Abstract
Background and Objectives
Methotrexate (MTX) is widely used for the treatment of a variety of neoplastic and autoimmune diseases. However, its toxicity and efficacy varied greatly among individuals, and they could be predicted by its pharmacokinetics. Many population pharmacokinetic models have been published to describe MTX pharmacokinetics. The objective of this systematic review was to summarize and discuss covariates with significant influence on MTX pharmacokinetics.
Methods
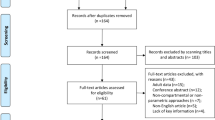
We searched PubMed and EMBASE databases from their inception to April 2021 for population pharmacokinetic of MTX. The articles were screened by inclusion and exclusion criteria. The characteristics of studies and information for model construction and validation were extracted, summarized and discussed.
Results
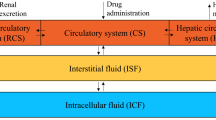
Thirty-five articles were included. The two-compartment model well described the pharmacokinetic behavior of MTX. For inter-individual variability, an exponential distribution error model was usually used for high-dose MTX population pharmacokinetic models, while a proportional distribution error model was used for low-dose MTX population pharmacokinetic models. Proportional and combined proportional and additive error models were used to describe residual error. Renal function was an independent indicator of MTX clearance. Body weight, age, gene polymorphisms (SLCO1B1, ABCC2, ABCB1, ABCG2 and MTHFR) and co-medications (proton pump inhibitors, non-steroidal anti-inflammatory drug, dexamethasone, vancomycin, penicillin and salicylic acid) could influence MTX clearance. Body weight, body surface area, age and dosage regimen have significant influence on MTX central compartment volume. Internal bootstrap test, external validation and visual predictive check were used to evaluate model predictive ability.
Conclusions
Various covariates could affect MTX pharmacokinetics, and their relationships have been summarized and discussed. This review will be helpful for researchers to develop their own population pharmacokinetic models and select appropriate models for individualized therapy of MTX.

Similar content being viewed by others
Abbreviations
- ALT :
-
Alanine transaminase
- BSA :
-
Body surface area
- BW :
-
Body weight
- CLCR :
-
Creatinine clearance
- CLMTX :
-
Methotrexate clearance
- GFR:
-
Glomerular filtration rate
- HD-MTX :
-
High-dose methotrexate
- IIV:
-
Inter-individual variability
- IOV:
-
Inter-occasion variability
- LD-MTX:
-
Low-dose methotrexate
- MTX:
-
Methotrexate
- PopPK:
-
Population pharmacokinetics
- QMTX :
-
Inter-compartmental clearance of methotrexate
- SCR:
-
Serum creatinine
- VcMTX :
-
Central compartment volume of methotrexate
- VpMTX :
-
Peripheral compartment volume of methotrexate
References
Guichard N, Guillarme D, Bonnabry P, Fleury-Souverain S. Antineoplastic drugs and their analysis: a state of the art review. Analyst. 2017;142(13):2273–321.
Godfrey C, Sweeney K, Miller K, Hamilton R, Kremer J. The population pharmacokinetics of long-term methotrexate in rheumatoid arthritis. Br J Clin Pharmacol. 1998;46(4):369–76.
Hui KH, Chu HM, Fong PS, Cheng WTF, Lam TN. Population pharmacokinetic study and individual dose adjustments of high-dose methotrexate in chinese pediatric patients with acute lymphoblastic leukemia or osteosarcoma. J Clin Pharmacol. 2019;59(4):566–77.
Zhang C, Zhai S, Yang L, Wu H, Zhang J, Ke X. Population pharmacokinetic study of methotrexate in children with acute lymphoblastic leukemia. Int J Clin Pharmacol Ther. 2010;48(1):11–21.
Grim J, Chládek J, Martínková J. Pharmacokinetics and pharmacodynamics of methotrexate in non-neoplastic diseases. Clin Pharmacokinet. 2003;42(2):139–51.
Joerger M, Huitema ADR, Van Den Bongard HJGD, Baas P, Schornagel JH, Schellens JHM, et al. Determinants of the elimination of methotrexate and 7-hydroxy-methotrexate following high-dose infusional therapy to cancer patients. Br J Clin Pharmacol. 2006;62(1):71–80.
Mei S, Li X, Jiang X, Yu K, Lin S, Zhao Z. Population pharmacokinetics of high-dose methotrexate in patients with primary central nervous system lymphoma. J Pharm Sci. 2018;107(5):1454–60.
Zhang W, Zhang Q, Tian X, Zhao H, Lu W, Zhen J, et al. Population pharmacokinetics of high-dose methotrexate after intravenous administration in Chinese osteosarcoma patients from a single institution. Chin Med J (Engl). 2015;128(1):111–8.
