Abstract
Purpose
The aim of the present study was to develop a population pharmacokinetic model for methotrexate (MTX) during high-dose treatment (HDMTX) in pediatric patients with acute lymphoblastic leukemia (ALL) and non-Hodgkin’s lymphoma (NHL) and to describe the influence of variability factors.
Methods
The study included 50 patients of both sexes (aged 1–18 years) who received 3 or 5 g/m2 of HDMTX. A nonlinear mixed effect modeling approach was applied for data analysis. Parameter estimation was performed by first-order conditional estimation method with interaction (FOCEI), whereas stepwise covariate modeling was used to assess variability factors.
Results
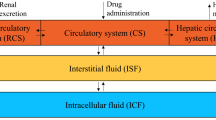
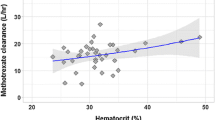
The final model is a two-compartment model that incorporates the effect of body surface area and the influence of hemoglobin and serum creatinine on MTX clearance (CL). Population pharmacokinetic values for a typical subject were estimated at 5.75 L/h/m2 for clearance (CL), 21.3 L/m2 for volume of the central compartment (V1), 8.2 L/m2 for volume of the peripheral compartment (V2), and 0.087 L/h/m2 for intercompartmental clearance (Q). According to the final model, MTX CL decreases with increasing serum creatinine, whereas a positive effect was captured for hemoglobin. A difference of almost 32% in MTX CL was observed among patients’ hemoglobin values reported in the study.
Conclusion
The developed population pharmacokinetic model can contribute to the therapy optimization during HDMTX in pediatric patients with ALL and NHL. In addition to renal function and body weight, it describes the influence of hemoglobin on CL, allowing better understanding of its contribution to the disposition of HDMTX.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
A randomized trial of the I-BFM-SG for the management of childhood non-B acute lymphoblastic leukemia (ALL IC-BFM 2009) (2009) Final version of therapy protocol from August-14-2009 [Internet]. https://www.bialaczka.org/wp-content/uploads/2016/10/ALLIC_BFM_2009.pdf. Accessed June 2023
Pillon M, Arico M, Mussolin L, Carraro E, Conter V, Sala A, Buffardi S, Garaventa A, D’Angelo P, Lo Nigro L, Santoro N, Piglione M, Lombardi A, Porta F, Cesaro S, Moleti ML, Casale F, Mura R, d’Amore ES, Basso G, Rosolen A (2015) Long-term results of the AIEOP LNH-97 protocol for childhood lymphoblastic lymphoma. Pediatr Blood Cancer 62(8):1388–1394. https://doi.org/10.1002/pbc.25469
International protocol for the treatment of childhood anaplastic large cell lymphoma (ALCL 99) (2000) [Internet]. https://www.skion.nl/workspace/uploads/alcl-99.pdf. Accessed June 2023
Zhang Y, Sun L, Chen X, Zhao L, Wang X, Zhao Z, Mei S (2022) A systematic review of population pharmacokinetic models of methotrexate. Eur J Drug Metab Pharmacokinet 47(2):143–164. https://doi.org/10.1007/s13318-021-00737-6
Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD (2016) Preventing and managing toxicities of high-dose methotrexate. Oncologist 21(12):1471–1482. https://doi.org/10.1634/theoncologist.2015-0164
Treon SP, Chabner BA (1996) Concepts in use of high-dose methotrexate therapy. Clin Chem 42(8 Pt 2):1322–1329
Nader A, Zahran N, Alshammaa A, Altaweel H, Kassem N, Wilby KJ (2017) Population pharmacokinetics of intravenous methotrexate in patients with hematological malignancies: utilization of routine clinical monitoring parameters. Eur J Drug Metab Pharmacokin 42(2):221–228. https://doi.org/10.1007/s13318-016-0338-1
Skoric B, Kuzmanovic M, Jovanovic M, Miljkovic B, Micic D, Jovic M, Jovanovic A, Vucicevic K (2023) Methotrexate concentrations and associated variability factors in high dose therapy of children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Pediatr Hematol Oncol 40(5):446–457. https://doi.org/10.1080/08880018.2023.2168809
Mould DR, Upton RN (2013) Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT: PSP 2(4):e38. https://doi.org/10.1038/psp.2013.14
