Abstract
Objectives
Methotrexate (MTX) is subject to therapeutic drug monitoring because of its high pharmacokinetic variability and safety risk outside the therapeutic window. This study aimed to develop a population pharmacokinetic model (popPK) of MTX for Brazilian pediatric acute lymphoblastic leukemia (ALL) patients who attended the Hospital de Clínicas de Porto Alegre, Brazil.
Methods
The model was developed using NONMEM 7.4 (Icon®), ADVAN3 TRANS4, and FOCE-I. To explain inter-individual variability, we evaluated covariates from demographic, biochemical, and genetic data (single nucleotide polymorphisms [SNPs] related to the transport and metabolism of drugs).
Results
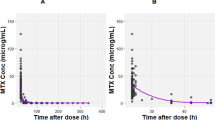
A two-compartment model was built using 483 data points from 45 patients (0.33–17.83 years of age) treated with MTX (0.25–5 g/m2) in different cycles. Serum creatinine (SCR), height (HT), blood urea nitrogen (BUN) and a low BMI stratification (according to the z-score defined by the World Health Organization [LowBMI]) were added as clearance covariates. The final model described MTX clearance as \(CL (L/h)= 8.02 \;x {\left(\frac{SCR}{{Median}_{SCR}}\right)}^{-0.401} \;x\; {{\left(\frac{HT}{{Median}_{HT}}\right)}^{0.957}\;x\; {\left(\frac{BUN}{{Median}_{BUN}}\right)}^{-0.181}\;x\; \left(1-0.266\right)}_{LowBMI}\). In the two-compartment structural model, the central and peripheral compartment volumes were 26.8 L and 8.47 L, respectively, and the inter-compartmental clearance was 0.218 L/h. External validation of the model was performed through a visual predictive test and metrics using data from 15 other pediatric ALL patients.
Conclusion
The first popPK model of MTX was developed for Brazilian pediatric ALL patients, which showed that inter-individual variability was explained by renal function and factors related to body size.



Similar content being viewed by others
Data Availability
The raw data used in this study is available upon direct request to the authors.
References
Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012;119(1):34–43. https://doi.org/10.1182/blood-2011-04-347872.
Chibber S, Hassan I, Farhan M, Naseem I. In vitro pro-oxidant action of Methotrexate in presence of white light. J Photochem Photobiol B. 2011;104(3):387–93. https://doi.org/10.1016/j.jphotobiol.2011.04.005.
Chan ES, Cronstein BN. Mechanisms of action of methotrexate. Bull Hosp Jt Dis. 2013;2013(71 Suppl 1):S5–8.
Gao B, Klumpen H-J, Gurney H. Defining the Starting Dose: Should It Be mg/kg, mg/m2, or Fixed? In: Rudek MA, editors. Handbook of Anticancer Pharmacokinetics and Pharmacodynamics. 2 ed. Baltimore, USA: Humana Press; 2014. p. 69–87.
Bleyer WA. The clinical pharmacology of methotrexate: new applications of an old drug. Cancer. 1978;41(1):36–51. https://doi.org/10.1002/1097-0142(197801)41:1%3c36::aid-cncr2820410108%3e3.0.co;2-i.
Gaies E, Jebabli N. Methotrexate side effects: Review article. J Drug Metab Toxicol. 2012;3(4). https://doi.org/10.4172/2157-7609.1000125.
Levêque D, Becker G, Toussaint E, Fornecker L-M, Paillard C. Clinical pharmacokinetics of methotrexate in oncology. Int J Pharmacokinet. 2017;2(2):137–47. https://doi.org/10.4155/ipk-2016-0022.
Campbell M, Kiss C, Zimmermann M, et al. Childhood acute lymphoblastic leukemia: Results of the randomized acute lymphoblastic leukemia intercontinental-Berlin-Frankfurt-Münster 2009 Trial. J Clin Oncol. 2023;JCO2201760. https://doi.org/10.1200/JCO.22.01760.
