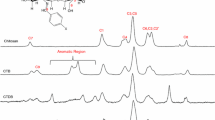
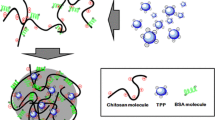
Abstract
Purpose
The work aimed to compare quality of a siRNA carrier prepared with chitosan of two different sources having similar degree of deacetylation and molecular weights. Differences were analyzed from thermodynamic characteristics of interactions with siRNA.
Methods
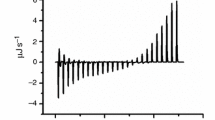
The siRNA carrier (chitosan-coated poly(isobutylcyanoacrylate) nanoparticles) was prepared with home-prepared, CSLab, and commercial, CSCom, chitosans. Chitosan counterion was identified and chitosans CSCommod1 and CSCommod2 were obtained from CSCom exchanging counterion with that found on CSLab. Carrier quality was checked considering the size, zeta potential and siRNA association capacity by gel electrophoresis. Thermodynamic parameters of interactions between siRNA and chitosans in solution or immobilized at the carrier surface were determined by isothermal titration calorimetry (ITC).
Results
CSLab and CSCommod2 having a high content of acetate counterion associated better siRNA than CSCom and CSCommod1 which counterion included mainly chloride. ITC measurements indicated that siRNA interactions with chitosan and the siRNA carrier were driven by entropic phenomena including dehydration, but thermodynamic parameters of interactions clearly differed according to the nature of the counterion of chitosan. The influence of chitosan counterions was interpreted considering their different lyotropic character.
Conclusion
Association of siRNA with our siRNA carrier was influenced by the nature of counterions associated with chitosan. Driven by entropic phenomena including dehydration, interactions were favored by acetate counterion. Although more work would be needed to decipher the influence of the counterion of chitosan during association with siRNA, it was pointed out as a new critical attribute of chitosan to consider while formulating siRNA carrier with this polysaccharide.






Similar content being viewed by others
Abbreviations
- CS:
-
Generic abbreviation for chitosans used in this work
- CSLab :
-
Chitosan prepared in our lab
- CSCom :
-
Commercial chitosan
- CSCom-mod1:
-
Commercial chitosan modified with method 1
- CSCom-mod2:
-
Commercial chitosan modified with method 2
- DeltaH :
-
Normalized enthalpy
- ΔH fit :
-
Interaction enthalpy deduced from the fit of the curve and using Eq. 5
- ΔH graph :
-
Interaction enthalpy evaluated from graphs
- ΔG :
-
Difference in free energy
- ΔS :
-
Entropy of the reaction
- D H :
-
Hydrodynamic diameter
- ITC:
-
Isothermal titration calorimetry
- K a :
-
Association constant
- K d :
-
Dissociation constant
- NP CSxxx :
-
Nanoparticles prepared with the specified chitosan
- NP CSxxx-P:
-
Nanoparticles prepared with the specified chitosan and poloxamer 188
- NP P:
-
Nanoparticles prepared with poloxamer 188
- PACA:
-
Poly(alkylcyanoacrylate)
- siRNA:
-
Small interfering RNA
- -TΔS :
-
Entropic therms of the reaction
References
Mello CC, Conte D Jr. Revealing the world of RNA interference. Nature. 2004;431(7006):338–42.
Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–55. https://doi.org/10.1016/j.cell.2009.01.035.
Ramon AL, Bertrand JR, Malvy C. Delivery of small interfering RNA. A review and an example of application to a junction oncogene. Tumori. 2008;94:254–63.
Strumberg D, Schultheis B, Traugott U, Vank C, Santel A, Keil O, et al. Phase I clinical development of Atu027, a siRNA formulation targeting PKN3 in patients with advanced solid tumors. Int J Clin Pharmacol Ther. 2012;50(1):76–8.
Lee MJ, Yoon TJ, Cho YS. Recent developments in nanoparticle-based siRNA delivery for cancer therapy. Biomed Res Int. 2013;2013:782041. https://doi.org/10.1155/2013/782041.
Ozcan G, Ozpolat B, Coleman RL, Sood AK, Lopez-Berestein G. Preclinical and clinical development of siRNA-based therapeutics. Adv Drug Deliv Rev. 2015;87:108–19. https://doi.org/10.1016/j.addr.2015.01.007.
Herrera VLM, Colby AH, Ruiz-Opazol N, Coleman DG, Grinstaff MW. Nucleic acid nanomedicines in phase II/III clinical trials: translation of nucleic acid therapies for reprogramming cells. Nanomedicine (Lond). 2018;13(16):2083–98. https://doi.org/10.2217/nnm-2018-0122.
