ABSTRACT
Purpose
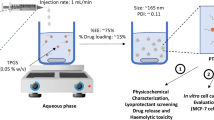
Preparation, optimization and in vitro evaluation of core-shell nanoparticles comprising of a hydrophilic core of BSA surrounded by a hydrophobic shell of PLGA for loading water-soluble drugs.
Methods
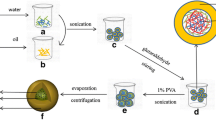
A double emulsion method was optimized for preparation of BSA-PLGA based core-shell nanoparticles. Proof of concept for core-shell type structure was established by visual techniques like confocal microscopy and TEM. Characterization was done for particle size, encapsulation efficiency, drug loading and in vitro drug release. Cellular uptake was assessed using confocal microscopy, bio-TEM and HPLC assay, and cytotoxic activity was tested by MTT assay in MG-63 osteosarcoma cells.
Results
The optimized core-shell nanoparticles showed a particle size of 243 nm (PDI-0.13) and encapsulation efficiency of 40.5% with a drug loading of 8.5% w/w. In vitro drug release studies showed a sustained release for 12 h. Cellular uptake studies indicated a rapid and efficient uptake within 2 h. TEM studies indicated that the core-shell nanoparticles were localized in cytoplasm region of the cells. Gemcitabine loaded core-shell nanoparticles showed enhanced cytotoxicity against MG-63 cells as compared to marketed formulation of gemcitabine (GEMCITE®).
Conclusion
These results indicate that core-shell nanoparticles can be a good carrier system for delivering hydrophilic drugs like gemcitabine successfully to the cells with enhanced efficacy.

Core-Shell Nanoparticles with a hydrophilic BSA core and hydrophobic PLGA shell for carrier system of hydrophilic drugs





Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumin
- PLGA:
-
poly(lactic acid-co-glycolic acid)
REFERENCES
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2012; In press. doi:10.1016/j.addr.2012.09.037
Ayen WY, Garkhal K, Kumar N. Doxorubicin-loaded (PEG)(3)-PLA nanopolymersomes: effect of solvents and process parameters on formulation development and in vitro study. Mol Pharm. 2011;8(2):466–78.
Ayen WY, Chintankumar B, Jain JP, Kumar N. Effect of PEG chain length and hydrophilic weight fraction on polymersomes prepared from branched (PEG)3-PLA co-polymers. Polym Adv Technol. 2011;22(1):158–65.
Jain JP, Kumar N. Self assembly of amphiphilic (PEG)(3)-PLA copolymer as polymersomes: preparation, characterization, and their evaluation as drug carrier. Biomacromolecules. 2010;11(4):1027–35.
Ayen WY, Kumar N. In vivo evaluation of doxorubicin-loaded (PEG)(3)-PLA nanopolymersomes (PolyDoxSome) using DMBA-induced mammary carcinoma rat model and comparison with marketed LipoDox. Pharm Res. 2012;29(9):2522–33.
Jeong Y-I, Cheon J-B, Kim S-H, Nah J-W, Lee Y-M, Sung Y-K, et al. Clonazepam release from core-shell type nanoparticles in vitro. J Control Release. 1998;51(2–3):169–78.
Chan JM, Zhang L, Yuet KP, Liao G, Rhee JW, Langer R, et al. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials. 2009;30(8):1627–34.
Chitkara D, Singh S, Kumar V, Danquah M, Behrman SW, Kumar N, et al. Micellar delivery of cyclopamine and Gefitinib for treating pancreatic cancer. Mol Pharm. 2012;9(8):2350–7.
Chitkara D, Nikalaje SK, Mittal A, Chand M, Kumar N. Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Dev Trans Res. 2012;2(2):112–23.
Lee JY, Lee SH, Oh MH, Kim JS, Park TG, Nam YS. Prolonged gene silencing by siRNA/chitosan-g-deoxycholic acid polyplexes loaded within biodegradable polymer nanoparticles. J Control Release. 2012;162(2):407–13.
Steinbach JM, Weller CE, Booth CJ, Saltzman WM. Polymer nanoparticles encapsulating siRNA for treatment of HSV-2 genital infection. J Control Release. 2012;162(1):102–10.