Stary J, Zimmermann M, Campbell M, et al. Intensive chemotherapy for childhood acute lymphoblastic leukemia: results of the randomized intercontinental trial ALL IC-BFM 2002. J Clin Oncol. 2014;32(3):174–184. https://doi.org/10.1200/JCO.2013.48.6522.
Brandalise SR, Pinheiro VR, Aguiar SS, et al. Benefits of the intermittent use of 6 mercaptopurine and methotrexate in maintenance treatment for low-risk acute lymphoblastic leukemia in children: randomized trial from the Brazilian Childhood Cooperative Group--protocol ALL-99. J Clin Oncol. 2010;28(11):1911–1918. https://doi.org/10.1200/JCO.2009.25.6115.
Pieters R, De Lorenzo P, Ancliffe P, et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the interfant-06 protocol: results from an international phase III randomized study. J Clin Oncol. 2019;37(25):2246–2256. https://doi.org/10.1200/JCO.19.00261.
Vilmer E, Suciu S, Ferster A, et al. Long-term results of three randomized trials (58831, 58832, 58881) in childhood acute lymphoblastic leukemia: a CLCG-EORTC report. Children Leukemia Cooperative Group. Leukemia. 2000;14(12):2257-2266. https://doi.org/10.1038/sj.leu.2401960.
Maloney KW, Devidas M, Wang C, et al. Outcome in children with standard-risk B-cell acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0331. J Clin Oncol. 2020;38(6):602–12. https://doi.org/10.1200/JCO.19.01086.
Evans W, Schentag JJ, Jusko WJ. Applied Pharmacokinetics: Principles of Therapeutic Drug Monitoring. 3 ed. Vancouver: Applied Therapeutics; 1992. p. 617–636.
Winter ME. Basic Clinical Pharmacokinetics. 3 ed. Vancouver: Applied Therapeutics; 1994. p. 266–288.
Aumente D, Buelga DS, Lukas JC, Gomez P, Torres A, García MJ. Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet. 2006;45(12):1227–38. https://doi.org/10.2165/00003088-200645120-00007.
Beechinor RJ, Thompson PA, Hwang MF, et al. The population pharmacokinetics of high-dose methotrexate in infants with acute lymphoblastic leukemia highlight the need for bedside individualized dose adjustment: a report from the Children’s Oncology Group. Clin Pharmacokinet. 2019;58(7):899–910. https://doi.org/10.1007/s40262-018-00734-0.
Buitenkamp TD, Mathôt RA, de Haas V, Pieters R, Zwaan CM. Methotrexate-induced side effects are not due to differences in pharmacokinetics in children with Down syndrome and acute lymphoblastic leukemia. Haematologica. 2010;95(7):1106–13. https://doi.org/10.3324/haematol.2009.019778.
Hui KH, Chu HM, Fong PS, Cheng WTF, Lam TN. Population pharmacokinetic study and individual dose adjustments of high-dose methotrexate in chinese pediatric patients with acute lymphoblastic leukemia or osteosarcoma. J Clin Pharmacol. 2019;59(4):566–77. https://doi.org/10.1002/jcph.1349.
FaganelKotnik B, Grabnar I, Bohanec Grabar P, Dolžan V, Jazbec J. Association of genetic polymorphism in the folate metabolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukaemia and malignant lymphoma. Eur J Clin Pharmacol. 2011;67(10):993–1006. https://doi.org/10.1007/s00228-011-1046-z.
Medellin-Garibay SE, Hernández-Villa N, Correa-González LC, et al. Population pharmacokinetics of methotrexate in Mexican pediatric patients with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2020;85(1):21–31. https://doi.org/10.1007/s00280-019-03977-1.
Odoul F, Le Guellec C, Lamagnère JP, et al. Prediction of methotrexate elimination after high dose infusion in children with acute lymphoblastic leukaemia using a population pharmacokinetic approach. Fundam Clin Pharmacol. 1999;13(5):595–604. https://doi.org/10.1111/j.1472-8206.1999.tb00366.x.