Mahmoodi Chalbatani G, Dana H, Gharagouzloo E, Grijalvo S, Eritja R, Logsdon CD, et al. Small interfering RNAs (siRNAs) in cancer therapy: a nano-based approach. Int J Nanomedicine. 2019;14:3111–28. https://doi.org/10.2147/IJN.S200253.
Zhang E, Xing R, Liu S, Qin Y, Li K, Li P. Advances in chitosan-based nanoparticles for oncotherapy. Carbohydr Polym. 2019;222:115004. https://doi.org/10.1016/j.carbpol.2019.115004.
Bruno K. Using drug-excipient interactions for siRNA delivery. Adv Drug Deliv Rev. 2011;63:1210–26.
Dolina JS, Sung SS, Novobrantseva TI, Nguyen TM, Hahn YS. Lipidoid nanoparticles containing PD-L1 siRNA delivered in vivo enter Kupffer cells and enhance NK and CD8(+) T cell-mediated hepatic antiviral immunity. Mol Ther Nucleic Acids. 2013;19(2):e72.
Forbes DC, Peppas NA. Oral delivery of small RNA and DNA. J Control Release. 2012;162:438–45.
Kole R, Leppert BJ. Targeting mRNA splicing as a potential treatment for Duchenne muscular dystrophy. Discov Med. 2012;14:59–69.
Gomes MJ, Martins S, Sarmento B. siRNA as a tool to improve the treatment of brain diseases: mechanism, targets and delivery. Ageing Res Rev. 2015;21:43–54. https://doi.org/10.1016/j.arr.2015.03.001.
Christie RJ, Nishiyama N, Kataoka K. (2010). Minireview: delivering the code: Polyplex carriers for deoxyribonucleic acid and ribonucleic acid interference therapies. Endocrinology. 2010;151:466–73.
Juliano RL. The delivery of therapeutic oligonucleotides. Nucleic Acids Res. 2016;44(14):6518–48. https://doi.org/10.1093/nar/gkw236.
Costa DF, Torchilin VP. Micelle-like nanoparticles as siRNA and miRNA carriers for cancer therapy. Biomed Microdevices. 2018;20(3):59. https://doi.org/10.1007/s10544-018-0298-0.
Cao Y, Tan YF, Wong YS, Liew MWJ, Venkatraman S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar Drugs. 2019;17(6). https://doi.org/10.3390/md17060381.
De Martimprey H, Vauthier C, Malvy C, Couvreur P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: oligonucleotides and siRNA. Eur J Pharm Biopharm. 2009;71:490–504.
Howard KA. Delivery of RNA interference therapeutics using polycation-based nanoparticles. Adv Drug Deliv Rev. 2009;61:710–20.
Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Del Rev. 2010;62:12–27.
Rudzinski WE, Aminabhavi TM. Chitosan as a carrier for targeted delivery of small interfering RNA. Int J Pharm. 2011;399:1–11. https://doi.org/10.1016/j.ijpharm.2010.08.022.
Singha K, Namgung R, Kim WJ. Polymers in small-interfering RNA delivery. Nuc Ac Therap. 2011;21:133–47.
Al-Qadi S, Grenha A, Remunan-Lopez C. Chitosan and its derivatives as nanocarriers for siRNA delivery. J Drug Del Sci Technol. 2012;22:29–42.
Rodrigues S, Dioniso M, Remunan-Lopez C, Grenha A. Biocompatibility of chitosan carriers with applications in drug delivery. J Funct Biomater. 2012;3:615–41.
Buschmann MD, Merzouki A, Lavertu M, Thibault M, Jean M, Darras V. Chitosans for delivery of nucleic acids. Adv Drug Deliv Rev. 2013;65(9):1234–70. https://doi.org/10.1016/j.addr.2013.07.005.
Kanasty R, Dorkin JR, Arturo Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–77.
Vauthier C, Zandanel C, Ramon AL. Chitosan-based nanoparticles for in vivo delivery of interfering agents including siRNA. Curr Opin Coll Interf Sci. 2013;18:406–18. https://doi.org/10.10106/j.cocis.2013.06.005.
Ragelle H, Vendermeulen G, Préat V. Chitosan-based siRNA delivery systems. J Control Release. 2013;172:207–18. https://doi.org/10.1016/j.jconrel.2013.08.005.
Bravo-Anaya LM, Soltero JFA, Rinaudo M. DNA/chitosan electrostatic complex. Int J Biol Macromol. 2016;88:345–53.
