Abstract
Purpose
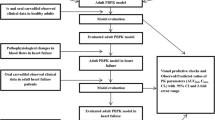
This study was designed to verify a virtual population representing patients with nonalcoholic fatty liver disease (NAFLD) to support the implementation of a physiologically based pharmacokinetic (PBPK) modeling approach for prediction of disease-related changes in drug pharmacokinetics.
Methods
A virtual NAFLD patient population was developed in GastroPlus (v.9.8.2) by accounting for pathophysiological changes associated with the disease and proteomics-informed alterations in the abundance of metabolizing enzymes and transporters pertinent to drug disposition. The NAFLD population model was verified using exemplar drugs where elimination is influenced predominantly by cytochrome P450 (CYP) enzymes (chlorzoxazone, caffeine, midazolam, pioglitazone) or by transporters (rosuvastatin, 11C-metformin, morphine and the glucuronide metabolite of morphine).
Results
PBPK model predictions of plasma concentrations of all the selected drugs and hepatic radioactivity levels of 11C-metformin were consistent with the clinically-observed data. Importantly, the PBPK simulations using the virtual NAFLD population model provided reliable estimates of the extent of changes in key pharmacokinetic parameters for the exemplar drugs, with mean predicted ratios (NAFLD patients divided by healthy individuals) within 0.80- to 1.25-fold of the clinically-reported values, except for midazolam (prediction-fold difference of 0.72).
Conclusion
A virtual NAFLD population model within the PBPK framework was successfully developed with good predictive capability of estimating disease-related changes in drug pharmacokinetics. This supports the use of a PBPK modeling approach for prediction of the pharmacokinetics of new investigational or repurposed drugs in patients with NAFLD and may help inform dose adjustments for drugs commonly used to treat comorbidities in this patient population.










Similar content being viewed by others
Data availability
No new experimental data were generated in this simulation study. The PBPK models used to verify the virtual NAFLD patient population are available from the corresponding authors upon reasonable request.
Abbreviations
- AUC:
-
Area under the plasma concentration–time curve
- BCRP:
-
Breast cancer resistance protein
- BMI:
-
Body mass index
- CBT:
-
13C-caffeine breath test
- CL/F:
-
Oral clearance
- Cmax :
-
Peak plasma concentrations
- Css,min :
-
Trough concentrations
- CYP:
-
Cytochrome P450
- M3G:
-
Morphine-3-glucuronide
- MATE:
-
Multidrug and toxin extrusion
- MRP:
-
Multidrug resistance-associated protein
- NAFL:
-
Nonalcoholic fatty liver or simple steatosis
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- OATP:
-
Organic anion transporting polypeptides
- OCT1:
-
Organic cation transporter 1
- PBPK:
-
Physiologically based pharmacokinetic
- PO:
-
Per os (Oral administration)
- QSP:
-
Quantitative systems pharmacology
- SUV:
-
Standardized uptake values
- UGT:
-
Uridine 5’-diphospho-glucuronosyltransferase
References
Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–64.
Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–47.
Llovet JM, Willoughby CE, Singal AG, Greten TF, Heikenwalder M, El-Serag HB, et al. Nonalcoholic steatohepatitis-related hepatocellular carcinoma: pathogenesis and treatment. Nat Rev Gastroenterol Hepatol. 2023;20(8):487–503.
Younossi ZM, Stepanova M, Ong J, Trimble G, AlQahtani S, Younossi I, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2021;19(3):580–9.
Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. 2022;126(154925):1–18.
Murphy WA, Adiwidjaja J, Sjöstedt N, Yang K, Beaudoin JJ, Spires J, et al. Considerations for physiologically based modeling in liver disease: from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH). Clin Pharmacol Ther. 2023;113(2):275–97.
Kawaguchi-Suzuki M, Bril F, Kalavalapalli S, Cusi K, Frye RF. Concentration-dependent response to pioglitazone in nonalcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;46(1):56–61.