Roganović M, Homšek A, Jovanović M, Topić Vučenović V, Ćulafić M, Miljković B, Vučićević K (2021) Concept and utility of population pharmacokinetic and pharmacokinetic/pharmacodynamic models in drug development and clinical practice. Arh farm 71(4):336–353. https://doi.org/10.5937/arhfarm71-32901
Mei S, Li X, Jiang X, Yu K, Lin S, Zhao Z (2018) Population pharmacokinetics of high-dose methotrexate in patients with primary central nervous system lymphoma. J Pharm Sci 107(5):1454–1460. https://doi.org/10.1016/j.xphs.2018.01.004
Faltaos DW, Hulot JS, Urien S, Morel V, Kaloshi G, Fernandez C, Xuan k H, Leblond V, Lechat P (2006) Population pharmacokinetic study of methotrexate in patients with lymphoid malignancy. Cancer Chemother Pharmacol 58(5):626–633. https://doi.org/10.1007/s00280-006-0202-0
Shi ZY, Liu YO, Gu HY, Xu XQ, Yan C, Yang XY, Yan D (2020) Population pharmacokinetics of high-dose methotrexate in Chinese pediatric patients with medulloblastoma. Biopharm Drug Dispos 41(3):101–110. https://doi.org/10.1002/bdd.2221
Fukuhara K, Ikawa K, Morikawa N, Kumagai K (2008) Population pharmacokinetics of high-dose methotrexate in Japanese adult patients with malignancies: a concurrent analysis of the serum and urine concentration data. J Clin Pharm Ther 33(6):677–684. https://doi.org/10.1111/j.1365-2710.2008.00966.x
Panetta JC, Roberts JK, Huang J, Lin T, Daryani VM, Harstead KE, Patel YT, Onar-Thomas A, Campagne O, Ward DA, Broniscer A, Robinson G, Gajjar A, Stewart CF (2020) Pharmacokinetic basis for dosing high-dose methotrexate in infants and young children with malignant brain tumours. Br J Clin Pharmacol 86(2):362–371. https://doi.org/10.1111/bcp.14160
Pai MP, Debacker KC, Derstine B, Sullivan J, Su GL, Wang SC (2020) Comparison of body size, morphomics, and kidney function as covariates of high-dose methotrexate clearance in obese adults with primary central nervous system lymphoma. Pharmacother 40(4):308–319. https://doi.org/10.1002/phar.2379
Taylor ZL, Mizuno T, Punt NC, Baskaran B, Navarro Sainz A, Shuman W, Felicelli N, Vinks AA, Heldrup J, Ramsey LB (2020) MTXPK.org: a clinical decision support tool evaluating high-dose methotrexate pharmacokinetics to inform post-infusion care and use of glucarpidase. Clin Pharmacol Ther 108(3):635–643. https://doi.org/10.1002/cpt.1957
Johansson AM, Hill N, Perisoglou M, Whelan J, Karlsson MO, Standing JF (2011) A population pharmacokinetic/pharmacodynamic model of methotrexate and mucositis scores in osteosarcoma. Ther Drug Monit 33(6):711–718. https://doi.org/10.1097/FTD.0b013e31823615e1
Kawakatsu S, Nikanjam M, Lin M, Le S, Saunders I, Kuo DJ, Capparelli EV (2019) Population pharmacokinetic analysis of high-dose methotrexate in pediatric and adult oncology patients. Cancer Chemother Pharmacol 84(6):1339–1348. https://doi.org/10.1007/s00280-019-03966-4
Jonsson P, Skarby T, Heldrup J, Schroder H, Hoglund P (2011) High dose methotrexate treatment in children with acute lymphoblastic leukaemia may be optimised by a weight-based dose calculation. Pediatric Blood Cancer 57(1):41–46. https://doi.org/10.1002/pbc.22999
Hui KH, Chu HM, Fong PS, Cheng WTF, Lam TN (2019) Population pharmacokinetic study and individual dose adjustments of high-dose methotrexate in Chinese pediatric patients with acute lymphoblastic leukemia or osteosarcoma. J Clin Pharmacol 59(4):566–577. https://doi.org/10.1002/jcph.1349
Wright KD, Panetta JC, Onar-Thomas A, Reddick WE, Patay Z, Qaddoumi I, Broniscer A, Robinson G, Boop FA, Klimo P Jr, Ward D, Gajjar A, Stewart CF (2015) Delayed methotrexate excretion in infants and young children with primary central nervous system tumors and postoperative fluid collections. Cancer Chemother Pharmacol 75(1):27–35. https://doi.org/10.1007/s00280-014-2614-6
Kotnik BF, Grabnar I, Grabar PB, Dolzan V, Jazbec J (2011) Association of genetic polymorphism in the folate metabolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukaemia and malignant lymphoma. Eur J Clin Pharmacol 67(10):993–1006. https://doi.org/10.1007/s00228-011-1046-z