Cavallaro G, Sardo C, Craparo EF, Porsio B, Giammona G. Polymeric nanoparticles for siRNA delivery: production and applications. Int J Pharm. 2017;525(2):313–33. https://doi.org/10.1016/j.ijpharm.2017.04.008.
Huh MS, Lee EJ, Koo H, Yhee JY, Oh KS, Son S, et al. Polysaccharide-based nanoparticles for gene delivery. Top Curr Chem (Cham). 2017;375(2):31. https://doi.org/10.1007/s41061-017-0114-y.
Serrano-Sevilla I, Artiga Á, Mitchell SG, De Matteis L, de la Fuente JM. Natural Polysaccharides for siRNA Delivery: Nanocarriers Based on Chitosan, Hyaluronic Acid, and Their Derivatives. Molecules. 2019;24(14). https://doi.org/10.3390/molecules24142570.
Xu S, Dong M, Liu X, Howard KA, Kjems J, Besenbacher F. Direct force measurements between siRNA and chitosan molecules using force spectroscopy. Biophys J. 2007;93:952–9.
Ma PL, Lavertu M, Winnik FM, Buschmann MD. New insights into chitosan−DNA interactions using isothermal titration Microcalorimetry. Biomacromol. 2009;10:1490–9.
Huang M, Khor E, Lim LY. Uptake and cytotoxicity of chitosan molecules and nanoparticles: effects of molecular weight and degree of Deacetylation. Pharm Res. 2004;21:344–53.
Alameh M, Lavertu M, Tran-Khanh N, Chang CY, Lesage F, Bail M, et al. siRNA delivery with chitosan: influence of chitosan molecular weight, degree of Deacetylation, and amine to phosphate ratio on in vitro silencing efficiency, Hemocompatibility, biodistribution, and in vivo efficacy. Biomacromolecules. 2018;19(1):112–31. https://doi.org/10.1021/acs.biomac.7b01297 Epub 2017 Dec 28.
Delas T, Mock-Joubert M, Faivre J, Hofmaier M, Sandre O, Dole F, et al. Effects of Chain Length of Chitosan Oligosaccharides on Solution Properties and Complexation with siRNA. Polymers (Basel). 2019;11(8). https://doi.org/10.3390/polym11081236.
Kiang T, Wen J, Lim HW, Leong KW. The effect of the degree of chitosan deacetylation on the efficiency of gene transfection. Biomaterials. 2004;25:5293–301.
Techaarpornkul S, Wongkupasert S, Opanasopit P, Apirakaramwong A, Nunthanid J, Ruktanonchai U. Chitosan-Mediated siRNA Delivery In Vitro: Effect of Polymer Molecular Weight, Concentration and Salt Forms AAPS PharmSciTech. 2010; 11(1): 64–72. Published online 2010 Jan 8. https://doi.org/10.1208/s12249-009-9355-6, 64, 72.
Kumirska J, Weinhold MX, Thöming J, Stepnowski P. Biomedical activity of chitin/chitosan based materials—influence of physicochemical properties apart from molecular weight and degree of N-acetylation. Polymers. 2011;3(4):1875–901. https://doi.org/10.3390/polym3041875.
Yang C, Gao S, Dagnæs-Hansen F, Jakobsen M, Kjems J. Impact of PEG chain length on the physical properties and bioactivity of PEGylated chitosan/siRNA nanoparticles in vitro and in vivo. ACS Appl Mater Interfaces. 2017;9(14):12203–16. https://doi.org/10.1021/acsami.6b16556.
De Martimprey H, Bertrand JR, Malvy C, Couvreur P, Vauthier C. New Core-Shell Nanoparticules for the intravenous delivery of siRNA to experimental thyroid papillary carcinoma. Pharm Res. 2010;27:498–509.
Ramon AL, Bertrand JR, De Martimprey H, Bernard G, Ponchel G, Malvy C, et al. SiRNA associated with immunonanoparticles directed against cd99 antigen improve gene expression inhibition in vivo in Ewing’s sarcoma. J Mol Recognit. 2013;26:318–29.
Gaudin A, Andrieux K, Couvreur P. Nanomedicines and stokes: towards translational research. J Drug Deliv Sci Technol. 2015;30:278–99.
Lakkireddy HR, Bazile D. Building the design, translation and development principles of polymeric nanomedicines using the case of clinically advanced poly(lactide(glycolide))-poly(ethylene glycol) nanotechnology as a model: an industrial viewpoint. Adv Drug Deliv Rev. 2016;107:289–332. https://doi.org/10.1016/j.addr.2016.08.012.