Ferslew BC, Johnston CK, Tsakalozou E, Bridges AS, Paine MF, Jia W, et al. Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther. 2015;97(4):419–27.
Tirona RG, Kassam Z, Strapp R, Ramu M, Zhu C, Liu M, et al. Apixaban and rosuvastatin pharmacokinetics in nonalcoholic fatty liver disease. Drug Metab Dispos. 2018;46(5):485–92.
Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37(3):544–50.
Siler SQ. Applications of quantitative systems pharmacology (QSP) in drug development for NAFLD and NASH and its regulatory application. Pharm Res. 2022;39(8):1789–802.
Young S, Tariq R, Provenza J, Satapathy SK, Faisal K, Choudhry A, et al. Prevalence and profile of nonalcoholic fatty liver disease in lean adults: systematic review and meta-analysis. Hepatol Commun. 2020;4(7):953–72.
VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME, et al. Longitudinal association of non-alcoholic fatty liver disease with changes in myocardial structure and function: the CARDIA study. J Am Heart Assoc. 2020;9(4): e014279.
Shigefuku R, Takahashi H, Kobayashi M, Ikeda H, Matsunaga K, Okuse C, et al. Pathophysiological analysis of nonalcoholic fatty liver disease by evaluation of fatty liver changes and blood flow using xenon computed tomography: can early-stage nonalcoholic steatohepatitis be distinguished from simple steatosis? J Gastroenterol. 2012;47(11):1238–47.
Sase S, Monden M, Oka H, Dono K, Fukuta T, Shibata I. Hepatic blood flow measurements with arterial and portal blood flow mapping in the human liver by means of xenon CT. J Comput Assist Tomogr. 2002;26(2):243–9.
Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transpl. 2005;11(12):1481–93.
Ghobadi C, Johnson TN, Aarabi M, Almond LM, Allabi AC, Rowland-Yeo K, et al. Application of a systems approach to the bottom-up assessment of pharmacokinetics in obese patients: expected variations in clearance. Clin Pharmacokinet. 2011;50(12):809–22.
Jamwal R, de la Monte SM, Ogasawara K, Adusumalli S, Barlock BB, Akhlaghi F. Nonalcoholic fatty liver disease and diabetes are associated with decreased CYP3A4 protein expression and activity in human liver. Mol Pharm. 2018;15(7):2621–32.
Sinha J, Duffull SB, Green B, Al-Sallami HS. Evaluating the relationship between lean liver volume and fat-free mass. Clin Pharmacokinet. 2020;59(4):475–83.
Berton M, Bettonte S, Stader F, Battegay M, Marzolini C. Repository describing the anatomical, physiological, and biological changes in an obese population to inform physiologically based pharmacokinetic models. Clin Pharmacokinet. 2022;61(9):1251–70.
Jamwal R. Effect of non-alcoholic fatty liver disease (NAFLD) on hepatic drug metabolism enzymes in human. Thesis, The University of Rhode Island, 2018, https://doi.org/10.23860/diss-jamwal-rohitash-2018 (accessed on January 9, 2024).
Hardwick RN, Ferreira DW, More VR, Lake AD, Lu Z, Manautou JE, et al. Altered UDP-glucuronosyltransferase and sulfotransferase expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2013;41(3):554–61.
Vildhede A, Kimoto E, Pelis RM, Rodrigues AD, Varma MVS. Quantitative proteomics and mechanistic modeling of transporter-mediated disposition in nonalcoholic fatty liver disease. Clin Pharmacol Ther. 2020;107(5):1128–37.
Fierbinteanu-Braticevici C, Plesca DA, Tribus L, Panaitescu E, Braticevici B. The role of 13C-methacetin breath test for the non-invasive evaluation of nonalcoholic fatty liver disease. J Gastrointestin Liver Dis. 2013;22(2):149–56.
Jones H, Rowland-Yeo K. Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacometrics Syst Pharmacol. 2013;2(8):e63:1–12.