Kim IW, Yun HY, Choi B, Han N, Park SY, Lee ES, et al. ABCB1 C3435T genetic polymorphism on population pharmacokinetics of methotrexate after hematopoietic stem cell transplantation in Korean patients: a prospective analysis. Clin Ther. 2012;34(8):1816–26.
Maksimovic V, Pavlovic-Popovic Z, Vukmirovic S, Cvejic J, Mooranian A, Al-Salami H, et al. Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol Biol Rep. 2020;47(6):4699–708.
Yang L, Wu H, de Winter BCM, Sheng CC, Qiu HQ, Cheng Y, et al. Pharmacokinetics and pharmacogenetics of high-dose methotrexate in Chinese adult patients with non-Hodgkin lymphoma: a population analysis. Cancer Chemother Pharmacol. 2020;85(5):881–97.
Shi ZY, Liu YO, Gu HY, Xu XQ, Yan C, Yang XY, et al. Population pharmacokinetics of high-dose methotrexate in Chinese pediatric patients with medulloblastoma. Biopharm Drug Dispos. 2020;41(3):101–10.
Pai MP, Debacker KC, Derstine B, Sullivan J, Su GL, Wang SC. Comparison of body size, morphomics, and kidney function as covariates of high-dose methotrexate clearance in obese adults with primary central nervous system lymphoma. Pharmacotherapy. 2020;40(4):308–19.
Garneau AP, Riopel J, Isenring P. Acute Methotrexate-induced crystal nephropathy. N Engl J Med. 2015;373(27):2691–3.
Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11(6):694–703.
Karami F, Ranjbar S, Ghasemi Y, Negahdaripour M. Analytical methodologies for determination of methotrexate and its metabolites in pharmaceutical, biological and environmental samples. J Pharm Anal. 2019;9(6):373–91.
Yamamoto T, Shikano K, Nanki T, Kawai S. Folylpolyglutamate synthase is a major determinant of intracellular methotrexate polyglutamates in patients with rheumatoid arthritis. Sci Rep. 2016;6:35615.
Meesters RJ, den Boer E, de Jonge R, Lindemans J, Luider TM. Assessment of intracellular methotrexate and methotrexate-polyglutamate metabolite concentrations in erythrocytes by ultrafast matrix-assisted laser desorption/ionization triple quadrupole tandem mass spectrometry. Rapid Commun Mass Spectrom. 2011;25(20):3063–70.
den Boer E, de Rotte MC, Pluijm SM, Heil SG, Hazes JM, de Jonge R. Determinants of erythrocyte methotrexate polyglutamate levels in rheumatoid arthritis. J Rheumatol. 2014;41(11):2167–78.
Kawakatsu S, Nikanjam M, Lin M, Le S, Saunders I, Kuo DJ, et al. Population pharmacokinetic analysis of high-dose methotrexate in pediatric and adult oncology patients. Cancer Chemother Pharmacol. 2019;84(6):1339–48.
Crom WR, Glynn-Barnhart AM, Rodman JH, Teresi ME, Kavanagh RE, Christensen ML, et al. Pharmacokinetics of anticancer drugs in children. Clin Pharmacokinet. 1987;12(3):168–213.
Groninger E, Proost JH, de Graaf SS. Pharmacokinetic studies in children with cancer. Crit Rev Oncol Hematol. 2004;52(3):173–97.
Pilkington CA, Wedderburn LR. Paediatric idiopathic inflammatory muscle disease: recognition and management. Drugs. 2005;65(10):1355–65.
Skärby T, Jönsson P, Hjorth L, Behrentz M, Björk O, Forestier E, et al. High-dose methotrexate: on the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol. 2003;51(4):311–20.
Wall AM, Gajjar A, Link A, Mahmoud H, Pui CH, Relling MV. Individualized methotrexate dosing in children with relapsed acute lymphoblastic leukemia. Leukemia. 2000;14(2):221–5.
Faganel Kotnik B, Grabnar I, Bohanec Grabar P, Dolžan V, Jazbec J. Association of genetic polymorphism in the folate metabolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukaemia and malignant lymphoma. Eur J Clin Pharmacol. 2011;67(10):993–1006.
Williams PJ, Ette EI. The role of population pharmacokinetics in drug development in light of the Food and Drug Administration’s “Guidance for Industry: population pharmacokinetics.” Clin Pharmacokinet. 2000;39(6):385–95.
Koch HJ. Population methods in drug development and related fields. Clin Pharmacokinet. 1996;31(2):164.
