–677 (2012). doi:10.1586/erp.12.66
Health Insurance Institute of Slovenia: Therapeutic reference pricing system in Slovenia—explanation for the insured population [in Slovenian]. http://www.zzzs.si/zzzs/internet/zzzs.nsf/o/6A6BD0CD7CB327B3C1257BD900451900?OpenDocument (2013). Accessed 5 June 2016
Forum of International Research and Development Pharmaceutical Companies EIG: Health insurance institute of Slovenia has introduced the first therapeutic class of medicines [in Slovenian]. http://firdpc.com/sl/Aktualno/ZZZS_uvaja_prvo_terapevtsko_skupino_zdravil_dopolnjeno/ (2013). Accessed 26 Nov 2016
Wallace, L.S.: A view of health care around the world. Ann. Fam. Med. 11(1), 84 (2013). doi:10.1370/afm.1484
A World Health Organization: Essential medicines and health products information portal, experiences with reference pricing. http://apps.who.int/medicinedocs/en/d/Js4912e/3.3.html (2003). Accessed 20 Oct 2016
Forum of International Research and Development Pharmaceutical Companies EIG: Opinion on therapeutic reference pricing [in Slovenian]. http://www.firdpc.com/sl/Sodelovanje_z_delezniki/ (2014). Accessed 26 Nov 2016
Forum of International Research and Development Pharmaceutical Companies EIG: Therapeutic reference pricing is harmful to patients [in Slovenian]. http://www.firdpc.com/sl/Dogodki_in_novice/Terapevtske_skupine_zdravil/ (2013). Accessed 26 Nov 2016
Kalo, Z., Abonyi-Toth, Z., Bartfai, Z., Voko, Z.: Pitfalls associated with the therapeutic reference pricing practice of asthma medication. BMC Pulm. Med. 12, 35 (2012). doi:10.1186/1471-2466-12-35
Health Insurance Institute of Slovenia: [Introduction of the therapeutic reference pricing into Slovenian health care system—explanation for the insured population] Uvedba sistema najvišjih priznanih vrednosti za terapevtske skupine zdravil—pojasnilo za zavarovane osebe [In Slovenian]. http://www.zzzs.si/zzzs/internet/zzzs.nsf/o/6A6BD0CD7CB327B3C1257BD900451900?OpenDocument (2013). Accessed 4 Mar 2016
Brekke, K.R., Konigbauer, I., Straume, O.R.: Reference pricing of pharmaceuticals. J. Health Econ. 26(3), 613–642 (2007). doi:10.1016/j.jhealeco.2006.11.003
Kalo, Z., Muszbek, N., Bodrogi, J., Bidlo, J.: Does therapeutic reference pricing always result in cost-containment? The Hungarian evidence. Health Policy 80(3), 402–412 (2007). doi:10.1016/j.healthpol.2006.04.002
Organisation for Economic Co-operation and Development (OECD): Pharmaceutical pricing policies in a global market. http://apps.who.int/medicinedocs/documents/s19834en/s19834en.pdf (2008). Accessed 20 Oct 2016
Dylst, P., Vulto, A., Simoens, S.: The impact of reference-pricing systems in Europe: a literature review and case studies. Expert Rev. Pharm. Out. Res. 11(6), 729–737 (2011). doi:10.1586/erp.11.70
Online Database of Medicinal Products in Slovenia. http://www.cbz.si/cbz/bazazdr2.nsf/Search/$searchForm?SearchView (2016). Accessed 10 Aug 2016
Wagner, A.K., Soumerai, S.B., Zhang, F., Ross-Degnan, D.: Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 27(4), 299–309 (2002)
Leopold, C., Zhang, F., Mantel-Teeuwisse, A.K., Vogler, S., Valkova, S., Ross-Degnan, D., Wagner, A.K.: Impact of pharmaceutical policy interventions on utilization of antipsychotic medicines in Finland and Portugal in times of economic recession: interrupted time series analyses. Int. J. Equity Health 13, 53 (2014). doi:10.1186/1475-9276-13-53
WHO Collaborating Centre for Drug Statistics Methodology: Definition and general considerations. http://www.whocc.no/ddd/definition_and_general_considera/ (2016). Accessed 9 Aug 2016
Statistical Office of the Republic of Slovenia: Population number and structure. http://www.stat.si/StatWeb/en/home (2016). Accessed 9 Aug 2016
