Abstract
Objective
This study aimed to determine the impact of formulation (gel vs cream) and microneedle characteristics (length, number) on permeation of metronidazole through excised microneedle-treated skin. The long-term goal is to apply these results towards a pharmacokinetic study in human subjects with diverse skin types, using in vitro flux data to determine dosing conditions and ultimately establish in vitro-in vivo correlations.
Methods
Metronidazole release from 0.75% gel and cream was quantified with flow-through diffusion cells, using a cellulose membrane. Excised porcine skin was treated with stainless steel microneedles (500 or 800 μm length), to create 50 or 100 micropores. Metronidazole gel or cream was applied to microneedle-treated skin and replaced every 48 h for up to 7 days. Metronidazole permeation was quantified using HPLC. Intact skin (no microneedle treatment) served as controls.
Results
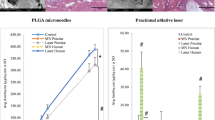
Metronidazole release was faster from the gel vs cream. At 7 days there was no difference between gel vs cream in total metronidazole permeated through intact skin. For both formulations, metronidazole permeation was significantly higher (vs intact skin) following microneedle application, regardless of microneedle length or micropore number. Increasing microneedle length and micropore number enhanced MTZ permeation multiple fold for both gel and cream. The greatest enhancement in total permeation for both formulations was achieved with the 800 μm MN, 100 micropore condition.
Conclusions
Formulation and microneedle conditions both impacted metronidazole permeation. These data will be used to estimate in vivo serum concentrations after applying metronidazole to microneedle-treated skin in humans.





Similar content being viewed by others
References
Phatale V, Vaiphei KK, Jha S, Patil D, Agrawal M, Alexander A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J Control Release. 2022;351:361–80.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–8.
Bolla PK, Clark BA, Juluri A, Cheruvu HS, Renukuntla J. Evaluation of formulation parameters on permeation of ibuprofen from topical formulations using Strat-M® membrane. Pharmaceutics. 2020;12(2):151.
Tokumoto S, Higo N, Sugibayashi K. Effect of electroporation and pH on the iontophoretic transdermal delivery of human insulin. Int J Pharm. 2006;326(1–2):13–9.
Vávrová K, Lorencová K, Klimentová J, Novotný J, Hrabálek A. Transdermal and dermal delivery of adefovir: effects of pH and permeation enhancers. Eur J Pharm Biopharm. 2008;69(2):597–604.
Djekic L, Martinovic M, Stepanović-Petrović R, Micov A, Tomić M, Primorac M. Formulation of hydrogel-thickened nonionic microemulsions with enhanced percutaneous delivery of ibuprofen assessed in vivo in rats. Eur J Pharm Sci. 2016;92:255–65.
Tobin KV, Fiegel J, Brogden NK. Thermosensitive Gels Used to Improve Microneedle-Assisted Transdermal Delivery of Naltrexone. Polymers (Basel). 2021;13(6):933.
Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–7.
Rath S, Kanfer I. A Validated IVRT Method to Assess Topical Creams Containing Metronidazole Using a Novel Approach. Pharmaceutics. 2020;12(2):119.
Russo J, Fiegel J, Brogden NK. Effect of Salt Form on Gelation and Drug Delivery Properties of Diclofenac-Loaded Poloxamer Gels for Delivery to Impaired Skin. Pharm Res. 2022;39(10):2515–27.
Hoare TR, Kohane DS. Hydrogels in drug delivery: Progress and challenges. Polymer. 2008;49(8):1993–2007.
Patel NA, Patel NJ, Patel RP. Comparative development and evaluation of topical gel and cream formulations of psoralen. Drug Discov Ther. 2009;3(5):234–42.
Jabir SA, Sulaiman HT, Ayash N. Preparation, Characterization and In-Vitro Diffusion Study of Different Topical Flurbiprofen Semisolids. Int J Drug Deliv Technol. 2020;10(1):81–7.
Russo J, Fiegel J, Brogden NK. Rheological and Drug Delivery Characteristics of Poloxamer-Based Diclofenac Sodium Formulations for Chronic Wound Site Analgesia. Pharmaceutics. 2020;12(12):1214.
Cho YK, Choi HK. Enhancement of percutaneous absorption of ketoprofen: effect of vehicles and adhesive matrix. Int J Pharm. 1998;169(1):95–104.
Gao X, Brogden NK. Development of Hydrogels for Microneedle-Assisted Transdermal Delivery of Naloxone for Opioid-Induced Pruritus. J Pharm Sci. 2019;108(11):3695–703.
Tobin KV, Brogden NK. Thermosensitive biomaterial gels with chemical permeation enhancers for enhanced microneedle delivery of naltrexone for managing opioid and alcohol dependency. Biomater Sci. 2023;11(17):5846–58.
Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105(6):2058–63.
Kirkby M, Hutton ARJ, Donnelly RF. Microneedle Mediated Transdermal Delivery of Protein, Peptide and Antibody Based Therapeutics: Current Status and Future Considerations. Pharm Res. 2020;37(6):117.
Alkilani AZ, McCrudden MT, Donnelly RF. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics. 2015;7(4):438–70.
Nalluri BN, Uppuluri C, Devineni J, Nayak A, Nair KJ, Whiteside BR, et al. Effect of microneedles on transdermal permeation enhancement of amlodipine. Drug Deliv Transl Res. 2017;7(3):383–94.
Puri A, Frempong D, Mishra D, Dogra P. Microneedle-mediated transdermal delivery of naloxone hydrochloride for treatment of opioid overdose. Int J Pharm. 2021;604:120739.
Oh JH, Park HH, Do KY, Han M, Hyun DH, Kim CG, et al. Influence of the delivery systems using a microneedle array on the permeation of a hydrophilic molecule, calcein. Eur J Pharm Biopharm. 2008;69(3):1040–5.
Song Y, Hemmady K, Puri A, Banga AK. Transdermal delivery of human growth hormone via laser-generated micropores. Drug Deliv Transl Res. 2018;8(2):450–60.
Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011;154(2):148–55.
Ogunjimi AT, Carr J, Lawson C, Ferguson N, Brogden NK. Micropore closure time is longer following microneedle application to skin of color. Sci Rep. 2020;10(1):1–14.
Ogunjimi AT, Lawson C, Carr J, Patel KK, Ferguson N, Brogden NK. Micropore Closure Rates following Microneedle Application at Various Anatomical Sites in Healthy Human Subjects. Skin Pharmacol Physiol. 2021;34(4):214–28.
Singh I, Morris AP. Performance of transdermal therapeutic systems: Effects of biological factors. Int J Pharm Investig. 2011;1(1):4.
Brogden NK, Banks SL, Crofford LJ, Stinchcomb AL. Diclofenac enables unprecedented week-long microneedle-enhanced delivery of a skin impermeable medication in humans. Pharm Res. 2013;30(8):1947–55.
Acknowledgements
The authors are grateful to Kevin Tobin, Valeria Cota, Jackson Russo, and Heena Maithania for providing technical and scientific support, as well as Yub Raj Neupane for editorial and writing assistance.
Funding
This work was supported by the National Institutes of Health, awards R35GM124551 and R35GM149337. Also, the funding statement is listed twice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, K.K., Brogden, N.K. Impact of Formulation and Microneedle Length on Transdermal Metronidazole Permeation through Microneedle-Treated Skin. Pharm Res 41, 355–363 (2024). https://doi.org/10.1007/s11095-023-03640-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03640-8