Abstract
Introduction
With an increased adoption of continuous manufacturing for pharmaceutical production, the ConsiGma® CTL25 wet granulation and tableting line has reached widespread use. In addition to the continuous granulation step, the semi-continuous six-segmented fluid bed dryer is a key unit in the line. The dryer is expected to have an even distribution of the inlet air between the six drying cells. However, process observations during manufacturing runs showed a repeatable pattern in drying time, which suggests a variability in the drying performance between the different cells of the dryer. The aim of this work is to understand the root-cause of this variability.
Materials and methods
In a first step, the variability in the air temperature and air flow velocity between the dryer cells was measured on an empty dryer. In a second step, the experimental data were interpreted with the help of results from computational fluid dynamics (CFD) simulations to better understand the reasons for the observed variability.
Results
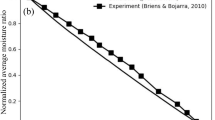
The CFD simulations were used to identify one cause of the measured difference in the air temperature, showing the impact of the air inlet design on the temperature distribution in the dryer.
Conclusions
Although the simulation could not predict the exact temperature, the trend was similar to the experimental observations, demonstrating the added value of this type of simulation to guide process development, engineering decisions and troubleshoot equipment performance variability.














Similar content being viewed by others
References
Vanhoorne V, Vervaet C. Recent progress in continuous manufacturing of oral solid dosage forms. Int J Pharm [Internet]. 2020;579(February):119194. Available from: https://doi.org/10.1016/j.ijpharm.2020.119194
Leane M, Pitt K, Reynolds GK, Dawson N, Ziegler I, Szepes A, et al. Manufacturing classification system in the real world: factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm Dev Technol [Internet]. 2018;23(10):964–77. Available from: https://doi.org/10.1080/10837450.2018.1534863
Portier C, Vervaet C, Vanhoorne V. Continuous Twin Screw Granulation: A Review of Recent Progress and Opportunities in Formulation and Equipment Design. Pharmaceutics [Internet]. 2021 May 7 [cited 2022 Feb 24];13(5):668. Available from: https://www.mdpi.com/1999-4923/13/5/668
Fülöp G, Domokos A, Galata D, Szabó E, Gyürkés M, Szabó B, et al. Integrated twin-screw wet granulation, continuous vibrational fluid drying and milling: A fully continuous powder to granule line. Int J Pharm. 2021;594(August 2020).
Vercruysse J, Delaet U, Van Assche I, Cappuyns P, Arata F, Caporicci G, et al. Stability and repeatability of a continuous twin screw granulation and drying system. Eur J Pharm Biopharm [Internet]. 2013;85(3 PART B):1031–8. Available from: https://doi.org/10.1016/j.ejpb.2013.05.002
Vercruysse J, Peeters E, Fonteyne M, Cappuyns P, Delaet U, Van Assche I, et al. Use of a continuous twin screw granulation and drying system during formulation development and process optimization. Eur J Pharm Biopharm [Internet]. 2015;89:239–47. Available from: https://doi.org/10.1016/j.ejpb.2014.12.017
De Leersnyder F, Vanhoorne V, Bekaert H, Vercruysse J, Ghijs M, Bostijn N, et al. Breakage and drying behaviour of granules in a continuous fluid bed dryer: Influence of process parameters and wet granule transfer. Eur J Pharm Sci [Internet]. 2018;115(January):223–32. Available from: https://doi.org/10.1016/j.ejps.2018.01.037
Ryckaert A, Ghijs M, Portier C, Djuric D, Funke A, Vervaet C, et al. The influence of equipment design and process parameters on granule breakage in a semi-continuous fluid bed dryer after continuous twin-screw wet granulation. Pharmaceutics. 2021;13(2):1–14.
Destro F, Salmon AJ, Facco P, Pantelides CC, Bezzo F, Barolo M. Monitoring a segmented fluid bed dryer by hybrid data-driven/knowledge-driven modeling. IFAC-PapersOnLine. 2020;53(2):11638–43.
Fonteyne M, Gildemyn D, Peeters E, Mortier STFC, Vercruysse J, Gernaey K V., et al. Moisture and drug solid-state monitoring during a continuous drying process using empirical and mass balance models. Eur J Pharm Biopharm [Internet]. 2014;87(3):616–28. Available from: https://doi.org/10.1016/j.ejpb.2014.02.015
Fonteyne M, Arruabarrena J, de Beer J, Hellings M, Van Den Kerkhof T, Burggraeve A, et al. NIR spectroscopic method for the in-line moisture assessment during drying in a six-segmented fluid bed dryer of a continuous tablet production line: Validation of quantifying abilities and uncertainty assessment. J Pharm Biomed Anal [Internet]. 2014;100:21–7. Available from: https://doi.org/10.1016/j.jpba.2014.07.012
Rehrl J, Sacher S, Horn M, Khinast J. End-point prediction of granule moisture in a ConsiGma™-25 segmented fluid bed dryer. Pharmaceutics. 2020;12(5):1–15.
Šibanc R, Rehrl J, Hörmann T, Hsiao W, Franke M. Experimental investigation of the drying step in a ConsiGma 25 twin screw granulation line. Freiburg: 2nd APV Continuous Manufacturing Conference; p. 1.
Fonteyne M, Vercruysse J, Díaz DC, Gildemyn D, Vervaet C, Remon JP, et al. Real-time assessment of critical quality attributes of a continuous granulation process. Pharm Dev Technol. 2013;18(1):85–97.
Ansys. Ansys FLUENT Theory Guide, Release 2020R2. ANSYS Inc; 2020.
Jayamaha SEG, Wijeysundera NE, Chou SK. Measurement of the heat transfer coefficient for walls. Build Environ. 1996;31(5):399–407.
Kunii D, Levenspiel O. Fluidization engineering. New York: Wiley; 1969.
Acknowledgements
Special thanks go to Eyke Slama for the design and manufacturing of the probe holder and to Patrick Vorraber for testing it prior to our experimental work.
Funding
The Research Center Pharmaceutical Engineering (RCPE) is funded within the framework of COMET—Competence Centers for Excellent Technologies by BMK, BMDW, Land Steiermark, and SFG. The COMET program is managed by the FFG.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grelier, A., Zadravec, M., Remmelgas, J. et al. Model-Guided Development of a Semi-Continuous Drying Process. Pharm Res 39, 2005–2016 (2022). https://doi.org/10.1007/s11095-022-03361-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03361-4