Peters SA. Physiologically based pharmacokinetic (PBPK) modeling and simulations: principles, methods, and applications in the pharmaceutical industry. 2nd ed. John Wiley & Sons, Inc.; 2022.
Ghosh J, Lawless MS, Waldman M, Gombar V, Fraczkiewicz R. Modeling ADMET. Methods Mol Biol. 2016;1425:63–83.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50(Suppl 1):S41-67.
Hens B, Bolger MB. Application of a dynamic fluid and pH model to simulate intraluminal and systemic concentrations of a weak base in GastroPlus™. J Pharm Sci. 2019;108(1):305–15.
Lukacova V, Parrott NJ, Lave T, Fraczkiewicz G, Bolger MB, Woltosz WS. General approach to calculation of tissue:plasma partition coefficients for physiologically based pharmacokinetic (PBPK) modeling. American Association of Pharmaceutical Scientists (AAPS) Meeting, 2008. https://www.simulations-plus.com/assets/Lukacova-General_Approach_Calc_Tissue_Plasma_Partition_Coefficients_PBPK_Modeling-AAPS-2008-1.pdf (accessed on January 9, 2024).
Rodgers T, Rowland M. Mechanistic approaches to volume of distribution predictions: understanding the processes. Pharm Res. 2007;24(5):918–33.
Yamamura Y, Koyama N, Umehara K. Comprehensive kinetic analysis and influence of reaction components for chlorzoxazone 6-hydroxylation in human liver microsomes with CYP antibodies. Xenobiotica. 2015;45(4):353–60.
Lucas D, Ferrara R, Gonzalez E, Bodenez P, Albores A, Manno M, et al. Chlorzoxazone, a selective probe for phenotyping CYP2E1 in humans. Pharmacogenetics. 1999;9(3):377–88.
Smit C, De Hoogd S, Bruggemann RJM, Knibbe CAJ. Obesity and drug pharmacology: a review of the influence of obesity on pharmacokinetic and pharmacodynamic parameters. Expert Opin Drug Metab Toxicol. 2018;14(3):275–85.
Darakjian LI, Kaddoumi A. Physiologically based pharmacokinetic/pharmacodynamic model for caffeine disposition in pregnancy. Mol Pharm. 2019;16(3):1340–9.
Park GJ, Wiseman E, George J, Katelaris PH, Seow F, Fung C, et al. Non-invasive estimation of liver fibrosis in non-alcoholic fatty liver disease using the 13C-caffeine breath test. J Gastroenterol Hepatol. 2011;26(9):1411–6.
Schmilovitz-Weiss H, Niv Y, Pappo O, Halpern M, Sulkes J, Braun M, et al. The 13C-caffeine breath test detects significant fibrosis in patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2008;42(4):408–12.
Perera V, Gross AS, McLachlan AJ. Measurement of CYP1A2 activity: a focus on caffeine as a probe. Curr Drug Metab. 2012;13(5):667–78.
Park GJ, Katelaris PH, Jones DB, Seow F, Le Couteur DG, Ngu MC. Validity of the 13C-caffeine breath test as a noninvasive, quantitative test of liver function. Hepatology. 2003;38(5):1227–36.
Ulvestad M, Skottheim IB, Jakobsen GS, Bremer S, Molden E, Asberg A, et al. Impact of OATP1B1, MDR1, and CYP3A4 expression in liver and intestine on interpatient pharmacokinetic variability of atorvastatin in obese subjects. Clin Pharmacol Ther. 2013;93(3):275–82.
Jaakkola T, Laitila J, Neuvonen PJ, Backman JT. Pioglitazone is metabolised by CYP2C8 and CYP3A4 in vitro: potential for interactions with CYP2C8 inhibitors. Basic Clin Pharmacol Toxicol. 2006;99(1):44–51.
Jaakkola T, Backman JT, Neuvonen M, Neuvonen PJ. Effects of gemfibrozil, itraconazole, and their combination on the pharmacokinetics of pioglitazone. Clin Pharmacol Ther. 2005;77(5):404–14.