Barichello JM, Morishita M, Takayama K, Nagai T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev Ind Pharm. 1999;25(4):471–6.
Zhang Z, Huey Lee S, Feng SS. Folate-decorated poly(lactide-co-glycolide)-vitamin E TPGS nanoparticles for targeted drug delivery. Biomaterials. 2007;28(10):1889–99.
Tewes F, Munnier E, Antoon B, Ngaboni Okassa L, Cohen-Jonathan S, Marchais H, et al. Comparative study of doxorubicin-loaded poly(lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur J Pharm Biopharm. 2007;66(3):488–92.
Leo E, Brina B, Forni F, Vandelli MA. In vitro evaluation of PLA nanoparticles containing a lipophilic drug in water-soluble or insoluble form. Int J Pharm. 2004;278(1):133–41.
Ubrich N, Bouillot P, Pellerin C, Hoffman M, Maincent P. Preparation and characterization of propranolol hydrochloride nanoparticles: a comparative study. J Control Release. 2004;97(2):291–300.
Khiati S, Luvino D, Oumzil K, Chauffert B, Camplo M, Barthelemy P. Nucleoside-lipid-based nanoparticles for cisplatin delivery. ACS Nano. 2011;5(11):8649–55.
Kashi TS, Eskandarion S, Esfandyari-Manesh M, Marashi SM, Samadi N, Fatemi SM, et al. Improved drug loading and antibacterial activity of minocycline-loaded PLGA nanoparticles prepared by solid/oil/water ion pairing method. Int J Nanomedicine. 2012;7(1):221–34.
Cavalli R, Bargoni A, Podio V, Muntoni E, Zara GP, Gasco MR. Duodenal administration of solid lipid nanoparticles loaded with different percentages of tobramycin. J Pharm Sci. 2003;92(5):1085–94.
Chavanpatil MD, Khdair A, Patil Y, Handa H, Mao G, Panyam J. Polymer-surfactant nanoparticles for sustained release of water-soluble drugs. J Pharm Sci. 2007;96(12):3379–89.
Vrignaud S, Benoit JP, Saulnier P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials. 2011;32(33):8593–604.
Chen Y, Chen H, Zeng D, Tian Y, Chen F, Feng J, et al. Core/shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug delivery. ACS Nano. 2010;4(10):6001–13.
Hu SH, Chen SY, Gao X. Multifunctional nanocapsules for simultaneous encapsulation of hydrophilic and hydrophobic compounds and on-demand release. ACS Nano. 2012;6(3):2558–65.
Liu M, Gan L, Chen L, Xu Z, Zhu D, Hao Z, et al. Supramolecular core-shell nanosilica@liposome nanocapsules for drug delivery. Langmuir. 2012;28(29):10725–32.
Zhu J, Tang A, Law LP, Feng M, Ho KM, Lee DK, et al. Amphiphilic core-shell nanoparticles with poly(ethylenimine) shells as potential gene delivery carriers. Bioconjug Chem. 2005;16(1):139–46.
Oh KS, Han SK, Lee HS, Koo HM, Kim RS, Lee KE, et al. Core/shell nanoparticles with lecithin lipid cores for protein delivery. Biomacromolecules. 2006;7(8):2362–7.
Arias JL, Gallardo V, Ruiz MA, Delgado AV. Magnetite/poly(alkylcyanoacrylate) (core/shell) nanoparticles as 5-fluorouracil delivery systems for active targeting. Eur J Pharm Biopharm. 2008;69(1):54–63.
Reddy LH, Couvreur C. Novel approaches to deliver gemcitabine to cancer. Curr Pharm Des. 2008;14(11):1124–37.
Okino H, Maeyama R, Manabe T, Matsuda T, Tanaka M. Trans-tissue, sustained release of gemcitabine from photocured gelatin Gel inhibits the growth of heterotopic human pancreatic tumor in nude mice. Clin Cancer Res. 2003;9(15):5786–93.
Celano M, Cavalgno MG, Bulotta S, Paolino D, Arturi F. Cytotoxic effects of gemcitabine-loaded liposomes in human anaplastic thyroid carcinoma cells. BMC Cancer. 2004;4(1):63–70.
