Abstract
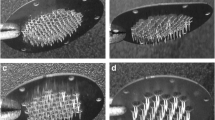
The present study aimed to investigate the effect of microneedle (MN) geometry parameters like length, density, shape and type on transdermal permeation enhancement of amlodipine (AMLO). Two types of MN devices viz. AdminPatch® arrays (ADM) (0.6, 1.2 and 1.5 mm lengths) and laboratory-fabricated polymeric MNs (PM) of 0.6 mm length were employed. In the case of PMs, arrays were applied thrice at different places within a 1.77-cm2 skin area (PM-3) to maintain the MN density closer to 0.6 mm ADM. Scaling analyses were done using dimensionless parameters like concentration of AMLO (Ct/Cs), thickness (h/L) and surface area of the skin (Sa/L2). Microinjection moulding technique was employed to fabricate PM. Histological studies revealed that the PM, owing to their geometry/design, formed wider and deeper microconduits when compared to ADM of similar length. Approximately 6.84- and 6.11-fold increase in the cumulative amount (48 h) of AMLO permeated was observed with 1.5 mm ADM and PM-3 treatments respectively, when compared to passive permeation amounts. Good correlations (R 2 > 0.89) were observed between different dimensionless parameters with scaling analyses. The enhancement in AMLO permeation was found to be in the order of 1.5 mm ADM ≥ PM-3 > 1.2 mm ADM > 0.6 mm ADM ≥PM-1 > passive. The study suggests that MN application enhances the AMLO transdermal permeation and the geometrical parameters of MNs play an important role in the degree of such enhancement.








Similar content being viewed by others
References
Brown MB, Gary PM, Stuart AJ, Franklin KA. Dermal and transdermal drug delivery systems: current and future prospects. Drug Delivery. 2006;13:175–87.
Prausnitz MR, Robert L. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–8.
Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the US: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief, No. 133. Hyattsville: National Center for Health Statistics, Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2013.
Lim SS, Vos T, Flaxman AD, Danaei G. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60.
Suchanova B, Kostiainen K, Ketola RA. Characterization of the in vitro metabolic profile of amlodipine in rat using liquid chromatography–mass spectrometry. Eur J Pharm Sci. 2008;33:91–9.
Hardman JG, Limbird LE. Goodman and Gilman’s the pharmacological basis of therapeutics, vol. 10. New Delhi: McGraw Hill Medical Publishing Division; 2001. p. 853–60.
McDaid DM, Deasy PB. Formulations development of a transdermal drug delivery system for amlodipine base. Int J Pharm. 1996;133:71–83.
Hemangi JP, Jitendra SP, Desai BG, Keyur DP. Permeability studies of anti-hypertensive drug amlodipine besilate for transdermal delivery. Asian Journal of Pharmaceutical and Clinical Research. 2010;3:31–4.
Sanju N, Kamal S, Bhavna Y, Benika S. Formulation and characterization of transdermal patch of amlodipine besylate. International Journal of Pharmaceutical and Chemical Sciences. 2012;1:953–69.
Lincy J, Arun K. Comparison of amlodipine transdermal patches using hydroxypropylmethylcellulose and chitosan. Asian J Pharm Clin Res. 2014;7:86–90.
Han T, Das DB. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: a review. Eur J Pharm Biopharm. 2015;89:312–28.
Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. 2008;24:585–94.
Jeong W, Lee A, Jung-Hwan PB. Prausnitz MR. Dissolving microneedles for transdermal drug delivery biomaterials. 2008;29:2113–24.
Zhou CP, Liu YL, Wang HL, Zhang PX, Zhang JL. Transdermal delivery of insulin using microneedle rollers in vivo. Int J Pharm. 2010;39:127–33.
Nalluri BN, Sai Sri Anusha V, Bramhini SR, Amulya J, Sultana AS, Teja UC, Das DB. In vitro skin permeation enhancement of sumatriptan by microneedle application. Curr Drug Deliv 2015;12:761–769.
Monika K, Kevin BI, Inna EP, Sanjai JP, Daniel AB. Microneedle-assisted delivery of verapamil hydrochloride and amlodipine besylate. Eur J Pharm Biopharm. 2014;86:284–91.
Gomaa YA, Morrow DI, Garland MJ, Donnelly RF, El-Khordagui LK, Meidan VM. Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: assessments by transepidermal water loss. Toxicol in Vitro. 2010;24:1971–8.
Chueng K, Han T, Das DB. Effect of force of microneedle insertion on the permeability of insulin in skin. J Diabetes Sci Technol. 2014;8:444–52.
Attia UM, Marsona S, Alcock JR. Micro-injection moulding of polymer microfluidic devices. Microfluidics and nano fluidics. 2009;7:1–28.
Nair KJ, Whiteside BR, Grant C, Patel R, Tuinea-Bobe C, Norris K, Paradkar AR. Investigation of plasma treatment on micro-injection moulded microneedle for drug delivery. Pharmaceutics. 2015;7:471–85.
Al-Qallaf B, Das DB, Mori D, Cui Z. Modelling transdermal delivery of high molecular weight drugs from microneedle systems. Phil Trans R Soc A. 2007;365:2951–67.
Leeladurga V, Teja UC, Ashraf SS, Sundeep K, Sai Sri Anusha V, Han T, Nalluri BN, Das DB. Application of microneedle arrays for enhancement of transdermal permeation of insulin: in vitro experiments, scaling analyses and numerical simulations. AAPS Pharma Sci Tech 2016;17(4):915–922.
Vadim VY. US Patent No. 7658728 B2. Washington DC: U.S. Patent and Trademark Office; 2010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was funded jointly by the Department of Science and Technology (DST), Ministry of Science and Technology, Govt. of India, and the British Council, London, UK, under DST-UKIERI scheme (DST/INT/UK/P-60/2014) to Buchi N. Nalluri and Diganta B. Das. All the authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nalluri, B.N., Uppuluri, C., Devineni, J. et al. Effect of microneedles on transdermal permeation enhancement of amlodipine. Drug Deliv. and Transl. Res. 7, 383–394 (2017). https://doi.org/10.1007/s13346-017-0361-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0361-z