Macwan JS, Lukacova V, Fraczkiewicz G. Physiologically based pharmacokinetic modeling of rosuvastatin and prediction of transporter-mediated drug-drug interactions involving rifampicin. BioMedical Transporters Conference, 2019. https://www.simulations-plus.com/assets/BMT-concference-RSTRIF-DDI-07-29-2019-Final-poster.pdf (accessed on January 9, 2024).
Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008;36(10):2014–23.
Bowman CM, Ma F, Mao J, Chen Y. Examination of physiologically-based pharmacokinetic models of rosuvastatin. CPT Pharmacometrics Syst Pharmacol. 2021;10(1):5–17.
Pfeifer ND, Yang K, Brouwer KLR. Hepatic basolateral efflux contributes significantly to rosuvastatin disposition I: characterization of basolateral versus biliary clearance using a novel protocol in sandwich-cultured hepatocytes. J Pharmacol Exp Ther. 2013;347(3):727–36.
Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2011;39(12):2395–402.
Drozdzik M, Szelag-Pieniek S, Post M, Zeair S, Wrzesinski M, Kurzawski M, et al. Protein abundance of hepatic drug transporters in patients with different forms of liver damage. Clin Pharmacol Ther. 2020;107(5):1138–48.
Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther. 2003;25(11):2822–35.
El-Khateeb E, Al-Majdoub ZM, Rostami-Hodjegan A, Barber J, Achour B. Proteomic quantification of changes in abundance of drug-metabolizing enzymes and drug transporters in human liver cirrhosis: different methods, similar outcomes. Drug Metab Dispos. 2021;49(8):610–8.
Emoto C, Fukuda T, Johnson TN, Neuhoff S, Sadhasivam S, Vinks AA. Characterization of contributing factors to variability in morphine clearance through PBPK modeling implemented with OCT1 transporter. CPT Pharmacometrics Syst Pharmacol. 2017;6(2):110–9.
Liang X, Giacomini KM. Transporters involved in metformin pharmacokinetics and treatment response. J Pharm Sci. 2017;106(9):2245–50.
Han TK, Everett RS, Proctor WR, Ng CM, Costales CL, Brouwer KLR, et al. Organic cation transporter 1 (OCT1/mOct1) is localized in the apical membrane of Caco-2 cell monolayers and enterocytes. Mol Pharmacol. 2013;84(2):182–9.
Goud NS, Bhattacharya A, Joshi RK, Nagaraj C, Bharath RD, Kumar P. Carbon-11: radiochemistry and target-based PET molecular imaging applications in oncology, cardiology, and neurology. J Med Chem. 2021;64(3):1223–59.
Sjöstedt N, Neuhoff S, Brouwer KLR. Physiologically-based pharmacokinetic model of morphine and morphine-3-glucuronide in nonalcoholic steatohepatitis. Clin Pharmacol Ther. 2021;109(3):676–87.
Jarvinen E, Deng F, Kiander W, Sinokki A, Kidron H, Sjöstedt N. The role of uptake and efflux transporters in the disposition of glucuronide and sulfate conjugates. Front Pharmacol. 2021;12(802539):1–31.
Elmokadem A, Zhang Y, Knab T, Jordie E, Gillespie WR. Bayesian PBPK modeling using R/Stan/Torsten and Julia/SciML/Turing.Jl. CPT Pharmacometrics Syst Pharmacol. 2023;12(3):300–10.
Guest EJ, Aarons L, Houston JB, Rostami-Hodjegan A, Galetin A. Critique of the two-fold measure of prediction success for ratios: application for the assessment of drug-drug interactions. Drug Metab Dispos. 2011;39(2):170–3.
Harrison SA, Thang C, Bolze S, Dewitt S, Hallakou-Bozec S, Dubourg J, et al. Evaluation of PXL065 - deuterium-stabilized (R)-pioglitazone in patients with NASH: a phase II randomized placebo-controlled trial (DESTINY-1). J Hepatol. 2023;78(5):914–25.
