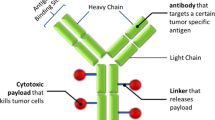
Abstract
Antibody drug conjugates (ADCs) are promising therapies currently in development for oncology with unique and challenging regulatory and scientific considerations. While there are currently no regulatory guidelines specific for the nonclinical development of ADCs, there are harmonized international guidelines (e.g., ICHS6(R1), ICHM3(R2), ICHS9) that apply to ADCs and provide a framework for their complex development with issues that apply to both small and large molecules. The regulatory and scientific perspectives on ADCs are evolving due to both the advances in ADC technology and a better understanding of the safety and efficacy of ADCs in clinical development. This paper introduces the key scientific and regulatory aspects of the nonclinical development of ADCs, discusses important regulatory and scientific issues in the nonclinical to clinical dose translation of ADCs, and introduces new concepts in the areas of pharmacokinetic/pharmacodynamic (PK/PD) modeling and simulation.

Similar content being viewed by others
Abbreviations
- ADC:
-
Antibody-drug conjugate
- ADME:
-
Absorption, distribution, metabolism and excretion
- ASCT:
-
Autologous stem cell transplant
- BW:
-
Body weight
- BSA:
-
Body surface area
- CNS:
-
Central nervous system
- DAR:
-
Drug-to-antibody ratio
- DM1:
-
Mertansine; N2′-deacetyl-N2′-(3-Mercapto-1-oxopropyl)-Maytansine
- DM4:
-
N2′-deacetyl-n2′-(4-Mercapto-4-Methyl-1-oxopentyl)-6-Methylmaytansine
- EMA:
-
European Medicines Agency
- FcRn:
-
Neonatal Fc receptor
- FDA:
-
US Food and Drug Administration
- FIH:
-
First-in-human
- HER2:
-
Human epidermal growth factor receptor 2
- hERG:
-
Human Ether-à-go-go-Related Gene
- HNSTD:
-
Highest non-severely toxic dose
- ICH:
-
The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- mAb:
-
Monoclonal antibodies
- MMAE:
-
Monomethyl auristatin E
- MMAF:
-
Desmethyl-auristatin F
- MOA:
-
Mechanism of action
- MTD:
-
Maximum tolerated dose
- PBD:
-
Pyrrolobenzodiazepine
- PD:
-
Pharmacodynamics
- PK:
-
Pharmacokinetics
- POC:
-
Proof-of-concept
- PNS:
-
Peripheral nervous system
- SMCC:
-
Succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate
- STD10:
-
Severely toxic dose in 10% of rodents
- TDC:
-
ThioMAb drug conjugate
- TK:
-
Toxicokinetics
References
Trail PA. Antibody drug conjugates as cancer therapeutics. Antibodies. 2013;2(1):113–29.
Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90.
Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28:2698–704.
Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25.
Goebl NA, Babbey CM, Datta-Mannan A, Witcher DR, Wroblewski VJ, Dunn KW. Neonatal Fc receptor mediates internalization of Fc in transfected human endothelial cells. Mol Biol Cell. 2008;19(12):5490–505.
Kuo TT, Baker K, Yoshida M, Qiao SW, Aveson VG, Lencer WI, et al. Neonatal Fc Receptor: from Immunity to Therapeutics. J Clin Immunol. 2010;30:777–89.
Dosio F, Stella B, Cerioni S, Gastaldi D, Arpicco S. Advances in anticancer antibody-drug conjugates and immunotoxins. Recent Patents Anti-Cancer Drug Discov. 2014;9:35–65.
Mullard A. Maturing antibody–drug conjugate pipeline hits 30. Nat Rev Drug Discov. 2013;12:329–32.
Flygare JA, Pillow TH, Aristoff P. Antibody‐drug conjugates for the treatment of cancer. Chem Biol Drug Des. 2013;81(1):113–21.
Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–32.
Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. mAbs. 2014;6(1):34–45.
Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–70.
Shen BQ, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30(2):184–9.
Kadcyla US Package Insert. 2014. Available from: http://www.gene.com/download/pdf/kadcyla_prescribing.pdf.
Adcetris US Package Insert. 2014. Available from: http://www.adcetris.com/pdf/ADCETRIS-brentuximab-vedotin-Prescribing-Information.pdf.
Food and Drug Administration, 2010. “FDA: Pfizer Voluntarily Withdraws Cancer Treatment Mylotarg from U.S. Market.” FDA News Release. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm216448.htm.