Lakkireddy HR, Bazile D. Nano-carriers for drug routeing - towards a new era. J Drug Target. 2019;27(5–6):525–41. https://doi.org/10.1080/1061186X.2018.1561891.
Satalkar P, Elger BS, Hunziker P, Shaw D. Challenges of clinical translation in nanomedicine: a qualitative study. Nanomedicine. 2016;12(4):893–900. https://doi.org/10.1016/j.nano.2015.12.376.
Agrahari V, Hiremath P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine (Lond). 2017;12(8):819–23. https://doi.org/10.2217/nnm-2017-0039.
Anchordoquy TJ, Barenholz Y, Boraschi D, Chorny M, Decuzzi P, Dobrovolskaia MA, et al. Mechanisms and barriers in Cancer Nanomedicine: addressing challenges. Looking for Solutions ACS Nano. 2017;11(1):12–8. https://doi.org/10.1021/acsnano.6b08244.
Hua S, de Matos MBC, Metselaar JM, Storm G. Current trends and challenges in the clinical translation of Nanoparticulate Nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790. https://doi.org/10.3389/fphar.2018.00790.Clinicaltranslation.
Dormont F, Rouquette M, Mahatsekake C, Gobeaux F, Peramo A, Brusini R, et al. Translation of nanomedicines from lab to industrial scale synthesis: the case of squalene-adenosine nanoparticles. J Control Release. 2019;307:302–14. https://doi.org/10.1016/j.jconrel.2019.06.040.
Soma E, Attali P, Merle P. A clinically relevant case study: the development of Livatag® for the treatment of advanced hepatocellular carcinoma. In: Alonso MJ, Csaba NS, editors. RSC drug discovery series N°22: nanostructured biomaterials for overcoming biological barriers. Dorchester: The Royal Society of Chemistry; 2012. p. 591–600. https://doi.org/10.1039/9781849735292-00591.
Merle P, Pelletier G, Habersetzer F, Luca Frassineti G, Pageaux GP, Borbath I, et al. A multicentre, randomised, open-label study comparing the efficacy and safety of two doses of Doxorubicin Transdrug™ to best standard of care in patients with advanced Hepatocellular Carcinoma (HCC) after sorafenib. The relive study. P1334. J. Hepatology. 2015;62(Supp. 2):S856. https://doi.org/10.1016/S0168-8278(15)31513-0.
Merle P, Camus P, Abergel A, Pageaux GP, Masliah C, Bronowicki JP, et al. Safety and efficacy of intra-arterial hepatic chemotherapy with doxorubicin-loaded nanoparticles in hepatocellular carcinoma. ESMO Open. 2017;2(4):e000238. https://doi.org/10.1136/esmoopen-2017-000238.
Rinaudo M. Chitin and chitosan: properties and applications. Prog Polymer Sci. 2006;31:603–32.
Domard A. A perspective on 30 years research on chitin and chitosan. Carbohydr Polym. 2011;84:696–703. https://doi.org/10.1016/j.carbpol.2010.04.083.
Liu X, Howard KA, Dong M, Andersen MØ, Rahbek UL, Johnsen MG, et al. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials. 2007;28:1280–8.
Holzerny P, Ajdini B, Heusermann W, Bruno K, Schuleit M, Meinel L, et al. Biophysical properties of chitosan/siRNA polyplexes: profiling the polymer/siRNA interactions and bioactivity. J Control Release. 2012;157:297–304. https://doi.org/10.1016/j.jconrel.2011.08.023.
Bravo-Anaya LM, Fernández-Solís KG, Rosselgong J, Nano-Rodríguez JLE, Carvajal F, Rinaudo M. Chitosan-DNA polyelectrolyte complex: influence of chitosa characteristics and mechanism of complex formation. Int J Biol Macromol. 2019;126:1037–49.
Katas H, Alpar HO. Development and characterization of chitosan nanoparticles for siRNA delivery. J Control Release. 2006;115:216–25.
Weecharangsan W, Opanasopit P, Ngawhirunpart T, Apirakaramwong A, Rojanarata T, Ruktanonchai U, et al. Evaluation of chitosan salts as non-viral gene vectors in CHO-K1 cells. Int J Pharm. 2008;348:161–8.
Patel HR, Patel SP, Patel MM. Poloxamers: a pharmaceutical excipients with therapeutic behaviors. Int J Pharm Tech Res. 2009;1(2):299–303.