Jacques V, Bolze S, Hallakou-Bozec S, Czarnik AW, Divakaruni AS, Fouqueray P, et al. Deuterium-stabilized (R)-pioglitazone (PXL065) is responsible for pioglitazone efficacy in NASH yet exhibits little to no PPARγ activity. Hepatol Commun. 2021;5(8):1412–25.
Newman EM, Rowland A. A physiologically based pharmacokinetic model to predict the impact of metabolic changes associated with metabolic associated fatty liver disease on drug exposure. Int J Mol Sci. 2022;23(19),11751:1–13.
Nolin TD, Gastonguay MR, Bies RR, Matzke GR, Frye RF. Impaired 6-hydroxychlorzoxazone elimination in patients with kidney disease: implication for cytochrome P450 2E1 pharmacogenetic studies. Clin Pharmacol Ther. 2003;74(6):555–68.
Emery MG, Fisher JM, Chien JY, Kharasch ED, Dellinger EP, Kowdley KV, et al. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology. 2003;38(2):428–35.
El-Khateeb E, Achour B, Al-Majdoub ZM, Barber J, Rostami-Hodjegan A. Non-uniformity of changes in drug-metabolizing enzymes and transporters in liver cirrhosis: implications for drug dosage adjustment. Mol Pharm. 2021;18(9):3563–77.
El-Khateeb E, Achour B, Scotcher D, Al-Majdoub ZM, Athwal V, Barber J, et al. Scaling factors for clearance in adult liver cirrhosis. Drug Metab Dispos. 2020;48(12):1271–82.
O’Malley M, King AN, Conte M, Ellingrod VL, Ramnath N. Effects of cigarette smoking on metabolism and effectiveness of systemic therapy for lung cancer. J Thorac Oncol. 2014;9(7):917–26.
Woolsey SJ, Beaton MD, Choi YH, Dresser GK, Gryn SE, Kim RB, et al. Relationships between endogenous plasma biomarkers of constitutive cytochrome P450 3A activity and single-time-point oral midazolam microdose phenotype in healthy subjects. Basic Clin Pharmacol Toxicol. 2016;118(4):284–91.
Stefan N, Cusi K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–96.
Sigler MA, Congdon L, Edwards KL. An evidence-based review of statin use in patients with nonalcoholic fatty liver disease. Clin Med Insights Gastroenterol. 2018;11(1179552218787502):1–9.
Deng F, Tuomi SK, Neuvonen M, Hirvensalo P, Kulju S, Wenzel C, et al. Comparative hepatic and intestinal efflux transport of statins. Drug Metab Dispos. 2021;49(9):750–9.
Tan SPF, Chan ECY, Chan JCY. Predicting human tissue exposures to xenobiotics using a bottom-up physiologically-based biokinetic model. Altex. 2021;38(2):253–68.
Iwaki Y, Lee W, Sugiyama Y. Comparative and quantitative assessment on statin efficacy and safety: insights into inter-statin and inter-individual variability via dose- and exposure-response relationships. Expert Opin Drug Metab Toxicol. 2019;15(11):897–911.
Sundelin E, Gormsen LC, Jensen JB, Vendelbo MH, Jakobsen S, Munk OL, et al. Genetic polymorphisms in organic cation transporter 1 attenuates hepatic metformin exposure in humans. Clin Pharmacol Ther. 2017;102(5):841–8.
Sundelin EIO, Gormsen LC, Heeboll S, Vendelbo MH, Jakobsen S, Munk OL, et al. Hepatic exposure of metformin in patients with non-alcoholic fatty liver disease. Br J Clin Pharmacol. 2019;85(8):1761–70.
Kuhlmann I, Noddebo Nyrup A, Bjerregaard Stage T, Hougaard Christensen MM, Korshoj Bergmann T, Damkier P, et al. Oral and intravenous pharmacokinetics of metformin with and without oral codeine intake in healthy subjects: a cross-over study. Clin Transl Sci. 2021;14(6):2408–19.