Plard C, Bressolle F, Fakhoury M, et al. A limited sampling strategy to estimate individual pharmacokinetic parameters of methotrexate in children with acute lymphoblastic leukemia [published correction appears in Cancer Chemother Pharmacol. 2007 Sep;60(4):621. Piard, Christine [corrected to Plard, Christine]]. Cancer Chemother Pharmacol. 2007;60(4):609–620. https://doi.org/10.1007/s00280-006-0394-3.
Schulte RR, Choi L, Utreja N, Van Driest SL, Stein CM, Ho RH. Effect of SLCO1B1 polymorphisms on high-dose methotrexate clearance in children and young adults with leukemia and lymphoblastic lymphoma. Clin Transl Sci. 2021;14(1):343–53. https://doi.org/10.1111/cts.12879.
Anderson BJ, Allegaert K, Holford NH. Population clinical pharmacology of children: general principles. Eur J Pediatr. 2006;165(11):741–6. https://doi.org/10.1007/s00431-006-0188-y.
Tagen M, Stewart CF. Clinical Pharmacology in Pediatrics. In: Teicher BA, Rudek MA, Chau CH, Figg WD, Mcleod HL, editors. Handbook of Anticancer Pharmacokinetics and Pharmacodynamics. 2 ed. New York: Humana Press; 2014. 625 p.
De Mattia E, Toffoli G. C677T and A1298C MTHFR polymorphisms, a challenge for antifolate and fluoropyrimidine-based therapy personalisation. Eur J Cancer. 2009;45(8):1333–51. https://doi.org/10.1016/j.ejca.2008.12.004.
Lui G, Treluyer JM, Fresneau B, et al. A pharmacokinetic and pharmacogenetic analysis of osteosarcoma patients treated with high-dose methotrexate: data from the OS2006/Sarcoma-09 Trial. J Clin Pharmacol. 2018;58(12):1541–9. https://doi.org/10.1002/jcph.1252.
Kim IW, Yun HY, Choi B, et al. ABCB1 C3435T genetic polymorphism on population pharmacokinetics of methotrexate after hematopoietic stem cell transplantation in Korean patients: a prospective analysis. Clin Ther. 2012;34(8):1816–26. https://doi.org/10.1016/j.clinthera.2012.06.022.
Frenia ML, Long KS. Methotrexate and nonsteroidal antiinflammatory drug interactions. Ann Pharmacother. 1992;26(2):234–7. https://doi.org/10.1177/106002809202600219.
Inose R, Hashimoto N, Hosomi K, Yokoyama S, Takada M. Association between malignancy and methotrexate and biological disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Int J Clin Pharmacol Ther. 2020;58(3):131–8. https://doi.org/10.5414/CP203604.
Beorlegui B, Aldaz A, Ortega A, Aquerreta I, Sierrasesúmega L, Giráldez J. Potential interaction between methotrexate and omeprazole. Ann Pharmacother. 2000;34(9):1024–7. https://doi.org/10.1345/aph.19094.
Kitaoka S, Terasawa M, Goto E, Miyaji T, Tsuchiya S, Konno T. Trimethoprim interference in methotrexate assay by an enzyme inhibition assay kit. Tohoku J Exp Med. 1986;150(4):481–2. https://doi.org/10.1620/tjem.150.481.
Csordas K, Lautner-Csorba O, Semsei AF, et al. Associations of novel genetic variations in the folate-related and ARID5B genes with the pharmacokinetics and toxicity of high-dose methotrexate in paediatric acute lymphoblastic leukaemia. Br J Haematol. 2014;166(3):410–20. https://doi.org/10.1111/bjh.12886.
Wang SM, Sun LL, Zeng WX, Wu WS, Zhang GL. Influence of genetic polymorphisms of FPGS, GGH, and MTHFR on serum methotrexate levels in Chinese children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2014;74(2):283–9. https://doi.org/10.1007/s00280-014-2507-8.
Imanishi H, Okamura N, Yagi M, et al. Genetic polymorphisms associated with adverse events and elimination of methotrexate in childhood acute lymphoblastic leukemia and malignant lymphoma. J Hum Genet. 2007;52(2):166–71. https://doi.org/10.1007/s10038-006-0096-z.