Batey MA, Wright JG, Azzabi A, Newell DR, Lind MJ, Calvert AH, et al. Population pharmacokinetics of adjuvant cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Eur J Cancer. 2002;38(8):1081–9.
Aumente D, Buelga DS, Lukas JC, Gomez P, Torres A, García MJ. Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet. 2006;45(12):1227–38.
Faltaos DW, Hulot JS, Urien S, Morel V, Kaloshi G, Fernandez C, et al. Population pharmacokinetic study of methotrexate in patients with lymphoid malignancy. Cancer Chemother Pharmacol. 2006;58(5):626–33.
Colom H, Farré R, Soy D, Peraire C, Cendros JM, Pardo N, et al. Population pharmacokinetics of high-dose methotrexate after intravenous administration in pediatric patients with osteosarcoma. Ther Drug Monit. 2009;31(1):76–85.
Min Y, Qiang F, Peng L, Zhu Z. High dose methotrexate population pharmacokinetics and Bayesian estimation in patients with lymphoid malignancy. Biopharm Drug Dispos. 2009;30(8):437–47.
Johansson ÅM, Hill N, Perisoglou M, Whelan J, Karlsson MO, Standing JF. A population pharmacokinetic/pharmacodynamic model of methotrexate and mucositis scores in osteosarcoma. Ther Drug Monit. 2011;33(6):711–8.
Nader A, Zahran N, Alshammaa A, Altaweel H, Kassem N, Wilby KJ. Population pharmacokinetics of intravenous methotrexate in patients with hematological malignancies: utilization of routine clinical monitoring parameters. Eur J Drug Metab Pharmacokinet. 2017;42(2):221–8.
Lui G, Treluyer JM, Fresneau B, Piperno-Neumann S, Gaspar N, Corradini N, et al. A Pharmacokinetic and pharmacogenetic analysis of osteosarcoma patients treated with high-dose methotrexate: data from the OS2006/Sarcoma-09 Trial. J Clin Pharmacol. 2018;58(12):1541–9.
Beechinor RJ, Thompson PA, Hwang MF, Vargo RC, Bomgaars LR, Gerhart JG, et al. The population pharmacokinetics of high-dose methotrexate in infants with acute lymphoblastic leukemia highlight the need for bedside individualized dose adjustment: a report from the Children’s Oncology Group. Clin Pharmacokinet. 2019;58(7):899–910.
Zang YN, Wang SZ, Qin Y, Zhang JR, Zhao LB, Wang XL. Population pharmacokinetic study of delayed methotrexate excretion in children with acute lymphoblastic leukemia. Int J Clin Pharmacol Ther. 2019;57(8):402–7.
Gallais F, Oberic L, Faguer S, Tavitian S, Lafont T, Marsili S, et al. Body surface area dosing of high-dose methotrexate should be reconsidered, particularly in overweight, adult patients. Ther Drug Monit. 2020;43(3):408–415.
Medellin-Garibay SE, Hernández-Villa N, Correa-González LC, Morales-Barragán MN, Valero-Rivera KP, Reséndiz-Galván JE, et al. Population pharmacokinetics of methotrexate in Mexican pediatric patients with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2020;85(1):21–31.
Panetta JC, Roberts JK, Huang J, Lin T, Daryani VM, Harstead KE, et al. Pharmacokinetic basis for dosing high-dose methotrexate in infants and young children with malignant brain tumours. Br J Clin Pharmacol. 2020;86(2):362–71.
Taylor Z, Mizuno T, Vinks A, Heldrup J, Ramsey L. Development of a novel three-compartment high-dose methotrexate population pharmacokinetic model to guide glucarpidase dosing in pediatric acute lymphoblastic leukemia. Clin Pharmacol Ther. 2020;107:S65.
Schulte RR, Choi L, Utreja N, Van Driest SL, Stein CM, Ho RH. Effect of SLCO1B1 polymorphisms on high-dose methotrexate clearance in children and young adults with leukemia and lymphoblastic lymphoma. Clin Transl Sci. 2021;14(1):343–53.
Fukuhara K, Ikawa K, Morikawa N, Kumagai K. Population pharmacokinetics of high-dose methotrexate in Japanese adult patients with malignancies: a concurrent analysis of the serum and urine concentration data. J Clin Pharm Ther. 2008;33(6):677–84.
Simon N, Marsot A, Villard E, Choquet S, Khe HX, Zahr N, et al. Impact of ABCC2 polymorphisms on high-dose methotrexate pharmacokinetics in patients with lymphoid malignancy. Pharmacogenom J. 2013;13(6):507–13.