Brennan, 2014. US FDA, EMA offer similar explanations for ADC regulations. Available from: http://www.biopharma-reporter.com/Markets-Regulations/US-FDA-EMA-offer-similar-explanations-for-ADC-regulations.
International Conference on Harmonisation, Harmonised Tripartite Guideline, Preclinical Safety Evaluation of Biotechnology-derived Pharmaceuticals S6(R1). 2011. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S6_R1/Step4/S6_R1_Guideline.pdf.
International Conference on Harmonisation, Harmonised Tripartite Guideline, Guidance on Nonclinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals M3(R2). 2009. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M3_R2/Step4/M3_R2__Guideline.pdf.
International Conference on Harmonisation, Harmonised Tripartite Guideline, Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use S2(R1). 2011. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S2_R1/Step4/S2R1_Step4.pdf.
International Conference on Harmonisation, Safety Pharmacology Studies for Human Pharmaceuticals S7A. 2000. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7A/Step4/S7A_Guideline.pdf.
International Conference on Harmonisation, The Non-clinical Evaluation of the Potentential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals S7B. 2005. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S7B/Step4/S7B_Guideline.pdf.
International Conference on Harmonisation, Nonclinical Evaluation for Anticancer Pharmaceuticals S9. 2009. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S9/Step4/S9_Step4_Guideline.pdf.
International Conference on Harmonisation, Immunotoxicity Studies for Human Pharmaceuticals S8. 2005. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S8/Step4/S8_Guideline.pdf.
Boswell C, Mundo E, Zhang C, Bumbaca D, Valle N, Kozak K, et al. Impact of drug conjugation on pharmacokinetics and tissue distribution of Anti-STEAP1 Antibody–Drug Conjugates in Rats. Bioconjug Chem. 2011;22(10):1994–2004.
Drake PM, Albers AE, Baker J, Banas S, Barfield RM, Bhat AS, et al. Aldehyde tag coupled with HIPS chemistry enables the production of ADCs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and PK outcomes. Bioconjug Chem. 2014;25(7):1331–41.
Kaur S, Keyang X, Saad O, Dere R, Carrasco-Triguero M. Bioanalytical assay strategies for the development of antibody–drug conjugate biotherapeutics. Bioanalysis. 2013;5(2):201–26.
Food and Drug Administration Guidance for Industry, Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf.
Alley S, Bilic S, Booth B, Gorovits B, Kaur S, Oldfield P, et al. Considerations for the bioanalysis of antibody drug conjugates. AAPS ADC working group position paper. Bioanalysis. 2013;5:997–1006.
Roberts SA, Andrews PA, Blanset D, Flagella KM, Gorovits B, Lynch CM, et al. Considerations for the nonclinical safety evaluation of antibody drug conjugates for oncology. Regul Toxicol Pharmacol. 2013;67(3):382–91.
Saber H, Leighton JK. An FDA oncology analysis of antibody-drug conjugates. Regul Toxicol Pharmacol. 2015;71(3):444–52.
International Conference on Harmonisation, Final Concept Paper S9: Q&As on Nonclinical Evaluation for Anticancer Pharmaceuticals. 2014. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Safety/S9/S9_Q_As_Final_Concept_Paper_October_23_2014.pdf.
Krippendorff B-F, Kuester K, Kloft C, Huisinga W. Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis. J Pharmacokinet Pharmacodyn. 2009;36:239–60.
Shah D, Haddish-Berhane N, Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn. 2012;39:643–59.
Sorger, PK, Allerheiligen, SR, Abernethy, DR, Altman, RB, Brouwer, KL, Califano, A, … & Ward, R. Quantitative and systems pharmacology in the post-genomic era: new approaches to discovering drugs and understanding therapeutic mechanisms. in An NIH white paper by the QSP workshop group. Bethesda, MD. 2011. Available at: http://www.nigms.nih.gov/training/documents/systemspharmawpsorger2011.pdf.
Khurana, M., Mehrotra, N., & Zadezensky, I. Clinical Pharmacology Assessment: Adequacy of Natpara Dosage Regimen in Treatment of Hypoparathyroidism. 2014. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM413617.pdf.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lansita, J.A., Burke, J.M., Apgar, J.F. et al. An Introduction to the Regulatory and Nonclinical Aspects of the Nonclinical Development of Antibody Drug Conjugates. Pharm Res 32, 3584–3592 (2015). https://doi.org/10.1007/s11095-015-1742-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1742-y