Chiabai MA, Lins TC, Pogue R, Pereira RW. Population analysis of pharmacogenetic polymorphisms related to acute lymphoblastic leukemia drug treatment. Dis Markers. 2012;32(4):247–53. https://doi.org/10.3233/DMA-2011-0884.
Giletti A, Esperon P. Genetic markers in methotrexate treatments. Pharmacogenomics J. 2018;18(6):689–703. https://doi.org/10.1038/s41397-018-0047-z.
Simon N, Marsot A, Villard E, et al. Impact of ABCC2 polymorphisms on high-dose methotrexate pharmacokinetics in patients with lymphoid malignancy. Pharmacogenomics J. 2013;13(6):507–13. https://doi.org/10.1038/tpj.2012.37.
Owen JS, Fiedler-Kelly J. Introduction to population pharmacokinetic/pharmacodynamic analysis with nonlinear mixed effects models. Hoboken, NJ: Wiley; 2014.
Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–37. https://doi.org/10.1681/ASN.2008030287.
Body mass inder-for-age (BMI-for-age). World Health Organization. https://www.who.int/toolkits/child-growth-standards/standards/body-mass-index-for-age-bmi-for-age. Accessed 20 Oct 2021.
El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther. 2007;320(1):229–35. https://doi.org/10.1124/jpet.106.110379.
Colebatch AN, Marks JL, van der Heijde DM, Edwards CJ. Safety of nonsteroidal antiinflammatory drugs and/or paracetamol in people receiving methotrexate for inflammatory arthritis: a Cochrane systematic review. J Rheumatol Suppl. 2012;90:62–73. https://doi.org/10.3899/jrheum.120345.
Wiczer T, Dotson E, Tuten A, Phillips G, Maddocks K. Evaluation of incidence and risk factors for high-dose methotrexate-induced nephrotoxicity. J Oncol Pharm Pract. 2016;22(3):430–6. https://doi.org/10.1177/1078155215594417.
Bezabeh S, Mackey AC, Kluetz P, Jappar D, Korvick J. Accumulating evidence for a drug-drug interaction between methotrexate and proton pump inhibitors. Oncologist. 2012;17(4):550–4. https://doi.org/10.1634/theoncologist.2011-0431.
Ma SN, Zaman Huri H, Yahya F. Drug-related problems in patients with rheumatoid arthritis. Ther Clin Risk Manag. 2019;15:505–524. Published 2019 Mar 21. https://doi.org/10.2147/TCRM.S194921.
Bagatini F, Blatt CR, Maliska G, et al. Potential drug interactions in patients with rheumatoid arthritis. Rev Bras Reumatol. 2011;51(1):20–39.
Johansson ÅM. Methodology for handling missing data in nonlinear mixed effects modelling. In: Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 189. Uppsala: Acta Universitatis Upsaliensis; 2014. p. 75.
Zhang Z. Missing data imputation: focusing on single imputation. Ann Transl Med. 2016;4(1):9. https://doi.org/10.3978/j.issn.2305-5839.2015.12.38.
Bloomfield C, et al. Assessing predictive performance of published population pharmacokinetic models of intravenous tobramycin in pediatric patients. Antimicrob Agents Chemother. 2016;60:3407–14. https://doi.org/10.1128/AAC.02654-15.
Wang S, et al. External evaluation of population pharmacokinetic models of methotrexate for model-informed precision dosing in pediatric patients with acute lymphoid leukemia. Pharmaceutics. 2023;15:569. https://doi.org/10.3390/pharmaceutics15020569.
Rausch CR, Jabbour EJ, Kantarjian HM, Kadia TM. Optimizing the use of the hyperCVAD regimen: clinical vignettes and practical management. Cancer. 2020;126(6):1152–60. https://doi.org/10.1002/cncr.32606.