Wang Z, Zhang N, Chen C, Chen S, Xu J, Zhou Y, et al. Influence of the OATP polymorphism on the population pharmacokinetics of methotrexate in Chinese patients. Curr Drug Metab. 2019;20(7):592–600.
Ye M, Fu Q, Li P, Zhu Z. High dose methotrexate population pharmacokinetics and bayesian estimation in patients with lymphoid malignancy. Biopharm Drug Dispos. 2009;30(8):437–47.
Piard C, Bressolle F, Fakhoury M, Zhang D, Yacouben K, Rieutord A, et al. A limited sampling strategy to estimate individual pharmacokinetic parameters of methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2007;60(4):609–20.
Wright KD, Panetta JC, Onar-Thomas A, Reddick WE, Patay Z, Qaddoumi I, et al. Delayed methotrexate excretion in infants and young children with primary central nervous system tumors and postoperative fluid collections. Cancer Chemother Pharmacol. 2015;75(1):27–35.
El Desoky ES, Ghazally MH, Singh RP, Abdelhamid ON, Derendorf H. Population pharmacokinetics of methotrexate in Egyptian children with lymphoblastic leukemia. Ther Drug Monit. 2011;33(4):548.
Yukawa E, Mori S, Ueda K, Nakada Y. Population pharmacokinetic investigation of low-dose methotrexate in rheumatoid arthritics Japanese patients. J Clin Pharm Ther. 2007;32(6):573–8.
Nagulu M, Uday Kiran V, Nalini Y, Narsimha Reddy Y, Rama KD. Population pharmacokinetics of methotrexate in Indian cancer patients. Asian Pac J Cancer Prev. 2010;11(2):403–7.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Kanasaki K, Kitada M, Kanasaki M, Koya D. The biological consequence of obesity on the kidney. Nephrol Dial Transpl. 2013;28(Suppl 4):1–7.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Dupuis C, Mercier C, Yang C, Monjanel-Mouterde S, Ciccolini J, Fanciullino R, et al. High-dose methotrexate in adults with osteosarcoma: a population pharmacokinetics study and validation of a new limited sampling strategy. Anticancer Drugs. 2008;19(3):267–73.
Odoul F, Le Guellec C, Lamagnère JP, Breilh D, Saux MC, Paintaud G, et al. Prediction of methotrexate elimination after high dose infusion in children with acute lymphoblastic leukaemia using a population pharmacokinetic approach. Fundam Clin Pharmacol. 1999;13(5):595–604.
Liu C, Wen J, Xiang J, Ouyang X, Yang Y, Lu W, et al. Age- and sex-specific reference intervals for the serum cystatin C/creatinine ratio in healthy children (0–18 years old). J Int Med Res. 2019;47(7):3151–9.
Goasguen JE, Dossot JM, Fardel O, Le Mee F, Le Gall E, Leblay R, et al. Expression of the multidrug resistance-associated P-glycoprotein (P-170) in 59 cases of de novo acute lymphoblastic leukemia: prognostic implications. Blood. 1993;81(9):2394–8.
Gorczyca L, Aleksunes LM. Transcription factor-mediated regulation of the BCRP/ABCG2 efflux transporter: a review across tissues and species. Expert Opin Drug Metab Toxicol. 2020;16(3):239–53.
Imanishi H, Okamura N, Yagi M, Noro Y, Moriya Y, Nakamura T, et al. Genetic polymorphisms associated with adverse events and elimination of methotrexate in childhood acute lymphoblastic leukemia and malignant lymphoma. J Hum Genet. 2007;52(2):166–71.
Wang YM, Fujimoto T. Clinical pharmacokinetics of methotrexate in children. Clin Pharmacokinet. 1984;9(4):335–48.
Acknowledgements
We thank all authors of the included studies for their hard work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
Zhigang Zhao was supported by the Beijing Municipal Administration of Hospitals (ZYLX201827). Shenghui Mei was supported by the National Key R&D Program of China (2020YFC2008306) and Beijing Municipal Health Bureau (2018000021469G238).
Conflict of interest
Yiming Zhang, Liyu Sun, Libo Zhao, Xiaoling Wang, Zhigang Zhao and Shenghui Mei have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Author contributions
ZZ and MS designed the review and planned the work of the manuscript. YM and SL performed the literature search. MS and YM analyzed the extracted data. YM, SL, ZL, WY, ZZ and MS drafted and revised the manuscript. All authors approved the final version of this article.
Rights and permissions
About this article
Cite this article
Zhang, Y., Sun, L., Chen, X. et al. A Systematic Review of Population Pharmacokinetic Models of Methotrexate. Eur J Drug Metab Pharmacokinet 47, 143–164 (2022). https://doi.org/10.1007/s13318-021-00737-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-021-00737-6