Cavallaro G, Mariano L, Salmaso S, Caliceti P, Gaetano G. Folate-mediated targeting of polymeric conjugates of gemcitabine. Int J Pharm. 2006;307(1–2):258–69.
Patra CR, Bhattacharya R, Wang E, Katarya A, Lau JS, Dutta S, et al. Targeted delivery of gemcitabine to pancreatic adenocarcinoma using cetuximab as a targeting agent. Cancer Res. 2008;68(6):1970–8.
Yang J, Park S-B, Yoon H-G, Huh Y-M, Haam S. Preparation of poly caprolactone nanoparticles containing magnetite for magnetic drug carrier. Int J Pharm. 2006;324(2):185–90.
Song C, Labhasetwar V, Cui X, Underwood T, Levy RJ. Arterial uptake of biodegradable nanoparticles for intravascular local drug delivery: results with an acute dog model. J Control Release. 1998;54(2):201–11.
Hosseinzadeh H, Atyabi F, Dinarvand R, Ostad SN. Chitosan-pluronic nanoparticles as oral delivery of anticancer gemcitabine: preparation and in vitro study. Int J Nanomedicine. 2012;7(1):1851–63.
Kandagal PB, Ashoka S, Seetharamappa J, Shaikh SMT, Jadegoud Y, Ijare OB. Study of the interaction of an anticancer drug with human and bovine serum albumin: Spectroscopic approach. J Pharm Biomed Anal. 2006;41(2):393–9.
Lamprecht A, Ubrich N, Pérez MH, Lehr C-M, Hoffman M, Maincent P. Influences of process parameters on nanoparticle preparation performed by a double emulsion pressure homogenization technique. Int J Pharm. 2000;196(2):177–82.
Guhagarkar SA, Malshe VC, Devarajan PV. Nanoparticles of polyethylene sebacate: a new biodegradable polymer. AAPS PharmSciTech. 2009;10(3):935–42.
Lamprecht A, Ubrich N, Yamamoto H, Schafer U, Takeuchi H, Lehr CM, et al. Design of rolipram-loaded nanoparticles: comparison of two preparation methods. J Control Release. 2001;71(3):297–306.
Braakhuis BJ, Ruiz van Haperen VW, Boven E, Veerman G, Peters GJ. Schedule-dependent antitumor effect of gemcitabine in in vivo model system. Semin Oncol. 1995;22(4 Suppl 11):42–6.
dos Santos T, Varela J, Lynch I, Salvati A, Dawson KA. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS One. 2011;6(9):e24438.
Alexander RL, Greene BT, Torti SV, Kucera GL. A novel phospholipid gemcitabine conjugate is able to bypass three drug-resistance mechanisms. Cancer Chemother Pharmacol. 2005;56(1):15–21.
ACKNOWLEDGMENTS AND DISCLOSURES
Financial support from the Department of Biotechnology, India is gratefully acknowledged. Authors are thankful to Department of Science and Technology, India for confocal laser scanning microscope and TEM facilities at NIPER, SAS Nagar. Ranbaxy Science Foundation (RSF), India is duly acknowledged for recognizing this work by awarding Ranbaxy Science Scholar Award-2011 to DC in the field of Pharmaceutical sciences. Support from Dr. Anupama Mittal in conducting cell culture experiments is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Figure S1
DSC thermograms of A) Gemcitabine, B) Blank core-shell nanoparticles, C) Gemcitabine loaded core-shell nanoparticles, D) PLGA and, E) BSA. (JPEG 142 kb)
Figure S2
XRD diagrams of (A) Gemcitabine, (B) PLGA, (C) BSA, (D) Gemcitabine loaded core-shell nanoparticles and (E) Blank core-shell nanoparticles (JPEG 228 kb)
Rights and permissions
About this article
Cite this article
Chitkara, D., Kumar, N. BSA-PLGA-Based Core-Shell Nanoparticles as Carrier System for Water-Soluble Drugs. Pharm Res 30, 2396–2409 (2013). https://doi.org/10.1007/s11095-013-1084-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1084-6