Eckert C, Parker C, Moorman AV, Irving JA, Kirschner-Schwabe R, Groeneveld-Krentz S, Révész T, Hoogerbrugge P, Hancock J, Sutton R, Henze G, Chen-Santel C, Attarbaschi A, Bourquin JP, Sramkova L, Zimmermann M, Krishnan S, von Stackelberg A, Saha V. Risk factors and outcomes in children with high-risk B-cell precursor and T-cell relapsed acute lymphoblastic leukaemia: combined analysis of ALLR3 and ALL-REZ BFM 2002 clinical trials. Eur J Cancer. 2021;151:175–89. https://doi.org/10.1016/j.ejca.2021.03.034.
Stanczyk M, Sliwinski T, Trelinska J, Cuchra M, Markiewicz L, Dziki L, Bieniek A, Bielecka-Kowalska A, Kowalski M, Pastorczak A, Szemraj J, Mlynarski W, Majsterek I. Role of base-excision repair in the treatment of childhood acute lymphoblastic leukaemia with 6-mercaptopurine and high doses of methotrexate. Mutat Res. 2012;741(1–2):13–21. https://doi.org/10.1016/j.mrgentox.2011.10.009.
Woillard JB, Debord J, Benz-de-Bretagne I, et al. A time-dependent model describes methotrexate elimination and supports dynamic modification of MRP2/ABCC2 activity. Ther Drug Monit. 2017;39(2):145–56. https://doi.org/10.1097/FTD.0000000000000381.
Triarico S, Rinninella E, Cintoni M, Capozza MA, Mastrangelo S, Mele MC, Ruggiero A. Impact of malnutrition on survival and infections among pediatric patients with cancer: a retrospective study. Eur Rev Med Pharmacol Sci. 2019;23(3):1165–75. https://doi.org/10.26355/eurrev_201901_17009.
Mendes MVC, Góes ACF, Brain FRM. Children and Adolescents in Cancer Treatment: an Analysis of the Vision of Postponing the Beginning or Interruption of School Education. Revista Brasileira de Cancerologia 2018; 64(3): 301–308. https://doi.org/10.32635/2176-9745.RBC.2018v64n3.27.
Bedoui Y, Guillot X, Sélambarom J, et al. Methotrexate an Old Drug with New Tricks. Int J Mol Sci. 2019;20(20):5023. Published 2019 Oct 10. https://doi.org/10.3390/ijms20205023.
Godfrey C, Sweeney K, Miller K, Hamilton R, Kremer J. The population pharmacokinetics of long-term methotrexate in rheumatoid arthritis. Br J Clin Pharmacol. 1998;46(4):369–76. https://doi.org/10.1046/j.1365-2125.1998.t01-1-00790.x.
Panetta JC, Roberts JK, Huang J, et al. Pharmacokinetic basis for dosing high-dose methotrexate in infants and young children with malignant brain tumours. Br J Clin Pharmacol. 2020;86(2):362–71. https://doi.org/10.1111/bcp.14160.
Makris K, Spanou L. Acute kidney injury: diagnostic approaches and controversies. Clin Biochem Rev. 2016;37(4):153–75.
Uchino S, Bellomo R, Goldsmith D. The meaning of the blood urea nitrogen/creatinine ratio in acute kidney injury. Clin Kidney J. 2012;5(2):187–91. https://doi.org/10.1093/ckj/sfs013.
Traivaree C, Likasitthananon N, Monsereenusorn C, Rujkijyanont P. The effect of intravenous hydration strategy on plasma methotrexate clearance during intravenous high-dose methotrexate administration in pediatric oncology patients. Cancer Manag Res. 2018;10:4471–8. https://doi.org/10.2147/CMAR.S172117.
Acknowledgements
The authors acknowledge Laura Ben Olivo for helping with model revision and external validation.
Funding
This work was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES, Finance Code 001) and by the PPSUS/FAPERGS, Rio Grande do Sul, Brazil (#21/2551–0000065-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Oliveira Henz, P., Pinhatti, A.V., Gregianin, L.J. et al. Population Pharmacokinetic Model of Methotrexate in Brazilian Pediatric Patients with Acute Lymphoblastic Leukemia. Pharm Res 40, 1777–1787 (2023). https://doi.org/10.1007/s11095-023-03544-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03544-7