Pierre V, Johnston CK, Ferslew BC, Brouwer KLR, Gonzalez D. Population pharmacokinetics of morphine in patients with nonalcoholic steatohepatitis (NASH) and healthy adults. CPT Pharmacometrics Syst Pharmacol. 2017;6(5):331–9.
Canet MJ, Merrell MD, Hardwick RN, Bataille AM, Campion SN, Ferreira DW, et al. Altered regulation of hepatic efflux transporters disrupts acetaminophen disposition in pediatric nonalcoholic steatohepatitis. Drug Metab Dispos. 2015;43(6):829–35.
Schrieber SJ, Wen Z, Vourvahis M, Smith PC, Fried MW, Kashuba AD, et al. The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos. 2008;36(9):1909–16.
Liu T, Ivaturi V, Gobburu J. Integrated model to describe morphine pharmacokinetics in humans. J Clin Pharmacol. 2019;59(8):1070–7.
Kuhlmann I, Hjelmar Petersen R, Overgaard M, Dornonville de la Cour K, Zwisler S, Bjerregaard Stage T, et al. No significant influence of OCT1 genotypes on the pharmacokinetics of morphine in adult surgical patients. Basic Clin Pharmacol Toxicol. 2022;130(1):93–102.
Verscheijden LFM, Litjens CHC, Koenderink JB, Mathijssen RHJ, Verbeek MM, de Wildt SN, et al. Physiologically based pharmacokinetic/pharmacodynamic model for the prediction of morphine brain disposition and analgesia in adults and children. PLoS Comput Biol. 2021;17(3):e1008786:1–21.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21.
Brouwer KLR, Evers R, Hayden E, Hu S, Li CY, Meyer Zu Schwabedissen HE, et al. Regulation of drug transport proteins-from mechanisms to clinical impact: a white paper on behalf of the International Transporter Consortium. Clin Pharmacol Ther. 2022;112(3):461–84.
Cunningham RP, Porat-Shliom N. Liver zonation - revisiting old questions with new technologies. Front Physiol. 2021;12(732929):1–17.
Woolsey SJ, Mansell SE, Kim RB, Tirona RG, Beaton MD. CYP3A activity and expression in nonalcoholic fatty liver disease. Drug Metab Dispos. 2015;43(10):1484–90.
Manitpisitkul P, Curtin CR, Shalayda K, Wang SS, Ford L, Heald D. Pharmacokinetic interactions between topiramate and pioglitazone and metformin. Epilepsy Res. 2014;108(9):1519–32.
Gormsen LC, Sundelin EI, Jensen JB, Vendelbo MH, Jakobsen S, Munk OL, et al. In vivo imaging of human 11C-metformin in peripheral organs: dosimetry, biodistribution, and kinetic analyses. J Nucl Med. 2016;57(12):1920–6.
Hohmann N, Blank A, Burhenne J, Suzuki Y, Mikus G, Haefeli WE. Simultaneous phenotyping of CYP2E1 and CYP3A using oral chlorzoxazone and midazolam microdoses. Br J Clin Pharmacol. 2019;85(10):2310–20.
Wang Z, Hall SD, Maya JF, Li L, Asghar A, Gorski JC. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol. 2003;55(1):77–85.
Perera V, Gross AS, Xu H, McLachlan AJ. Pharmacokinetics of caffeine in plasma and saliva, and the influence of caffeine abstinence on CYP1A2 metrics. J Pharm Pharmacol. 2011;63(9):1161–8.
Kamimori GH, Karyekar CS, Otterstetter R, Cox DS, Balkin TJ, Belenky GL, et al. The rate of absorption and relative bioavailability of caffeine administered in chewing gum versus capsules to normal healthy volunteers. Int J Pharm. 2002;234(1–2):159–67.