Poloxamer 188 Drug Bank #DB11333. 2019 September 17. Available from: https://www.drugbank.ca/drugs/DB11333.
Bertholon I, Lesieur S, Labarre D, Besnard M, Vauthier C. Characterization of dextran−poly(isobutylcyanoacrylate) copolymers obtained by redox radical and anionic emulsion polymerization. Macromolecules. 2006;39:3559–67.
Maghami GG, Roberts GAF. Evaluation of the viscometric constants for chitosan. Makromol Chem. 1988;189:195–200.
Khan TA, Peh KK, Ch'ng HS. Mechanical, bioadhesive strength and biological evaluations of chitosan films for wound dressing. J Pharm Pharm Sci. 2000;3(3):303–11.
Hirai A, Odani H, Nakajima A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym Bull. 1991;26:87–94.
Vauthier C, Schmidt C, Couveur P. Measurement of the density of polymeric Nanoparticulate drug carriers by Isopycnic centrifugation. J Nanopart Res. 1999;1:411–8.
Zandanel C, Vauthier C. Poly(isobutylcyanoacrylate) nanoparticles decorated with chitosan: effect of conformation of chitosan chains at the surface on complement activation properties. J Colloid Sci Biotechnol. 2012;1:68–81.
Ma PL, Lavertu M, Winnik FM, Buschmann MD. Stability and binding affinity of DNA/chitosan complexes by polyanion competition. Carbohydr Polym. 2017;176:167–76. https://doi.org/10.1016/j.carbpol.2017.08.002.
Zhang Y, Cremer PS. Interactions between macromolecules and ions: the Hofmeister series. Curr Opin Chem Biol. 2006;10(6):658–63. https://doi.org/10.1016/j.cbpa.2006.09.020.
Song W, Liu L, Liu G. Ion specificity of macromolecules in crowded environments. Soft Matter. 2015;11:5940–6.
Okur HI, Hladílková J, Rembert KB, Cho Y, Heyda J, Dzubiella J, et al. Beyond the Hofmeister series: ion-specific effects on proteins and their biological functions. J Phys Chem B. 2017;121(9):1997–2014. https://doi.org/10.1021/acs.jpcb.6b10797.
Zhang Y, Furyk S, Bergbreiter DE, Cremer PS. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J Am Chem Soc. 2005;127:14505–10. https://doi.org/10.1021/ja054642.
Mrácek A, Varhaníková J, Lehocký M, Grundelová L, Pokopcová A, Velebný V. The influence of Hofmeister series ions on hyaluronan swelling and viscosity. Molecules. 2008;13(5):1025–34.
Zajforoushan Moghaddam S, Thormann E. Hofmeister effect of salt mixtures on thermo-responsive poly(propylene oxide). Phys Chem Chem Phys. 2015;17(9):6359–66.
Zajforoushan Moghaddam S, Thormann E. Hofmeister effect on PNIPAM in bulk and at an Interface: surface partitioning of weakly hydrated anions. Langmuir. 2017;33(19):4806–15. https://doi.org/10.1021/acs.langmuir.7b00953.
Chen WY, Hsu MY, Tsai CW, Chang Y, Ruaan RC, Kao WH, et al. Kosmotrope-like hydration behavior of polyethylene glycol from microcalorimetry and binding isotherm measurements. Langmuir. 2013;29(13):4259–65. https://doi.org/10.1021/la304500w.
Smith MH, Lyon LA. Multifunctional nanogels for siRNA delivery. Acc Chem Res. 2012;45(7):985–93. https://doi.org/10.1021/ar200216f Poly(NIPAM) siRNA delivery.
Yang HY, van Ee RJ, Timmer K, Craenmehr EGM, Huang JH, Öner FC, et al. A novel injectable thermoresponsive and cytocompatible gel of poly(N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 2015;23:214–28. https://doi.org/10.1016/j.actbio.2015.05.018.
Antila HS, Van Tassel PR, Sammalkorpi M. Repulsion between oppositely charged rod-shaped macromolecules: role of overcharging and ionic confinement. J Chem Phys. 2017;147(12):124901. https://doi.org/10.1063/1.4993492.
ACKNOWLEDGMENTS AND DISCLOSURES
Financial support was obtained from INNABIOSANTE, Toulouse, France project NANOINTERFERENCE.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zandanel, C., Noiray, M. & Vauthier, C. Counterion of Chitosan Influences Thermodynamics of Association of siRNA with a Chitosan-Based siRNA Carrier. Pharm Res 37, 22 (2020). https://doi.org/10.1007/s11095-019-2751-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2751-z