Leveque D, Santucci R, Gourieux B, Herbrecht R (2011) Pharmacokinetic drug-drug interactions with methotrexate in oncology. Expert Rev Clin Pharmacol 4(6):743–750. https://doi.org/10.1586/ecp.11.57
Dupuis C, Mercier C, Yang C, Monjanel-Mouterde S, Ciccolini J, Fanciullino R, Pourroy B, Deville JL, Duffaud F, Bagarry-Liegey D, Durand A, Iliadis A, Favre R (2008) High-dose methotrexate in adults with osteosarcoma: a population pharmacokinetics study and validation of a new limited sampling strategy. Anticancer Drugs 19(3):267–273. https://doi.org/10.1097/cad.0b013e3282f21376
Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ (Eds). NONMEM users guides (7.5.0). ICON plc, Gaithersburg, Maryland (1989–2021) [Internet]. https://nonmem.iconplc.com. Accessed June 2023
R Core Team (2016) R: A language and environment for statistical computing. Vienna, Austria [Internet]. http://www.R-project.org. Accessed June 2023
Lexicomp Online, Lexi-Interact. Waltham, MA: UpToDate, Inc.; July 30, 2021 [Internet]. https://online.lexi.com. Accessed Oct 2022
Hooker AC, Staatz CE, Karlsson MO (2007) Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24(12):2187–2197. https://doi.org/10.1007/s11095-007-9361-x
Karlsson MO, Savic RM (2007) Diagnosing model diagnostics. Clin Pharmacol Ther 82(1):17–20. https://doi.org/10.1038/sj.clpt.6100241
Parke J, Holford NH, Charles BG (1999) A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed 59(1):19–29. https://doi.org/10.1016/s0169-2607(98)00098-4
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13(2):143–151. https://doi.org/10.1208/s12248-011-9255-z
Beechinor RJ, Thompson PA, Hwang MF, Vargo RC, Bomgaars LR, Gerhart JG, Dreyer ZE, Gonzalez D (2019) The population pharmacokinetics of high-dose methotrexate in infants with acute lymphoblastic leukemia highlight the need for bedside individualized dose adjustment: a report from the children’s oncology group. Clin Pharmacokinet 58(7):899–910. https://doi.org/10.1007/s40262-018-00734-0
Jovanović M, Vučićević K (2020) Pediatric pharmacokinetic considerations and implications for drug dosing. Arh farm 72(3):340–352. https://doi.org/10.5937/arhfarm72-37605
Ruhs H, Becker A, Drescher A, Panetta JC, Pui CH, Relling MV, Jaehde U (2012) Population PK/PD model of homocysteine concentrations after high-dose methotrexate treatment in patients with acute lymphoblastic leukemia. PLoS ONE 7(9):e46015. https://doi.org/10.1371/journal.pone.0046015
de Beaumais TA, Jacqz-Aigrain E (2012) Intracellular disposition of methotrexate in acute lymphoblastic leukemia in children. Curr Drug Metab 13(6):822–834. https://doi.org/10.2174/138920012800840400
Min Y, Qiang F, Peng L, Zhu Z (2009) High dose methotrexate population pharmacokinetics and Bayesian estimation in patients with lymphoid malignancy. Biopharm Drug Dispos 30(8):437–447. https://doi.org/10.1002/bdd.678
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation, Republic of Serbia, through Grant Agreement with the University of Belgrade-Faculty of Pharmacy (No: 451–03-47/2023–01/200161).
Author information
Authors and Affiliations
Contributions
Conceptualization, research design, and project supervision: M.K. and K.V.; data curation: B.Š.; population analysis: M.J. and B.Š.; interpretation of the results: M.K., K.V., M.J., and B.Š.; writing—original draft preparation: M.J. and B.Š.; review and editing: M.K., B.M., and K.V. M.J. and B. Š. contributed equally to this paper and share first authorship. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The Ethics Committee of the Institute for Mother and Child Healthcare of Serbia “Dr Vukan Čupić” approved the study protocol and retrospective collection of data from medical records.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Škorić, B., Jovanović, M., Kuzmanović, M. et al. Understanding hemoglobin contribution to high-dose methotrexate disposition—population pharmacokinetics in pediatric patients with hematological malignancies. Eur J Clin Pharmacol 80, 697–705 (2024). https://doi.org/10.1007/s00228-024-03642-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-024-03642-4