Frakt, A.: The incidental economist. The health services research blog. Market concentration and entry. http://theincidentaleconomist.com/wordpress/market-concentration-and-entry/. Accessed 16 Aug 2016
Investopedia: Herfindahl–Hirschman Index—HHI. http://www.investopedia.com/terms/h/hhi.asp#ixzz4H0ZEoISh (2016). Accessed 16 Aug 2016
Bobocea, L., Spiridon, S., Petrescu, L., Gheorghe, C.M., Purcarea, V.L.: The management of external marketing communication instruments in health care services. J. Med. Life 9(2), 137–140 (2016)
Health Insurance Institute of Slovenia: [Annual report of the Health Insurance Institute of Slovenia for 2014] Poslovno poročilo Zavoda za zdravstveno zavarovanje Slovenije za leto 2014 [In Slovenian]. http://www.zzzs.si/ZZZS/info/egradiva.nsf/o/9B52139ED62405F7C1257DFD00573AF8?OpenDocument (2015). Accessed 4 Feb 2016
National Institute of Public Health: Medicine use in an ambulatory healthcare in 2014 [in Slovenian]. http://www.nijz.si/sites/www.nijz.si/files/datoteke/poraba_ambulantno_predpisanih_zdravil_v_sloveniji_2014.pdf (2015). Accessed 5 Mar 2017
Brekke, K.R., Canta, C., Straume, O.R.: Reference pricing, generic entry, and pharmaceutical prices. http://www.hec.unil.ch/documents/seminars/iems/1717.pdf.pdf (2005). Accessed 8 Mar 2017
Dylst, P., Vulto, A., Simoens, S.: Reference pricing systems in Europe: characteristics and consequences. Generics Biosimilar Initiat. J. 1(3–4), 127–131 (2012)
Third Slovenian Meeting on Clinical Pharmacology: The safe use of medicines—introduction of therapeutic reference pricing. http://ktf.si/dogodki/3-slovensko-srecanje-o-klinicni-farmakologiji-varna-uporaba-zdravil/ (2015). Accessed 21 Oct 2016
Dunne, S., Shannon, B., Dunne, C., Cullen, W.: Patient perceptions of generic medicines: a mixed-methods study. Patient 7(2), 177–185 (2014). doi:10.1007/s40271-013-0042-z
Himmel, W., Simmenroth-Nayda, A., Niebling, W., Ledig, T., Jansen, R.D., Kochen, M.M., Gleiter, C.H., Hummers-Pradier, E.: What do primary care patients think about generic drugs? Int. J. Clin. Pharmacol. Ther. 43(10), 472–479 (2005)
Quintal, C., Mendes, P.: Underuse of generic medicines in Portugal: an empirical study on the perceptions and attitudes of patients and pharmacists. Health Policy 104(1), 61–68 (2012). doi:10.1016/j.healthpol.2011.10.001
Mardetko, N., Kos, M.: Patients’ knowledge and attitude towards therapeutic reference pricing system in Slovenia. Int. J. Clin. Pharm. 38(5), 1301–1310 (2016). doi:10.1007/s11096-016-0370-x
Business News Daily: What is a BCG matrix? http://www.businessnewsdaily.com/5693-bcg-matrix.html (2016). Accessed 26 Nov 2016
Stargardt, T.: The impact of reference pricing on switching behaviour and healthcare utilisation: the case of statins in Germany. Eur. J. Health Econ. 11(3), 267–277 (2010). doi:10.1007/s10198-009-0172-3
Duel between the marketing authorisation holders and Health Insurance Institute of Slovenia. http://www.delo.si/zgodbe/ozadja/dvoboj-med-proizvajalci-zdravil-in-zzzs.html (2014). Accessed 20 Nov 2016
Worries about that the mortality would be higher due to therapeutic reference pricing [in Slovenian]. http://www.zurnal24.si/skrbi-jih-da-bo-umrljivost-bolnikov-visja-terapevtske-skupine-zdravil-hipertenzija-clanek-241455 (2014). Accessed 26 Nov 2016
Health Insurance Institute of Slovenia: The national health claims database. https://partner.zzzs.si/wps/portal/portali/aizv/zdravila_in_zivila_za_posebne_zdravstvene_namene/podatki_o_porabi_zdravil/!ut/p/z0/04_Sj9CPykssy0xPLMnMz0vMAfIjo8ziTQxdPd2N_Q08LSyCDQ0cjZzMzXz8XQ0sTAz0C7IdFQGdnpEx/ (2016). Accessed 8 Mar 2017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Marđetko, N., Kos, M. Introduction of therapeutic reference pricing in Slovenia and its economic consequences. Eur J Health Econ 19, 571–584 (2018). https://doi.org/10.1007/s10198-017-0903-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-017-0903-9