Lappin G, Kuhnz W, Jochemsen R, Kneer J, Chaudhary A, Oosterhuis B, et al. Use of microdosing to predict pharmacokinetics at the therapeutic dose: experience with 5 drugs. Clin Pharmacol Ther. 2006;80(3):203–15.
Halama B, Hohmann N, Burhenne J, Weiss J, Mikus G, Haefeli WE. A nanogram dose of the CYP3A probe substrate midazolam to evaluate drug interactions. Clin Pharmacol Ther. 2013;93(6):564–71.
Eap CB, Buclin T, Cucchia G, Zullino D, Hustert E, Bleiber G, et al. Oral administration of a low dose of midazolam (75 microg) as an in vivo probe for CYP3A activity. Eur J Clin Pharmacol. 2004;60(4):237–46.
Tornio A, Niemi M, Neuvonen PJ, Backman JT. Trimethoprim and the CYP2C8*3 allele have opposite effects on the pharmacokinetics of pioglitazone. Drug Metab Dispos. 2008;36(1):73–80.
Sirtori CR, Franceschini G, Galli-Kienle M, Cighetti G, Galli G, Bondioli A, et al. Disposition of metformin (N, N-dimethylbiguanide) in man. Clin Pharmacol Ther. 1978;24(6):683–93.
Hoskin PJ, Hanks GW, Aherne GW, Chapman D, Littleton P, Filshie J. The bioavailability and pharmacokinetics of morphine after intravenous, oral and buccal administration in healthy volunteers. Br J Clin Pharmacol. 1989;27(4):499–505.
Lotsch J, Skarke C, Schmidt H, Liefhold J, Geisslinger G. Pharmacokinetic modeling to predict morphine and morphine-6-glucuronide plasma concentrations in healthy young volunteers. Clin Pharmacol Ther. 2002;72(2):151–62.
Stuart-Harris R, Joel SP, McDonald P, Currow D, Slevin ML. The pharmacokinetics of morphine and morphine glucuronide metabolites after subcutaneous bolus injection and subcutaneous infusion of morphine. Br J Clin Pharmacol. 2000;49(3):207–14.
Osborne R, Joel S, Trew D, Slevin M. Morphine and metabolite behavior after different routes of morphine administration: demonstration of the importance of the active metabolite morphine-6-glucuronide. Clin Pharmacol Ther. 1990;47(1):12–9.
Acknowledgements
The authors are grateful to Drs. Viera Lukacova (PBPK Solutions, Simulations Plus, Inc.), and also Kyunghee Yang and James Beaudoin (Quantitative Systems Pharmacology Solutions, Simulations Plus, Inc.) for helpful suggestions and for providing feedback on the manuscript. We would like to thank Dr. Scott Siler (Quantitative Systems Pharmacology Solutions, Simulations Plus, Inc.) for scientific discussions on the NAFLD patient population. Dr. Ke Szeto (formerly at Simulations Plus, Inc.; current affiliation: Incyte Corporation) provided guidance and technical assistance during the initial stages of the project. Components of this work have been presented at the International Society for the Study of Xenobiotics (ISSX) and the Microsomes and Drug Oxidations (MDO) Joint Meeting in September 2022.
Funding
During this study, JA received financial support from Simulations Plus, Inc. for his postdoctoral training.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
JS is an employee and shareholder of Simulations Plus, Inc. Following the completion of this study, JA joined Simulations Plus, Inc. as an employee. KLRB has received financial support for a PhD Studentship awarded to Dr. William Murphy from a Certara Simcyp 2020/2021 Grant and Partnership Scheme (GPS).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adiwidjaja, J., Spires, J. & Brouwer, K.L.R. Physiologically Based Pharmacokinetic (PBPK) Model Predictions of Disease Mediated Changes in Drug Disposition in Patients with Nonalcoholic Fatty Liver Disease (NAFLD). Pharm Res 41, 441–462 (2024). https://doi.org/10.1007/s11095-024-03664-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-024-03664-8